Marine Natural Products as Breast Cancer Resistance Protein Inhibitors
Abstract
:1. Introduction
1.1. Breast Cancer Resistance Protein
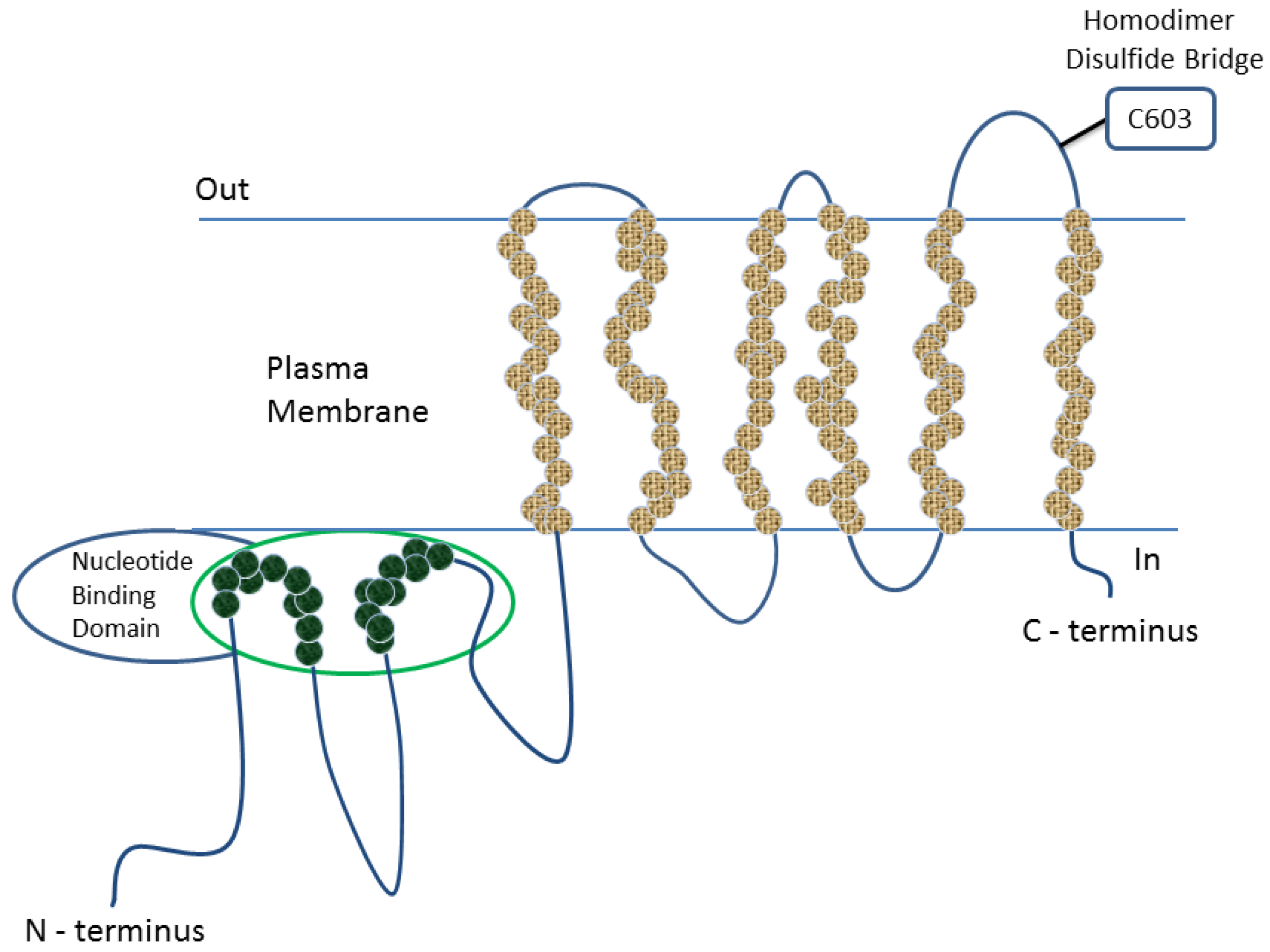
1.2. Functions of BCRP
1.3. Importance in Therapy
1.4. BCRP Inhibitors
| Inhibitor | Substrate | Cell Line | ATPase Activity | Photoaffinity Labeling | Specificity |
|---|---|---|---|---|---|
| FTC [14,15] | MX | S1-M1-3.2 | Inhibited | XX | Yes |
| Novobiocin [16] | TPT | PC6/SN2-5H2 | XX | XX | Yes |
| Elacridar [17,18,19] | MX | MCF-7 MX | Inhibited | Unaffected | No |
| Reserpine [20,21] | H33342 | SP | XX | Inhibited | No |
| Cyclosporin A [22,23,24] | PhA, MX | HEK/482R | Inhibited | Unaffected | No |
| Tariquidar [25] | MX | H460/MX20 | Stimulated | XX | No |
| Ortataxel [26] | MX | 8226/MR20 | Decreased | XX | No |
| Gefitinib [27] | H33342 | PLB-ABCG2 | Inhibited a | XX | No |
1.5. In Silico Studies with BCRP Inhibitors
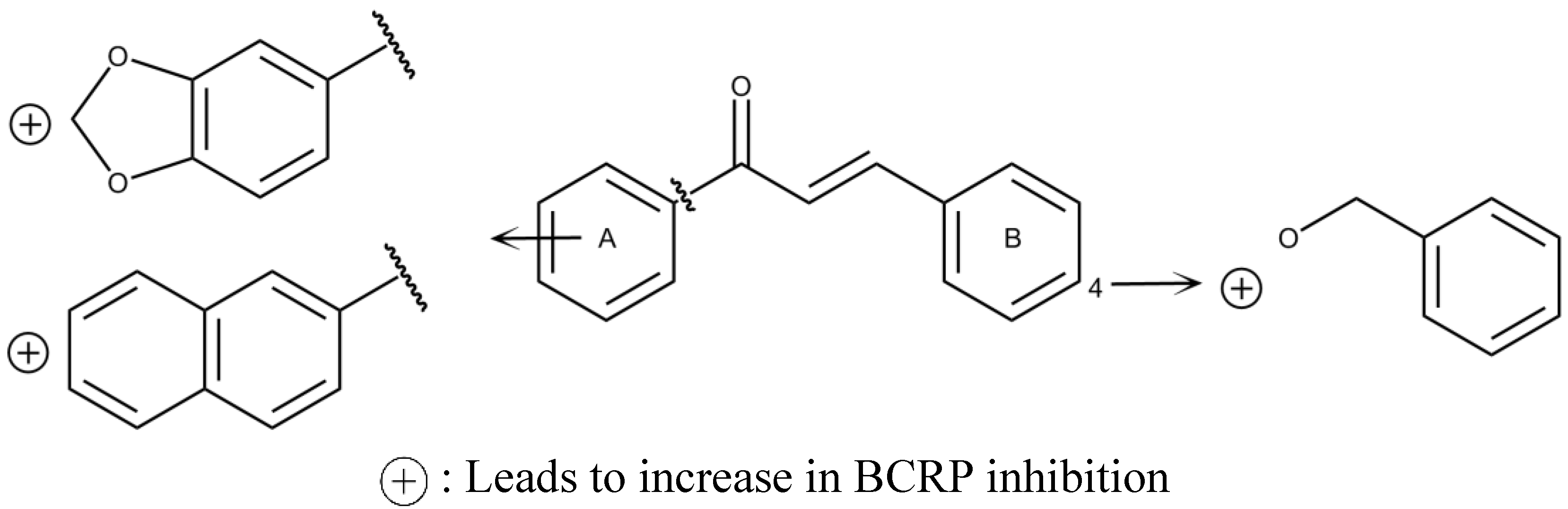
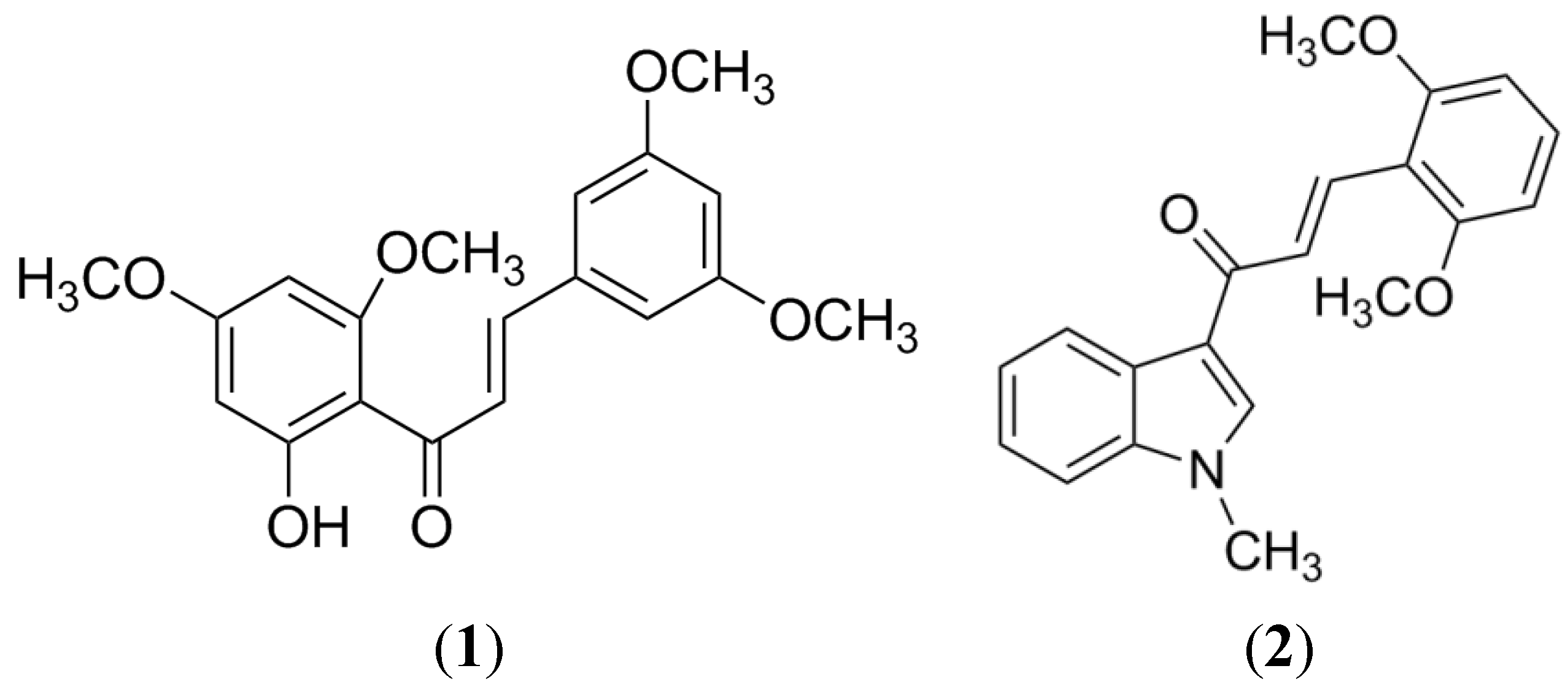
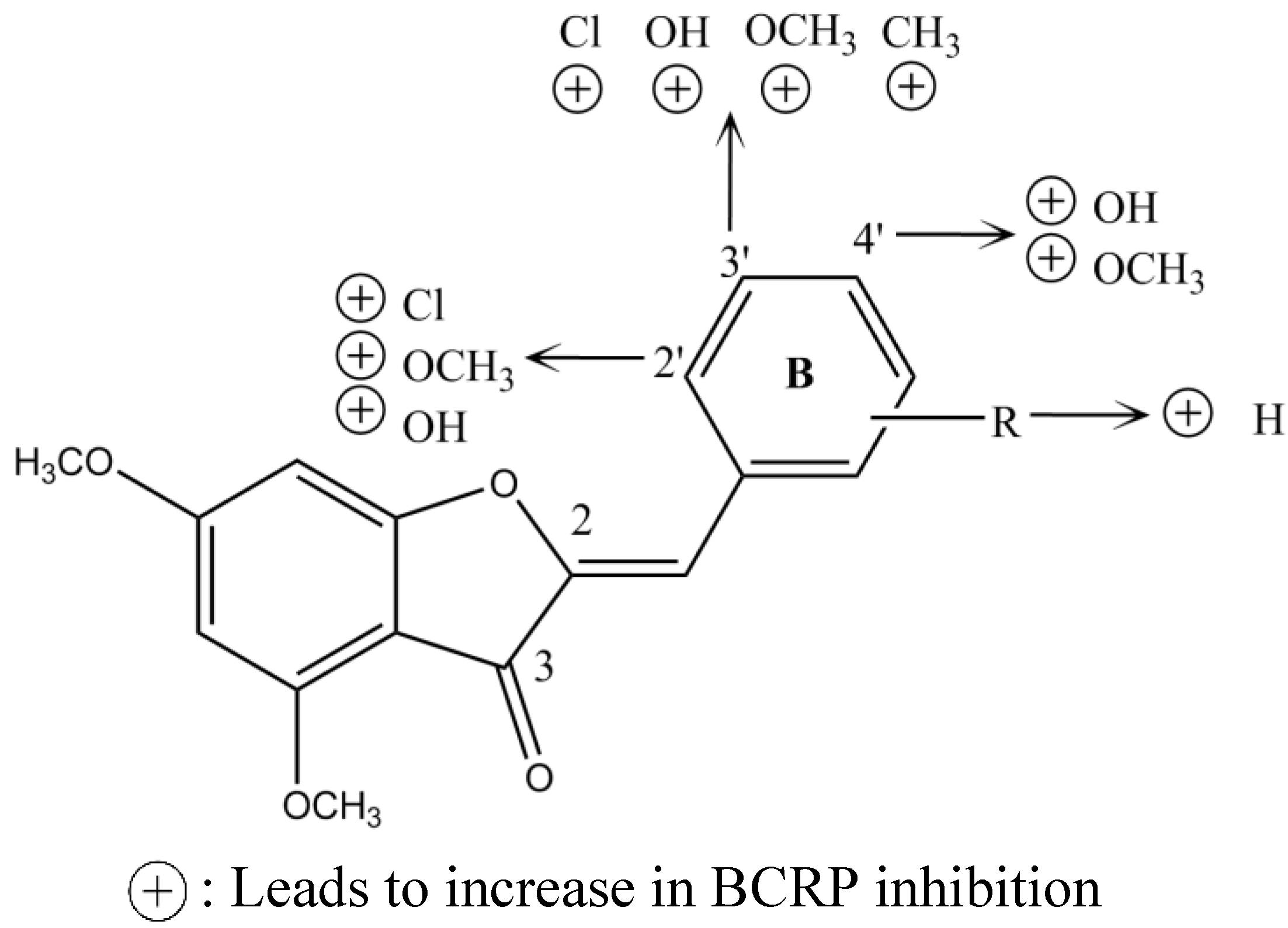
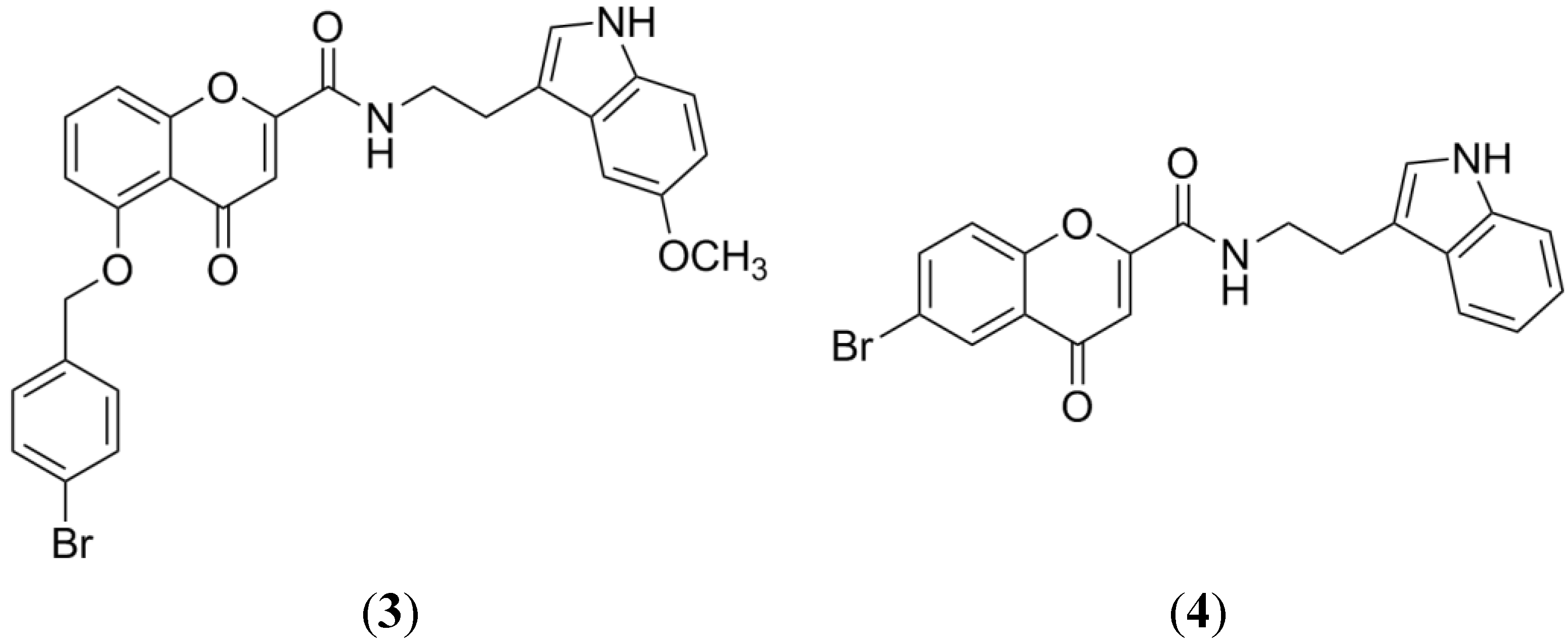
2. BCRP Inhibitors from Marine Sources
2.1. Fumitremorgin C
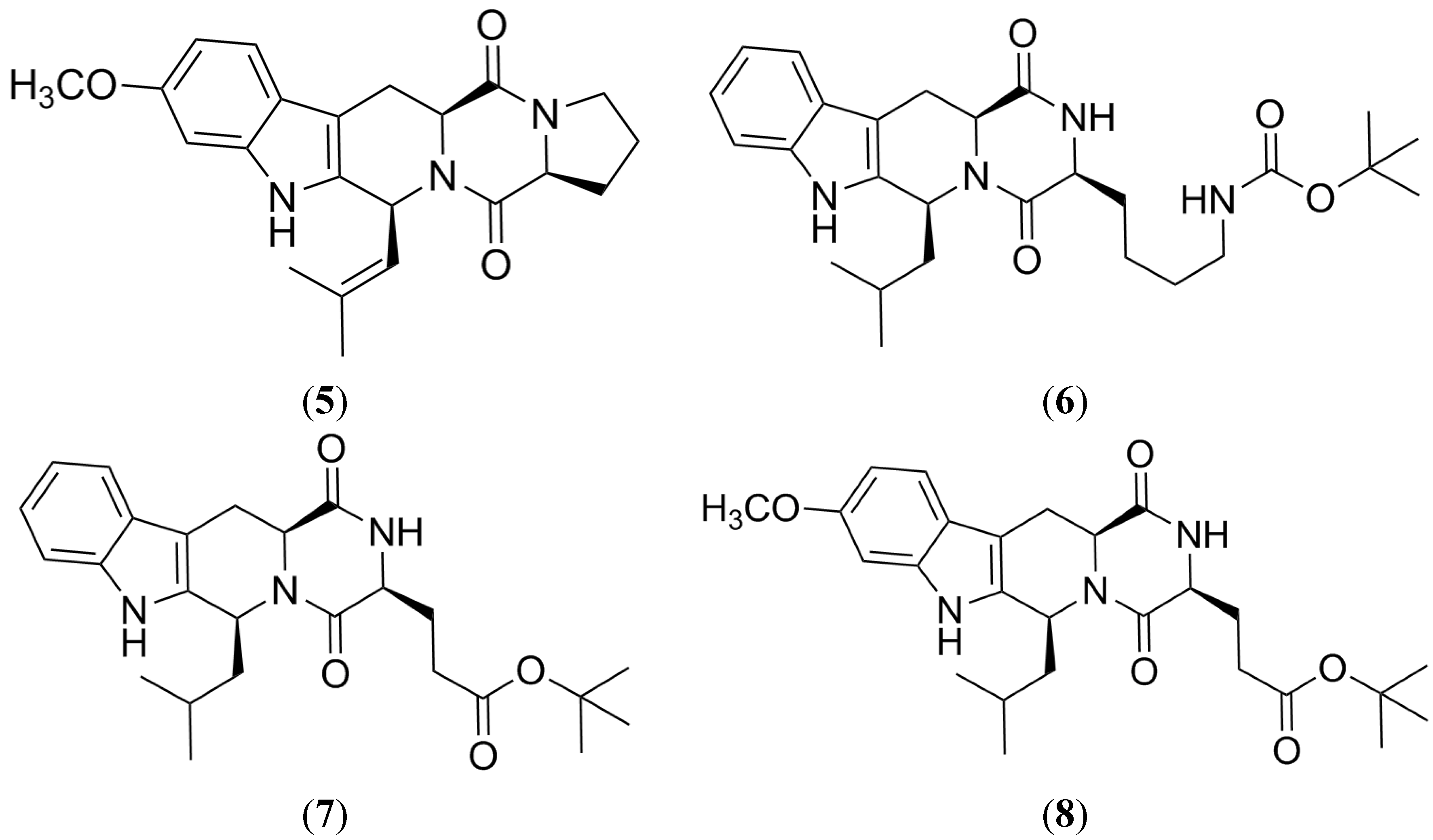
2.2. Tryprostatin A
2.3. Harmine
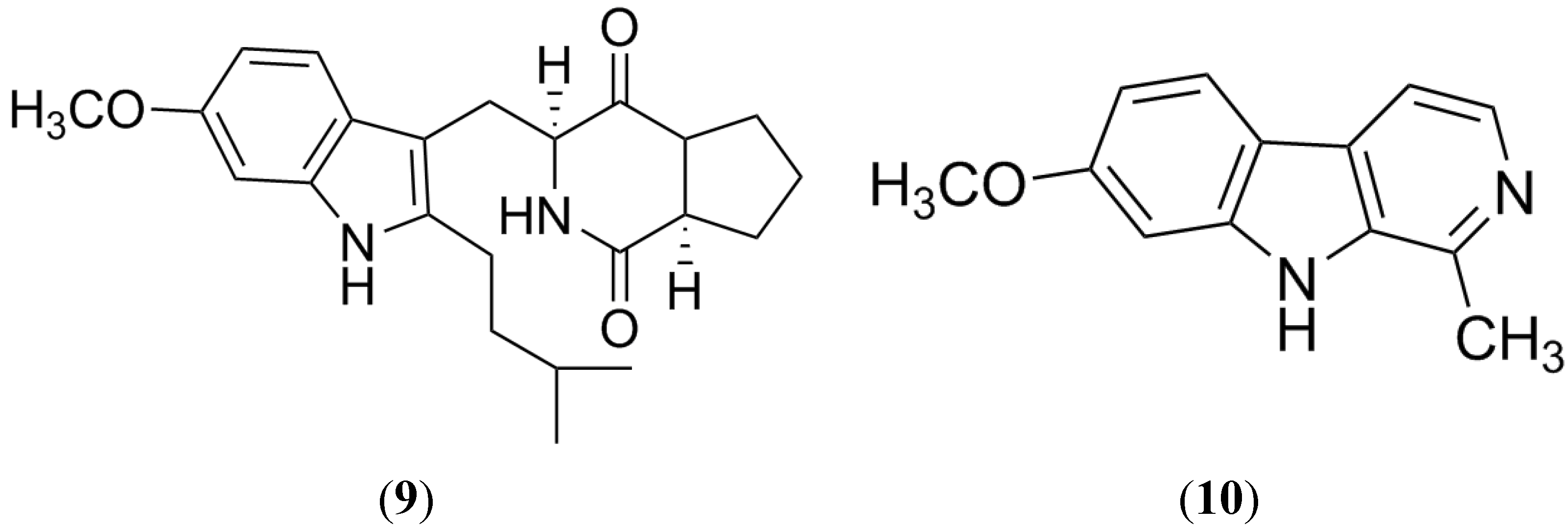
2.4. Botryllamides
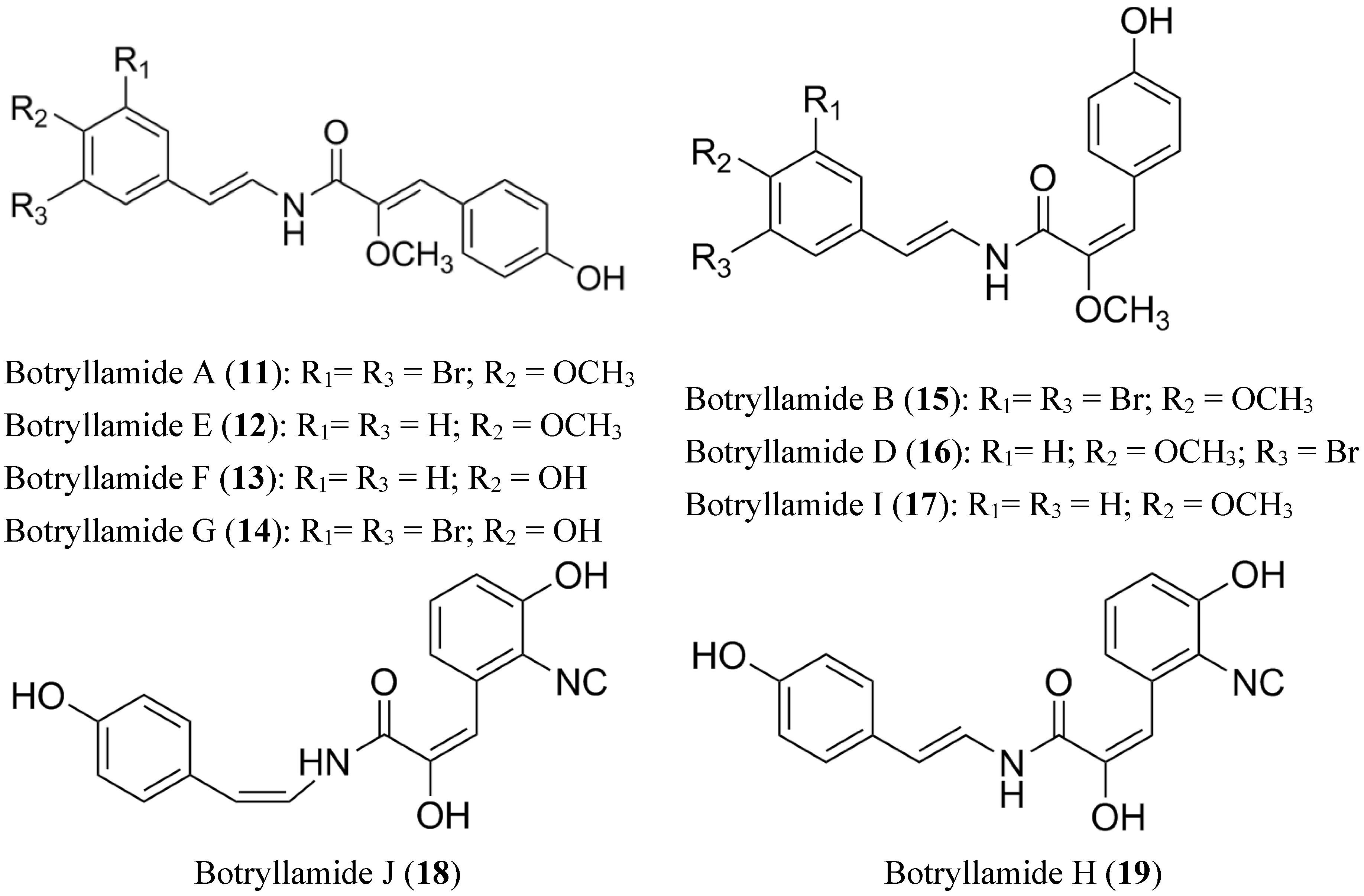
2.5. Lamellarin O
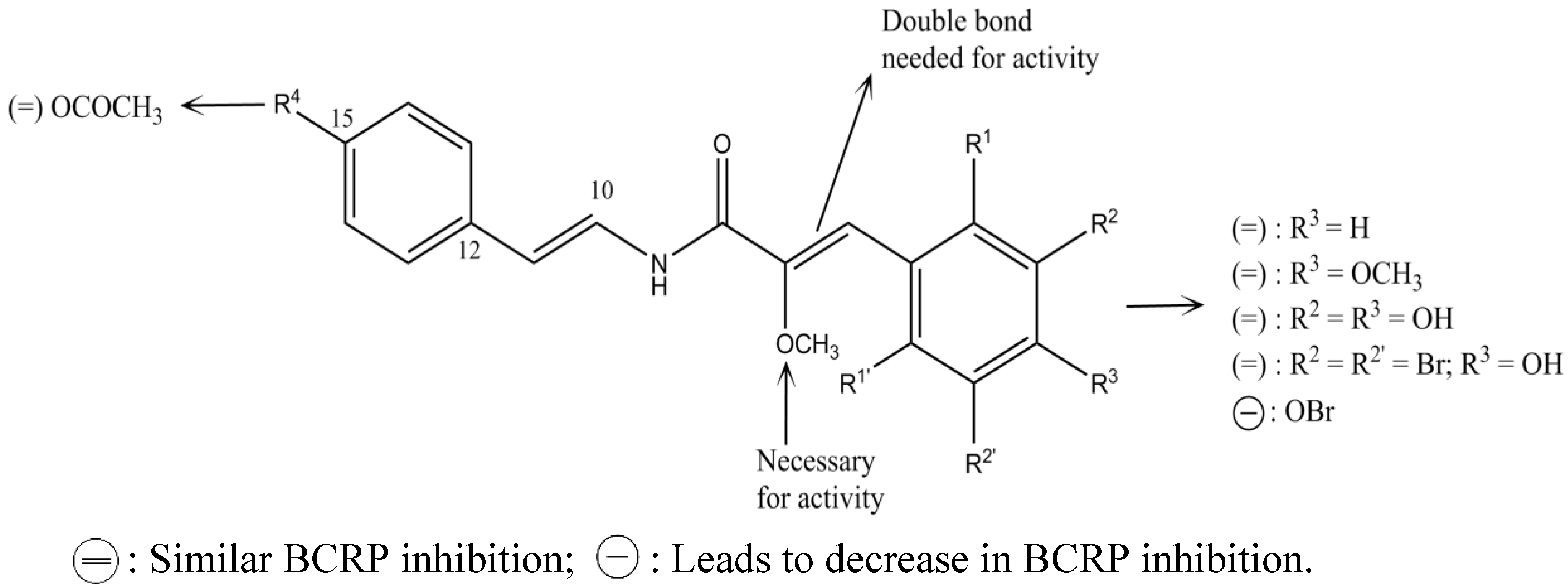
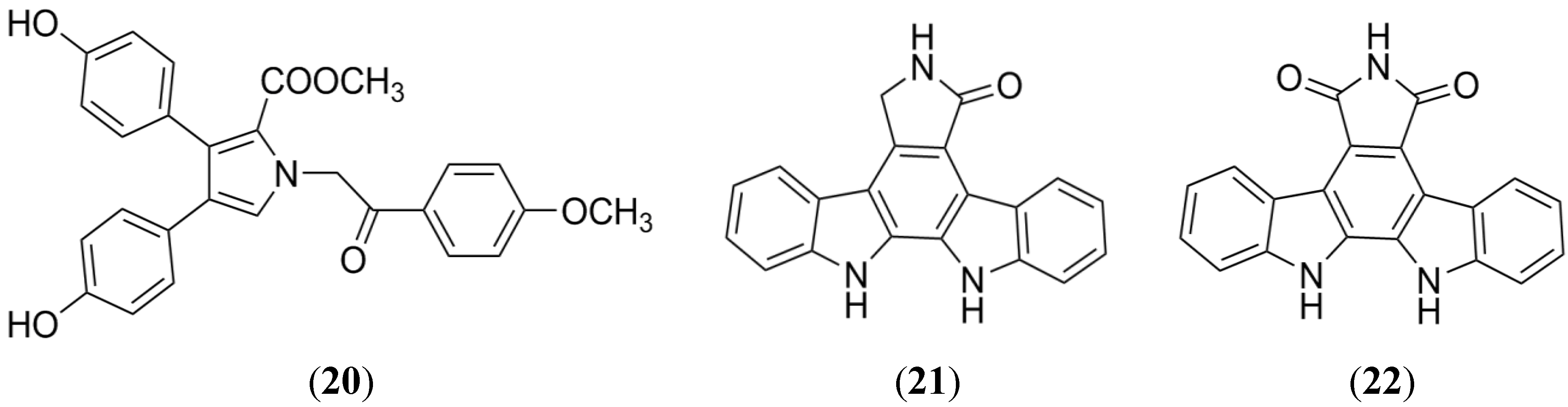
2.6. Indolocarbazole Alkaloids
2.7. Terrein
2.8. Secalonic Acid D
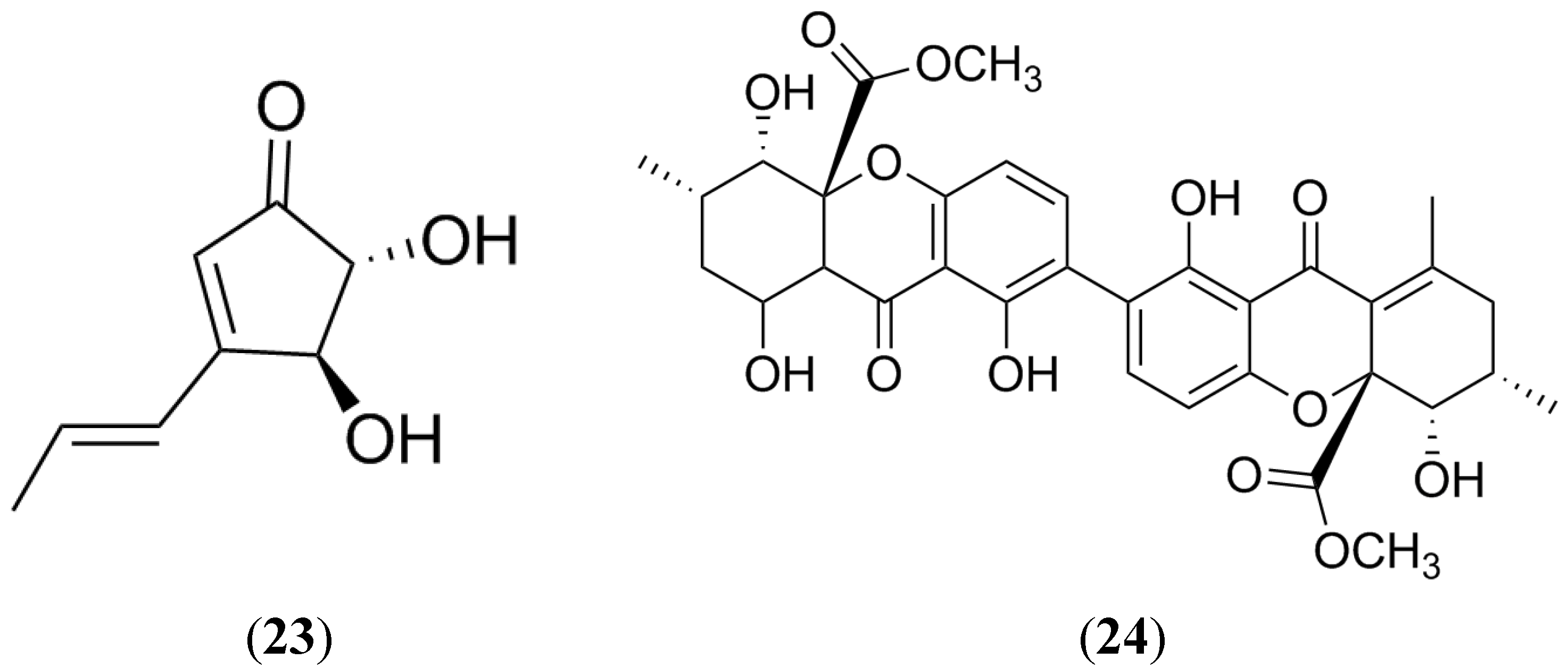
2.9. Naphthopyrones

3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lecerf-Schmidt, F.; Peres, B.; Valdameri, G.; Gauthier, C.; Winter, E.; Payen, L.; di Pietro, A.; Boumendjel, A. ABCG2: Recent discovery of potent and highly selective inhibitors. Future Med. Chem. 2013, 5, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Xie, Y.; Baer, M.R.; Ross, D.D. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem. Pharmacol. 2012, 83, 1084–1103. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Katayama, K.; Sugimoto, Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers. Med. 2014, 7, 53–64. [Google Scholar] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport-an update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.E.; Jansson, P.J.; Richardson, D.R. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol. Pharmacol. 2013, 84, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Mickley, L.; Litman, T.; Zhan, Z.; Robey, R.; Cristensen, B.; Brangi, M.; Greenberger, L.; Dean, M.; Fojo, T.; et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: Demonstration of homology to ABC transport genes. Cancer Res. 1999, 59, 8–13. [Google Scholar] [PubMed]
- Allikmets, R.; Schriml, L.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339. [Google Scholar] [PubMed]
- Wang, H.; Zhou, L.; Gupta, A.; Vethanayagam, R.R.; Zhang, Y.; Unadkat, J.D.; Mao, Q. Regulation of BCRP/ABCG2 expression by progesterone and 17 beta-estradiol in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E798–E807. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Potska, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Müller, F.; Fromm, M.F. Transporters and drug-drug interactions: Important determinants of drug disposition and effects. Pharmacol. Rev. 2013, 65, 944–966. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Honjo, Y.; van de Laar, A.; Miyake, K.; Regis, J.T.; Litman, T.; Bates, S.E. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim. Biophys. Acta 2001, 1512, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ozvegy, C.; Litman, T.; Szakács, G.; Nagy, Z.; Bates, S.; Váradi, A.; Sarkadi, B. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochem. Biophys. Res. Commun. 2001, 285, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, K.; Oka, M.; Soda, H.; Yoshikawa, M.; Ikegami, Y.; Tsurutani, J.; Nakatomi, K.; Nakamura, Y.; Doi, S.; Kitazaki, T.; et al. Reversal of breast cancer resistance protein (BCRP/ABCG2)-mediated drug resistance by novobiocin, a coumermycin antibiotic. Int. J. Cancer 2004, 108, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pick, A.; Müller, H.; Wiese, M. Structure-activity relationships of new inhibitors of breast cancer resistance protein (ABCG2). Bioorg. Med. Chem. 2008, 16, 8224–8236. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Pozza, A.; Muñoz-Martínez, F.; Bates, S.E.; Castanys, S.; Gamarro, F.; di Pietro, A.; Pérez-Victoria, J.M. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005, 65, 4852–4860. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.; Perez-Victoria, J.M.; Sardo, A.; Ahmed-Belkacem, A.; Di Pietro, A. Purification of breast cancer resistance protein ABCG2 and role of arginine-482. Cell Mol. Life Sci. 2006, 63, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Schuetz, J.D.; Bunting, K.D.; Colapietro, A.M.; Sampath, J.; Morris, J.J.; Lagutina, I.; Grosveld, G.C.; Osawa, M.; Nakauchi, H.; et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med. 2001, 7, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Alqawi, O.; Bates, S.; Georges, E. Arginine482 to threonine mutation in the breast cancer resistance protein ABCG2 inhibits rhodamine 123 transport while increasing binding. Biochem. J. 2004, 382, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dai, Y.; Vethanayagam, R.R.; Hebert, M.F.; Thummel, K.E.; Unadkat, J.D.; Ross, D.D.; Mao, Q. Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother. Pharmacol. 2006, 58, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Conseil, G.; Gupta, A.; Cole, S.P.; Unadkat, J.D. Functional expression of the human breast cancer resistance protein in Pichia pastoris. Biochem. Biophys. Res. Commun. 2004, 320, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Ejendal, K.F.; Hrycyna, C.A. Differential sensitivities of the human ATP-binding cassette transporters ABCG2 and P-glycoprotein to cyclosporin A. Mol. Pharmacol. 2005, 67, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Telu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Halldin, C.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS Chem. Neurosci. 2011, 2, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Minderman, H.; Brooks, T.A.; O’Loughlin, K.L.; Ojima, I.; Bernacki, R.J.; Baer, M.R. Broad-spectrum modulation of ATP-binding cassette transport proteins by the taxane derivatives ortataxel (IDN-5109, BAY 59-8862) and tRA96023. Cancer Chemother. Pharmacol. 2004, 53, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Ozvegy-Laczka, C.; Hegedus, T.; Várady, G.; Ujhelly, O.; Schuetz, J.D.; Váradi, A.; Kéri, G.; Orfi, L.; Német, K.; Sarkadi, B. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol. Pharmacol. 2004, 65, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Hoffmann, B.; Stoven, V.; Vert, J.P. Virtual screening of GPCRs: An in silico chemogenomics approach. BMC Bioinform. 2008, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.L.; Shih, Y.H.; Tsai, F.Y.; Leong, M.K. In silico prediction of inhibition of promiscuous breast cancer resistance protein (BCRP/ABCG2). PLoS ONE 2014, 9, e90689. [Google Scholar] [CrossRef] [PubMed]
- Villoutreix, B.O.; Kuenemann, M.A.; Poyet, J.L.; Bruzzoni-Giovanelli, H.; Labbé, C.; Lagorce, D.; Sperandio, O.; Miteva, M.A. Drug-like protein—Protein interaction modulators: Challenges and opportunities for drug discovery and chemical biology. Mol. Inform. 2014, 33, 414–437. [Google Scholar] [CrossRef] [PubMed]
- Hazai, E.; Bikádi, Z. Homology modeling of breast cancer resistance protein (ABCG2). J. Struct. Biol. 2008, 162, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Polgar, O.; Ierano, C.; Tamaki, A.; Stanley, B.; Ward, Y.; Xia, D.; Tarasova, N.; Robey, R.W.; Bates, S.E. Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry 2010, 49, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Bikadi, Z.; Rosenberg, M.F.; Mao, Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr. Drug Metab. 2010, 11, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Polgar, O.; Okada, M.; Esser, L.; Bates, S.E.; Xia, D. Towards understanding the mechanism of action of the multidrug resistance-linked half-ABC transporter ABCG2: A molecular modeling study. J. Mol. Graph. Model. 2007, 25, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Guha, R. On exploring structure-activity relationships. Methods Mol. Biol. 2013, 993, 81–94. [Google Scholar] [PubMed]
- Peltason, L.; Bajorath, J. Systematic computational analysis of structure-activity relationships: Concepts, challenges and recent advances. Future Med. Chem. 2009, 1, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Erić, S.; Kalinić, M.; Ilić, K.; Zloh, M. Computational classification models for predicting the interaction of drugs with P-glycoprotein and breast cancer resistance protein. SAR QSAR Environ. Res. 2014, 25, 955–982. [Google Scholar] [CrossRef]
- Rangel, L.P.; Winter, E.; Gauthier, C.; Terreux, R.; Chiaradia-Delatorre, L.D.; Mascarello, A.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B.; Macalou, S.; et al. New structure-activity relationships of chalcone inhibitors of breast cancer resistance protein: Polyspecificity toward inhibition and critical substitutions against cytotoxicity. Drug Des. Devel. Ther. 2013, 7, 1043–1052. [Google Scholar] [PubMed]
- Han, Y.; Riwanto, M.; Go, M.L.; Ee, P.L. Modulation of breast cancer resistance protein (BCRP/ABCG2) by non-basic chalcone analogues. Eur. J. Pharm. Sci. 2008, 35, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Tee, H.W.; Go, M.L. Functionalized chalcones as selective inhibitors of P-glycoprotein and breast cancer resistance protein. Bioorg. Med. Chem. 2008, 16, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Juvale, K.; Pape, V.F.; Wiese, M. Investigation of chalcones and benzochalcones as inhibitors of breast cancer resistance protein. Bioorg. Med. Chem. 2012, 20, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.M.; Lee, C.Y.; Ee, P.L.; Go, M.L. Dimethoxyaurones: Potent inhibitors of ABCG2 (breast cancer resistance protein). Eur. J. Pharm. Sci. 2008, 35, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; Nicolle, E.; Moraux, T.; Gerby, B.; Blanc, M.; Ronot, X.; Boutonnat, J. Piperazinobenzopyranones and phenalkylaminobenzopyranones: Potent inhibitors of breast cancer resistance protein (ABCG2). J. Med. Chem. 2005, 48, 7275–7281. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, E.; Boccard, J.; Guilet, D.; Dijoux-Franca, M.G.; Zelefac, F.; Macalou, S.; Grosselin, J.; Schmidt, J.; Carrupt, P.A.; di Pietro, A.; et al. Breast cancer resistance protein (BCRP/ABCG2): New inhibitors and QSAR studies by a 3D linear solvation energy approach. Eur. J. Pharm. Sci. 2009, 38, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdameri, G.; Genoux-Bastide, E.; Peres, B.; Gauthier, C.; Guitton, J.; Terreux, R.; Winnischofer, S.M.; Rocha, M.E.; Boumendjel, A.; di Pietro, A. Substituted chromones as highly potent nontoxic inhibitors, specific for the breast cancer resistance protein. J. Med. Chem. 2012, 55, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Valdameri, G.; Genoux-Bastide, E.; Gauthier, C.; Peres, B.; Terreux, R.; Winnischofer, S.M.; Rocha, M.E.; di Pietro, A.; Boumendjel, A. 6-halogenochromones bearing tryptamine: One-step access to potent and highly selective inhibitors of breast cancer resistance protein. Chem. Med. Chem. 2012, 7, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Honorat, M.; Guitton, J.; Gauthier, C.; Bouard, C.; Lecerf-Schmidt, F.; Peres, B.; Terreux, R.; Gervot, H.; Rioufol, C.; Boumendjel, A.; et al. MBL-II-141, a chromone derivative, enhances irinotecan (CPT-11) anticancer efficiency in ABCG2-positive xenografts. Oncotarget 2014, 5, 11957–11970. [Google Scholar] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Martin, J.; Harrington, C.; Dobson, A.D.; O’Gara, F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar. Drugs 2014, 12, 3516–3559. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer. J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Hatakeyama, H.; Harashima, H. Cancer multidrug resistance: Mechanisms involved and strategies for circumvention using a drug delivery system. Arch. Pharm. Res. 2014, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Martinez-Luis, S. Marine natural products with P-glycoprotein inhibitor properties. Mar. Drugs 2014, 12, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, Y.; Zhu, T.; Zhang, M.; Lin, A.; Gu, Q.; Zhu, W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron 2008, 64, 7986–7991. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.L.; Bai, J.; Zhang, L.M.; Wu, X.; Zhang, L.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. 2,5-diketopiperazines from the marine-derived fungus Aspergillus fumigatus YK-7. Chem. Biodivers. 2012, 9, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.L.; Fang, Y.C.; Zhu, T.J.; Gu, Q.Q.; Zhu, W.M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhang, Y.P.; Zhu, T.J.; Fang, Y.C.; Liu, H.B.; Gu, Q.Q.; Zhu, W.M. Studies on the indolyl diketopiperazine analogs produced by marine-derived fungus A-f-11. Chin. J. Antibiot. 2006, 31, 749–752. [Google Scholar]
- Rabindran, S.K.; He, H.; Singh, M.; Brown, E.; Collins, K.I.; Annable, T.; Greenberger, L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 1998, 58, 5850–5858. [Google Scholar] [PubMed]
- Hazlehurst, L.A.; Foley, N.E.; Gleason-Guzman, M.C.; Hacker, M.P.; Cress, A.E.; Greenberger, L.W.; de Jong, M.C.; Dalton, W.S. Multiple mechanisms confer drug resistance to mitoxantrone in the human 8226 myeloma cell line. Cancer Res. 1999, 59, 1021–1028. [Google Scholar] [PubMed]
- Rabindran, S.K.; Ross, D.D.; Doyle, L.A.; Yang, W.; Greenberger, L.M. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000, 60, 47–50. [Google Scholar] [PubMed]
- Plate, R.; Hermkens, P.H.H.; Behm, H.; Ottenheijm, H.C.J. Application of an isoxazolidine in a stereoselective approach to the fumitremorgin series. J. Org. Chem. 1987, 52, 560–564. [Google Scholar] [CrossRef]
- Van Loevezijn, A.; Allen, J.D.; Schinkel, A.H.; Koomen, G.J. Inhibition of BCRP-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg. Med. Chem. Lett. 2001, 11, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Van Maarseveen, J.H. Solid phase synthesis of heterocycles by cyclization/cleavage methodologies. Comb. Chem. High Throughput Screen. 1998, 1, 185–214. [Google Scholar] [PubMed]
- Allen, J.D.; van Loevezijn, A.; Lakhai, J.M.; van der Valk, M.; van Tellingen, O.; Reid, G.; Schellens, J.H.; Koomen, G.J.; Schinkel, A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol. Cancer Ther. 2002, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Okada, G.; Onose, R.; Ubukata, M.; Takahashi, I.; Isono, K.; Osada, H. Tryprostatins A and B, novel mammalian cell cycle inhibitors produced by Aspergillus fumigatus. J. Antibiot. 1995, 48, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.B.; Kakeya, H.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. II. Physico-chemical properties and structures. J. Antibiot. 1996, 49, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Woehlecke, H.; Osada, H.; Herrmann, A.; Lage, H. Reversal of breast cancer resistance protein-mediated drug resistance by tryprostatin A. Int. J. Cancer 2003, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M.; Noreen, S.; Imran, Z.P.; Choudhary, M.I. A new compound, jolynamine, from marine brown alga Jolyna laminarioides. Nat. Prod. Res. 2011, 25, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Zaker, F.; Oody, A.; Arjmand, A. A study on the antitumoral and differentiation effects of Peganum harmala derivatives in combination with ATRA on leukaemic cells. Arch. Pharm. Res. 2007, 30, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wink, M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother. Res. 2010, 24, 146–149. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.A.; Swersey, J.C.; Ireland, C.M.; Carroll, A.R.; Coll, J.C.; Bowden, B.F.; Fairchild, C.R.; Cornell, L. Botryllamides A-D, new brominated tyrosine derivatives from styelid ascidians of the genus Botryllus. Tetrahedron 1995, 51, 5237–5244. [Google Scholar] [CrossRef]
- Rao, M.R.; Faulkner, D.J. Botryllamides E–H, four new tyrosine derivatives from the ascidian Botrylloides tyreum. J. Nat. Prod. 2004, 67, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.J.; Carroll, A.R.; Quinn, R.J. Perspicamides A and B, quinolinecarboxylic acid derivatives from the Australian ascidian Botrylloides perspicuum. J. Nat. Prod. 2005, 68, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Henrich, C.J.; Robey, R.; Takada, K.; Bokesch, H.R.; Bates, S.E.; Shukla, S.; Ambudkar, S.V.; McMahon, J.B.; Gustafson, K.R. Botryllamides: Natural product inhibitors of ABCG2. ACS Chem. Biol. 2009, 4, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Imamura, N.; Gustafson, K.R.; Henrich, C.J. Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter. Bioorg. Med. Chem. Lett. 2010, 20, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.J.; Faulkner, D.J.; He, C.H.; van Duyne, G.D.; Clardy, J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J. Am. Chem. Soc. 1985, 107, 5492–5495. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W.; van Duyne, G.D.; Clardy, J. New alkaloids of the lamellarin class from the marine ascidian Didemnum chartaceum (Sluiter, 1909). J. Org. Chem. 1988, 53, 4570–4574. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Pierens, G.K.; Quinn, R.J. New lamellarin alkaloids from the Australian ascidian, Didemnum chartaceum. J. Nat. Prod. 1999, 62, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Urban, S.; Butler, M.S.; Capon, R.J. Lamellarins O and P: New aromatic metabolites from the Australian marine sponge Dendrilla cactos. Aust. J. Chem. 1994, 47, 1919–1924. [Google Scholar] [CrossRef]
- Urban, S.; Hobbs, L.; Hooper, J.N.A.; Capon, R.J. Lamellarins Q and R: New aromatic metabolites from an Australian marine sponge Dendrilla cactos. Aust. J. Chem. 1995, 48, 1491–1494. [Google Scholar] [CrossRef]
- Carroll, A.R.; Bowden, B.F.; Coll, J.C. Studies of Australian ascidians. I. Six new lamellarin-class alkaloids from a colonial ascidian, Didemnum sp. Austr. J. Chem. 1993, 46, 489–501. [Google Scholar] [CrossRef]
- Urban, S.; Capon, R.J. Lamellarin S: A new aromatic metabolite from an Australian tunicate Didemnum sp. Aust. J. Chem. 1996, 49, 711–713. [Google Scholar]
- Reddy, M.V.R.; Faulkner, D.J.; Venkateswarlu, Y.; Rao, M.R. New lamellarin alkaloids from an unidentified ascidian from the Arabian Sea. Tetrahedron 1997, 53, 3457–3466. [Google Scholar] [CrossRef]
- Bailly, C. Lamellarins, from A to Z: A family of anticancer marine pyrrole alkaloids. Curr. Med. Chem. Anticancer Agents 2004, 4, 363–378. [Google Scholar]
- Huang, X.C.; Xiao, X.; Zhang, Y.K.; Talele, T.T.; Salim, A.A.; Chen, Z.S.; Capon, R.J. Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar. Drugs 2014, 12, 3818–3837. [Google Scholar] [CrossRef] [PubMed]
- Tsubotani, S.H.; Tanida, S.; Harada, S. Structure determination of indolocarbazole alkaloids by NMR spectroscopy. Tetrahedron 1991, 47, 3565–3574. [Google Scholar] [CrossRef]
- Sánchez, C.; Zhu, L.; Braña, A.F.; Salas, A.P.; Rohr, J.; Méndez, C.; Salas, J.A. Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc. Natl. Acad. Sci. USA 2005, 102, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Méndez, C.; Salas, J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006, 23, 1007–1045. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhu, T.; Li, D.; Gu, J.; Xia, W.; Fang, Y.; Hongbing, L.; Zhu, W.; Gu, Q. Two indolocarbazole alkaloids with apoptosis activity from a marine-derived actinomycete Z(2)039-2. Arch. Pharm. Res. 2007, 30, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Shukla, S.; Steadman, K.; Obrzut, T.; Finley, E.M.; Ambudkar, S.V.; Bates, S.E. Inhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structure. Mol. Cancer Ther. 2007, 6, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, B.; Li, Z.; Zhang, B. Enhanced production of (+)-terrein in fed-batch cultivation of Aspergillus terreus strain PF26 with sodium citrate. World J. Microbiol. Biotechnol. 2013, 29, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, J.; Liu, P.; Wang, W.; Zhu, W. Three new compounds from Aspergillus terreus PT06–2 grown in a high salt medium. Mar. Drugs 2011, 9, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, J.; Christophersen, C.; Barrero, A.F.; Oltra, J.E.; Justicia, J.; Rosales, A. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. J. Nat. Prod. 2002, 65, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Cho, H.J.; Lee, H.K.; Lee, W.H.; Park, E.S.; Youn, S.W.; Park, K.C. Terrein, a fungal metabolite, inhibits the epidermal proliferation of skin equivalents. J. Dermatol. Sci. 2007, 46, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Phattanawasin, P.; Pojchanakom, K.; Sotanaphun, U.; Piyapolrungroj, N.; Zungsontiporn, S. Weed growth inhibitors from Aspergillus fischeri TISTR 3272. Nat. Prod. Res. 2007, 21, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Yu, M.K.; Lee, R.; Lee, Y.H.; Jeon, J.G.; Lee, M.H.; Jhee, E.C.; Yoo, I.D.; Yi, H.K. Terrein reduces pulpal inflammation in human dental pulp cells. J. Endodont. 2008, 34, 433–437. [Google Scholar] [CrossRef]
- Arakawa, M.; Someno, T.; Kawada, M.; Ikeda, D. A new terrain glucoside, a novel inhibitor of angiogenin secretion in tumor angiogenesis. J. Antibiot. 2008, 61, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, N.H.; Bhattarai, G.; Oh, Y.T.; Yu, M.K.; Yoo, I.D.; Jhee, E.C.; Yi, H.K. Enhancement of osteoblast biocompatibility on titanium surface with terrein treatment. Cell Biochem. Funct. 2010, 28, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.Y.; Shen, C.N.; Lin, L.H.; Yang, Y.L.; Han, H.Y.; Chen, J.W.; Kuo, S.C.; Wu, S.H.; Liaw, C.C. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic Aspergillus terreus. J. Nat. Prod. 2012, 75, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 1970, 26, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Yan, Y.Y.; Dai, C.L.; Xia, X.K.; She, Z.G.; Lin, Y.Ch.; Fu, L.W. Secalonic acid D induced leukemia cell apoptosis and cell cycle arrest of G1 with involvement of GSK-3β/β-catenin/c-Myc pathway. Cell Cycle 2009, 8, 2444–2450. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Shao, C.; She, Z.; Cai, X.; Liu, F.; Vrijimoed, L.L.; Lin, Y. 1H and 13C NMR assignments for two oxaphenalenones bacillosporin C and D from the mangrove endophytic fungus SBE-14. Magn. Reson. Chem. 2007, 45, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Tian, L.; Gu, Q.; Zhu, W. Secalonic acid D; A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch. Pharm. Res. 2006, 29, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Hong, R. Secalonic acid D as a novel DNA topoisomerase I inhibitor from marine lichen-derived fungus Gliocladium sp. T31. Pharm. Biol. 2011, 49, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.P.; Tao, L.Y.; Wang, F.; Zhang, J.Y.; Liang, Y.J.; Fu, L.W. Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem. Pharmacol. 2013, 85, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Bokesch, H.R.; Cartner, L.K.; Fuller, R.W.; Wilson, J.A.; Henrich, C.J.; Kelley, J.A.; Gustafson, K.R.; McMahon, J.B.; McKee, T.C. Inhibition of ABCG2-mediated drug efflux by naphthopyrones from marine crinoids. Bioorg. Med. Chem. Lett. 2010, 20, 3848–3850. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherigo, L.; Lopez, D.; Martinez-Luis, S. Marine Natural Products as Breast Cancer Resistance Protein Inhibitors. Mar. Drugs 2015, 13, 2010-2029. https://doi.org/10.3390/md13042010
Cherigo L, Lopez D, Martinez-Luis S. Marine Natural Products as Breast Cancer Resistance Protein Inhibitors. Marine Drugs. 2015; 13(4):2010-2029. https://doi.org/10.3390/md13042010
Chicago/Turabian StyleCherigo, Lilia, Dioxelis Lopez, and Sergio Martinez-Luis. 2015. "Marine Natural Products as Breast Cancer Resistance Protein Inhibitors" Marine Drugs 13, no. 4: 2010-2029. https://doi.org/10.3390/md13042010





