Coral-Derived Compound WA-25 Inhibits Angiogenesis by Attenuating the VEGF/VEGFR2 Signaling Pathway
Abstract
:1. Introduction
2. Results
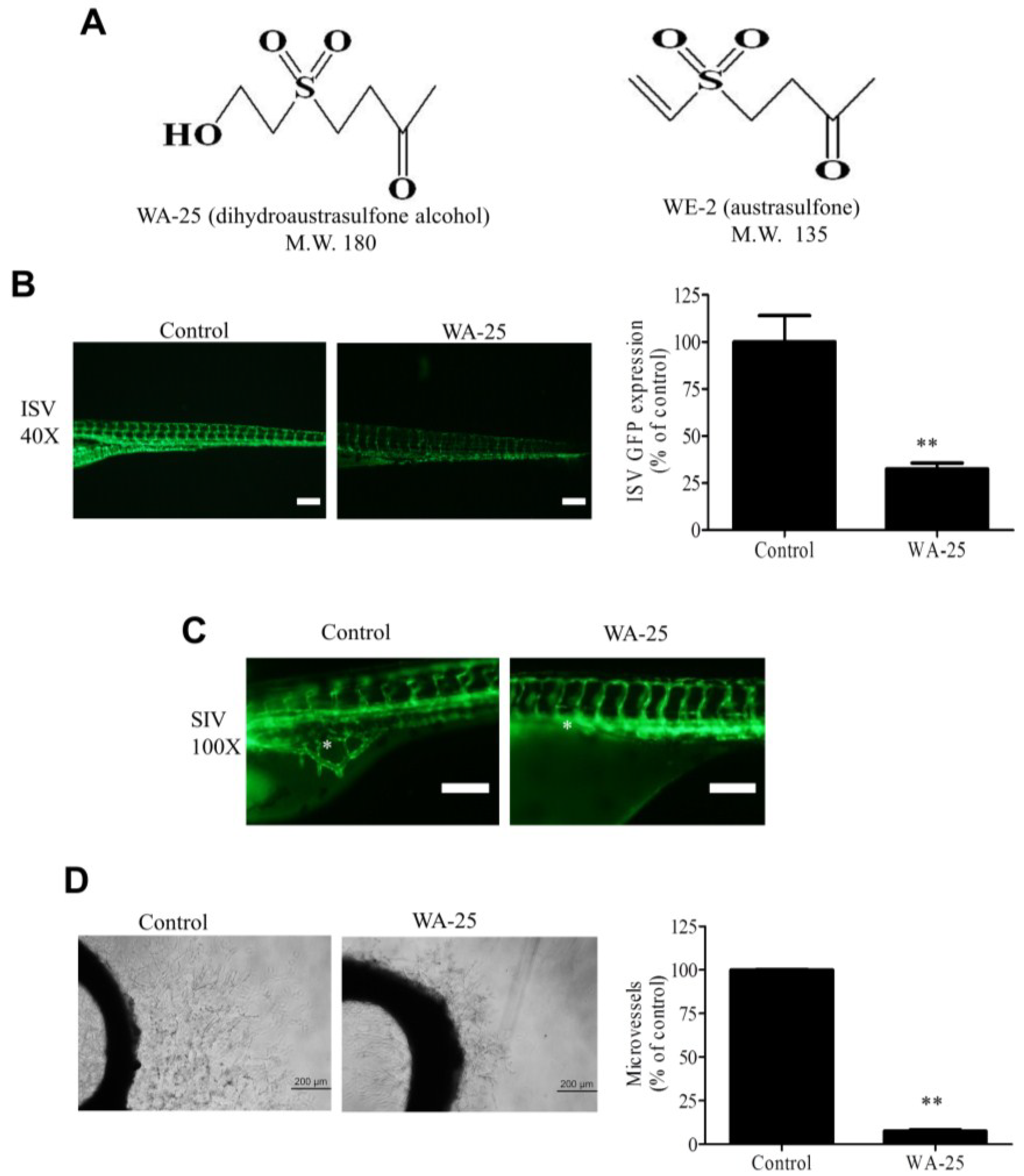
2.1. WA-25 Perturbs Vessel Development in Zebrafish and Rat Aortic Rings
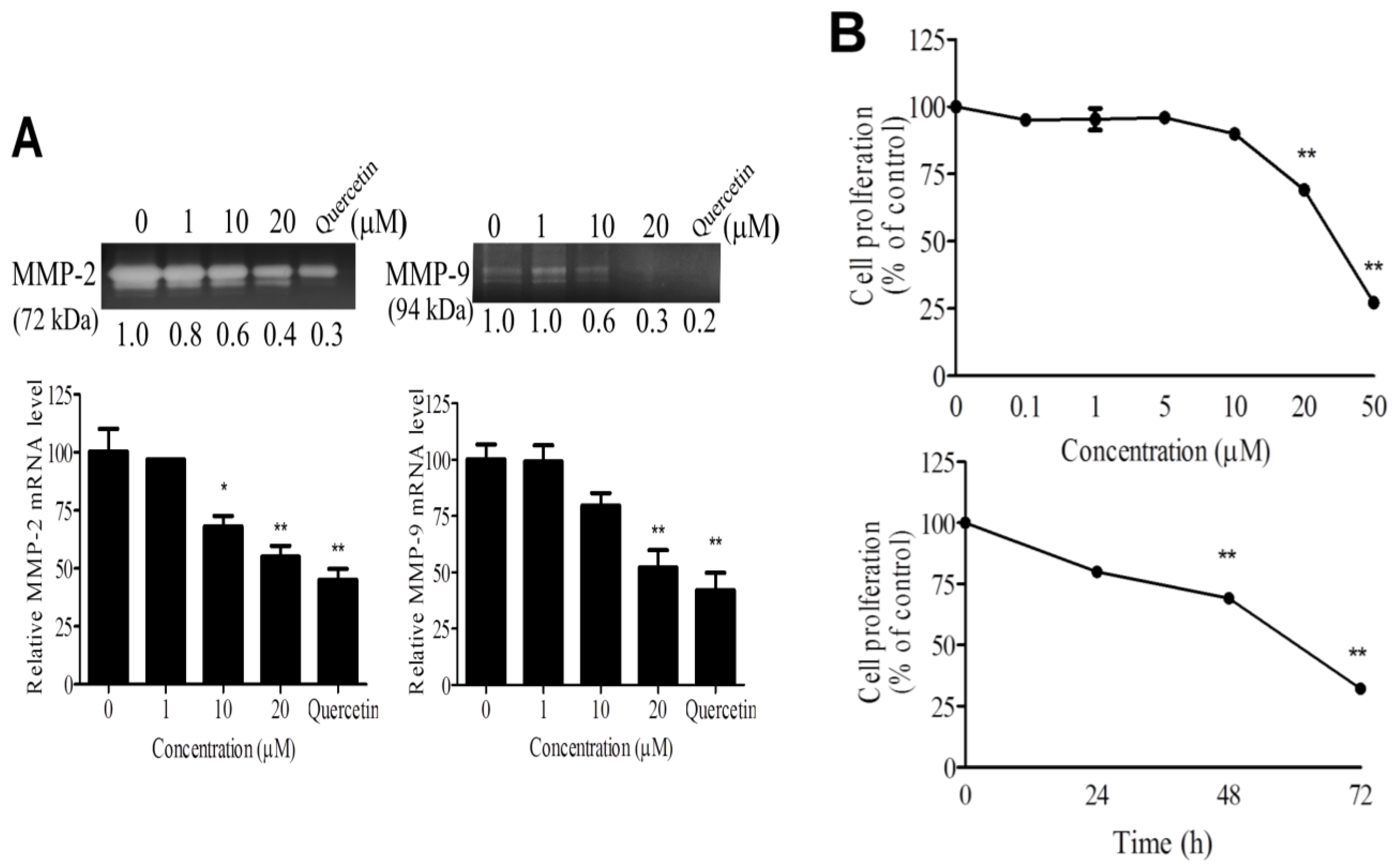
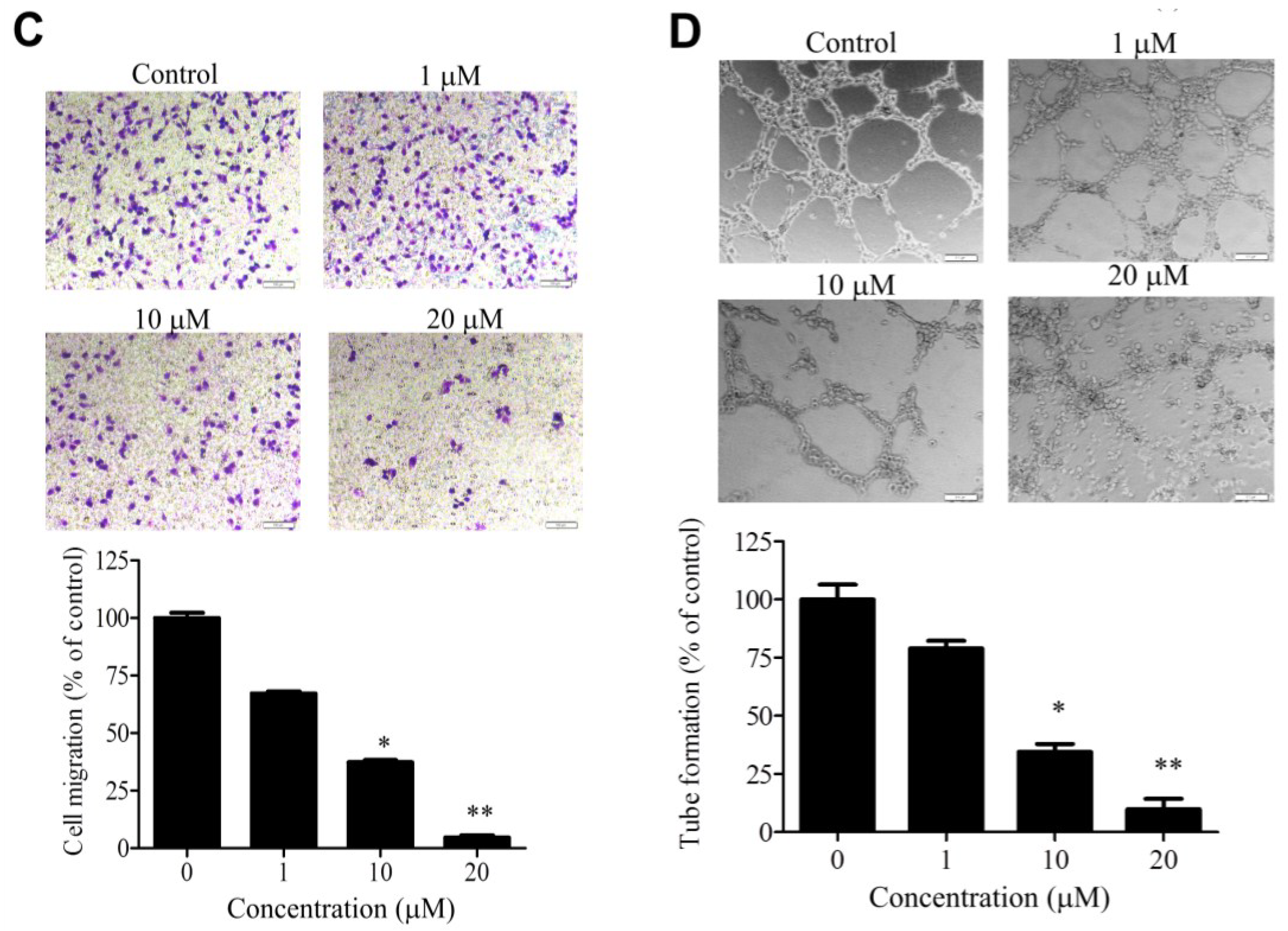
2.2. WA-25 Inhibits Matrix Metalloproteinase Secretion, Proliferation, Migration and Tube Formation of Cultured Endothelial Cells
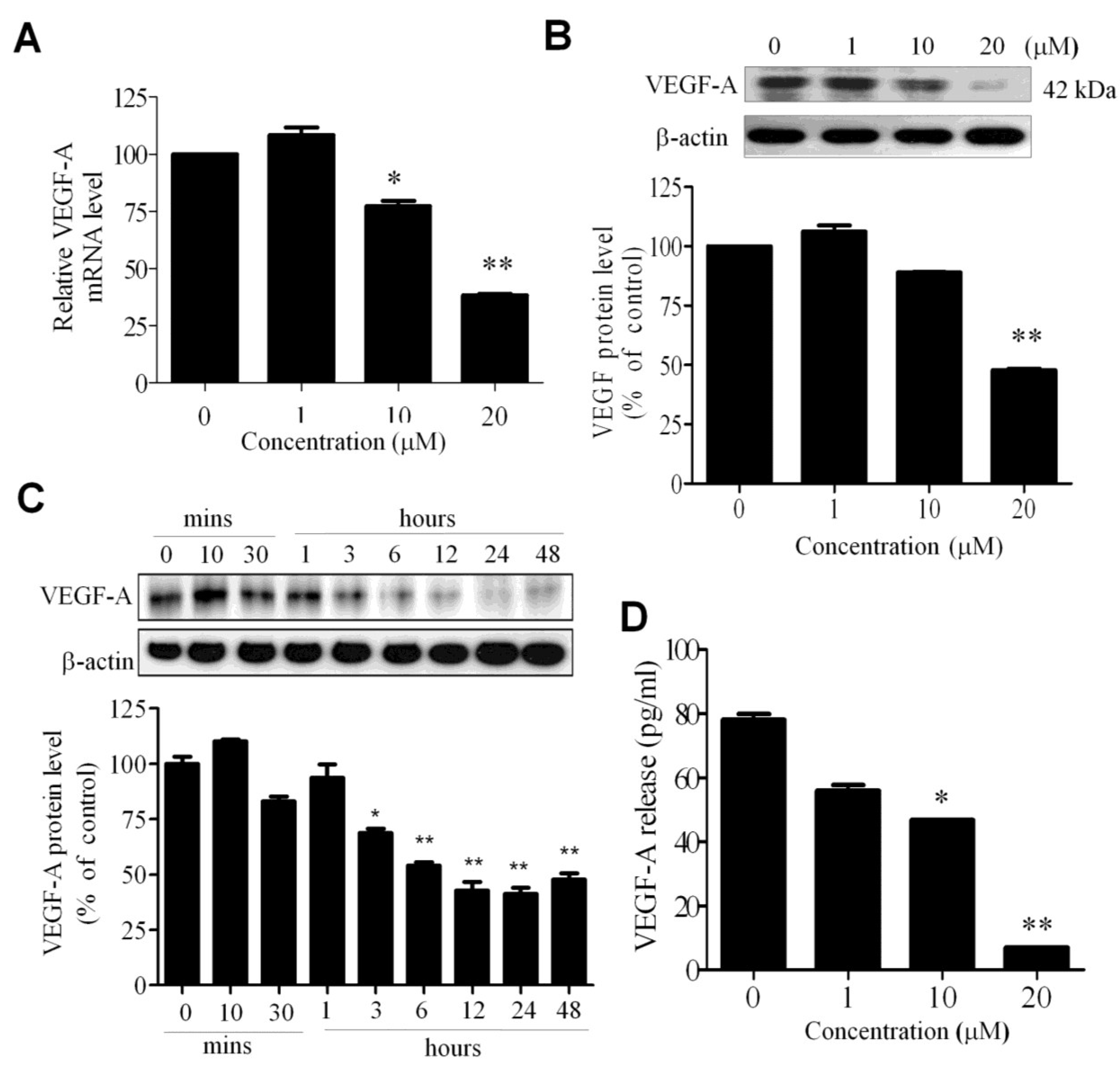
2.3. WA-25 Reduces the Release and Alleviates the Bioavailability of Vascular Endothelial Growth Factor-A in Endothelial Cells
2.4. WA-25 Reduces Vascular Endothelial Growth Factor Receptor 2 Expression in Endothelial Cells
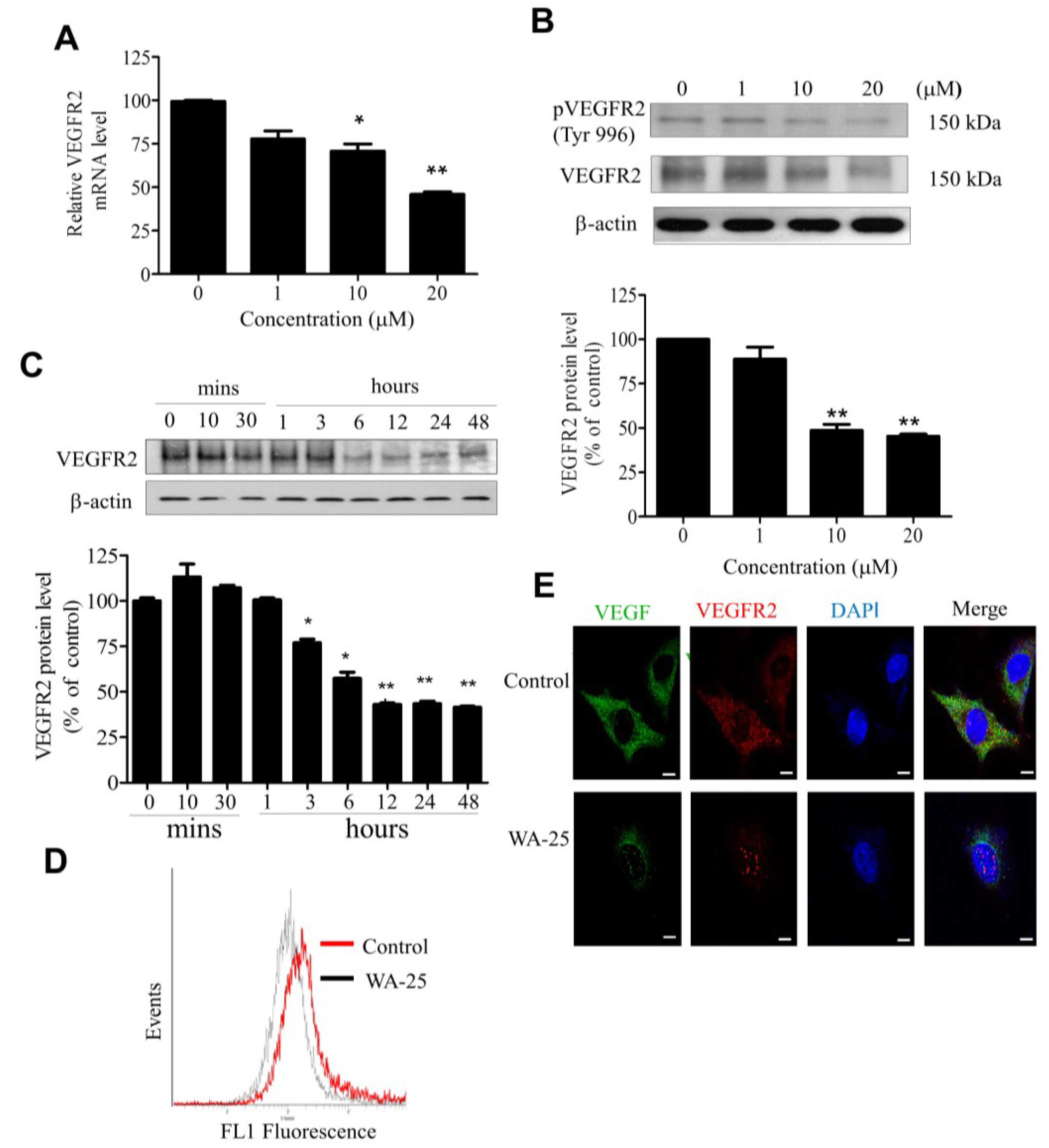
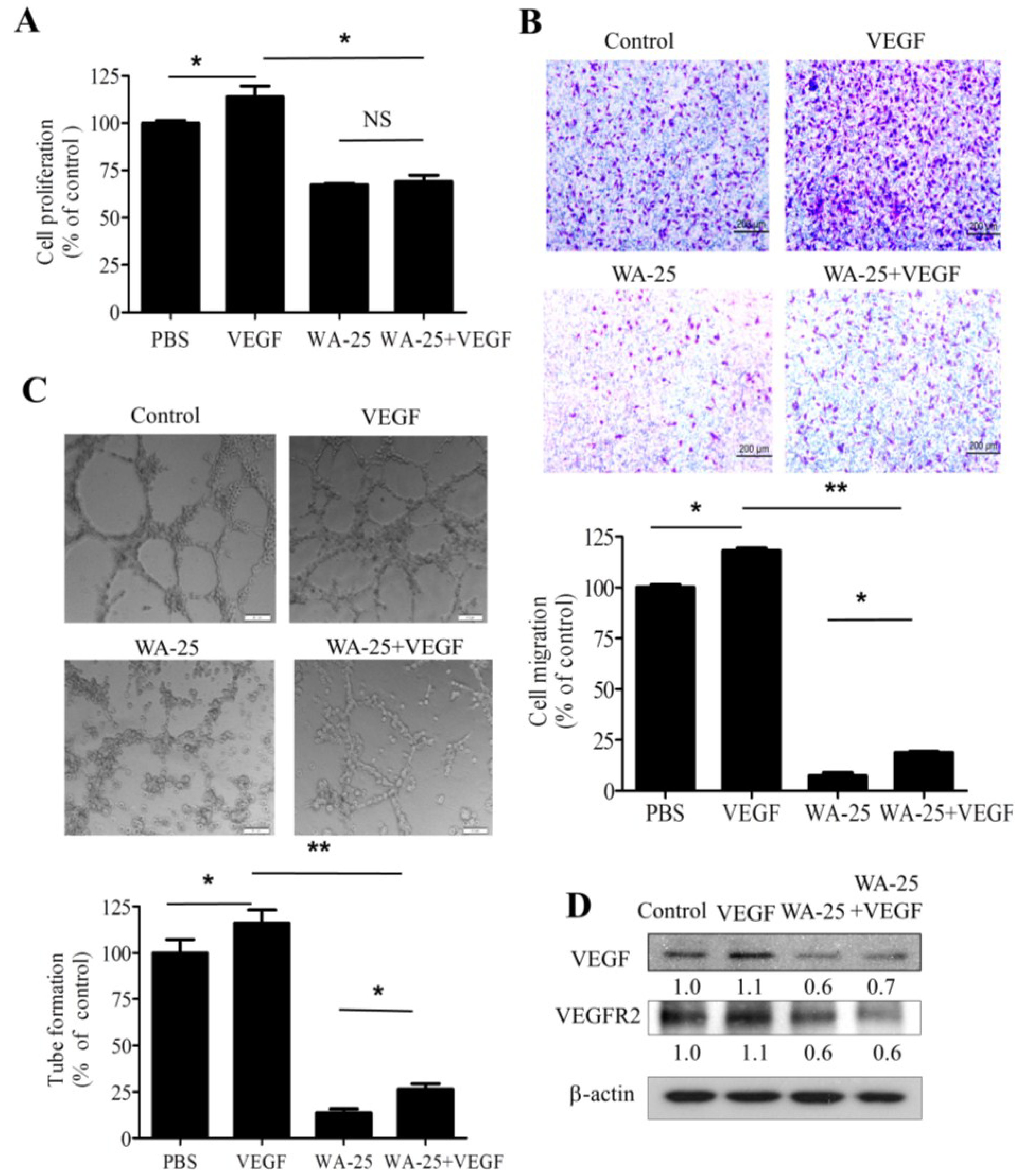
2.5. Vascular Endothelial Growth Factor-A Supply Partially Rescues the WA-25-Induced Neovascularization Blockade in Vitro
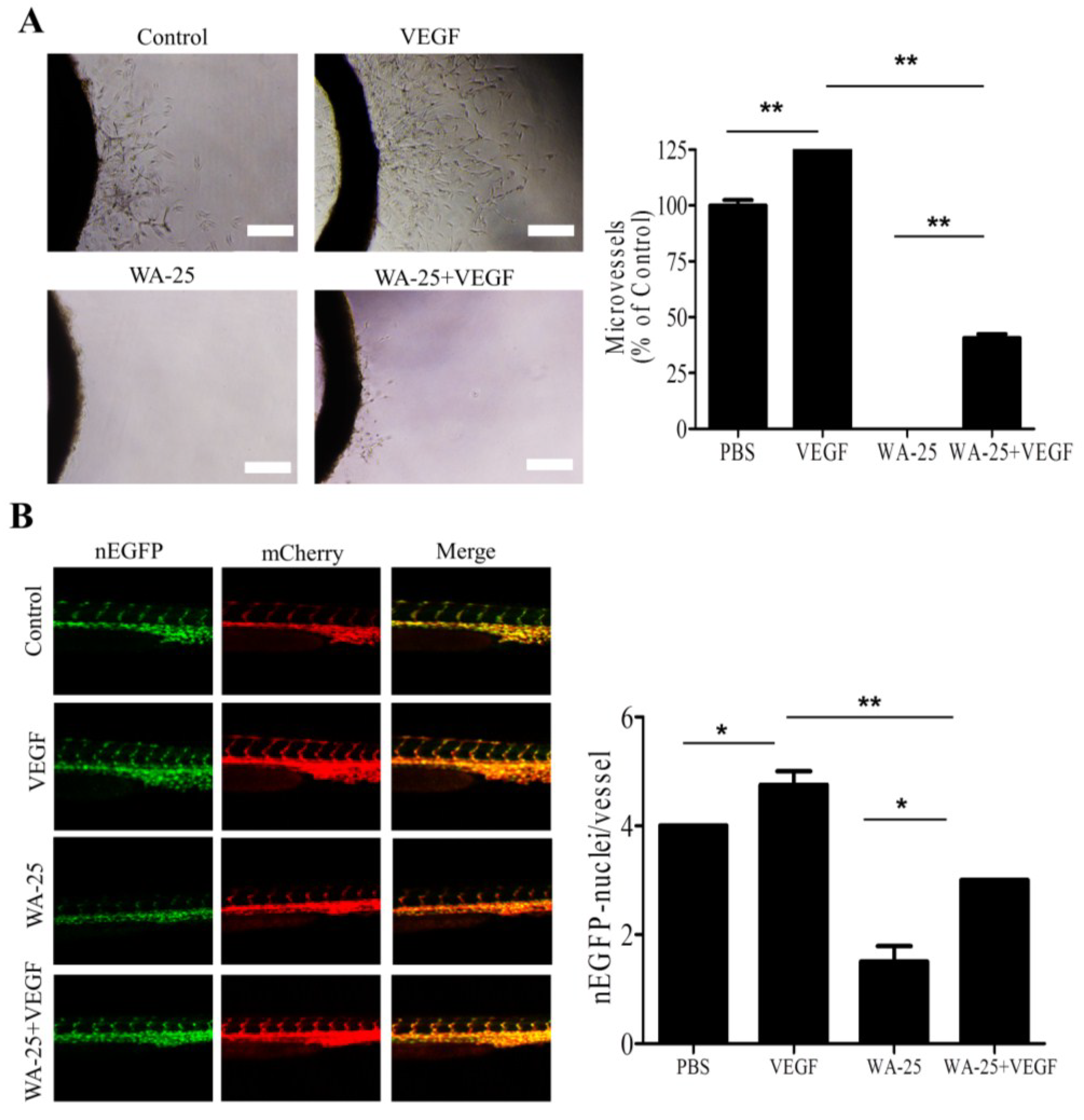
2.6. Vascular Endothelial Growth Factor-A Partially Rescues the WA-25-Induced Neovascularization Blockade in Vivo
3. Discussion
4. Methods and Materials
4.1. Coral Compounds and Antibodies
4.2. Aortic Ring Assay
4.3. Zebrafish Angiogenesis Model
4.4. Cell Culture
4.5. Gelatin Zymography
4.6. Proliferation Assay
4.7. Migration Assay
4.8. Tube Formation Assay
4.9. Immunofluorescence Assay
4.10. Flow Cytometric Analysis
4.11. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.12. Western Blot Analysis
4.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Bell, J.R.; Ramachandran, J.; Miljanich, G.P. Neuroanatomical distribution of receptors for a novel voltage-sensitive calcium-channel antagonist, SNX-230 (omega-conopeptide MVIIC). Brain Res. 1994, 653, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bowersox, S.S.; Gadbois, T.; Singh, T.; Pettus, M.; Wang, Y.X.; Luther, R.R. Selective N-type neuronal voltage-sensitive calcium channel blocker, SNX-111, produces spinal antinociception in rat models of acute, persistent and neuropathic pain. J. Pharmacol. Exp. Ther. 1996, 279, 1243–1249. [Google Scholar] [PubMed]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2010. Molecules 2011, 16, 5629–5646. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhang, X.; Tian, F.; Yi, Y.; Xu, Q.; Li, L.; Tong, L.; Lin, L.; Ding, J. Philinopside A, a novel marine-derived compound possessing dual anti-angiogenic and anti-tumor effects. Int. J. Cancer 2005, 114, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Maskey, R.P.; Helmke, E.; Kayser, O.; Fiebig, H.H.; Maier, A.; Busche, A.; Laatsch, H. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycete and their absolute stereochemistry. J. Antibiot. (Tokyo) 2004, 57, 771–779. [Google Scholar] [CrossRef]
- Su, J.H.; Chen, B.Y.; Hwang, T.L.; Chen, Y.H.; Huang, I.C.; Lin, M.R.; Chen, J.J.; Fang, L.S.; Wang, W.H.; Li, J.J.; et al. Excavatoids L-N, new 12-hydroxybriaranes from the cultured octocoral Briareum excavatum (Briareidae). Chem. Pharm. Bull. (Tokyo) 2010, 58, 662–665. [Google Scholar] [CrossRef]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Mencarelli, A.; D’Amore, C.; Renga, B.; Zampella, A.; Fiorucci, S. Total synthesis and pharmacological characterization of solomonsterol A, a potent marine pregnane-X-receptor agonist endowed with anti-inflammatory activity. J. Med. Chem. 2011, 54, 4590–4599. [Google Scholar] [CrossRef] [PubMed]
- Germano, G.; Frapolli, R.; Simone, M.; Tavecchio, M.; Erba, E.; Pesce, S.; Pasqualini, F.; Grosso, F.; Sanfilippo, R.; Casali, P.G.; et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res. 2010, 70, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, P.A.; Ng, Y.S. Won’t you be my neighbor? Local induction of arteriogenesis. Cell 2002, 110, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Applanat, M.; di Benedetto, M. Autocrine functions of VEGF in breast tumor cells: Adhesion, survival, migration and invasion. Cell Adhes. Migr. 2012, 6, 547–553. [Google Scholar] [CrossRef]
- Bates, D.O.; Curry, F.E. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am. J. Physiol. 1996, 271, H2520–H2528. [Google Scholar] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Chapuli, R.; Quesada, A.R.; Angel Medina, M. Angiogenesis and signal transduction in endothelial cells. Cell. Mol. Life Sci. CMLS 2004, 61, 2224–2243. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, M.; Zhao, Q.; Li, S.; Peng, Y.; Zhang, P.; Han, M. Vascular endothelial growth factor plays a critical role in the formation of the pre-metastatic niche via prostaglandin E2. Oncol. Rep. 2014, 32, 2477–2484. [Google Scholar] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Karamysheva, A.F. Mechanisms of angiogenesis. Biochemistry (Mosc.) 2008, 73, 751–762. [Google Scholar] [CrossRef]
- Jeong, S.J.; Koh, W.; Lee, E.O.; Lee, H.J.; Lee, H.J.; Bae, H.; Lu, J.; Kim, S.H. Antiangiogenic phytochemicals and medicinal herbs. Phytother. Res. PTR 2011, 25, 1–10. [Google Scholar] [CrossRef]
- Wen, Z.H.; Chao, C.H.; Wu, M.H.; Sheu, J.H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-kappaB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Cao, H.H.; Tse, A.K.; Kwan, H.Y.; Yu, H.; Cheng, C.Y.; Su, T.; Fong, W.F.; Yu, Z.L. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem. Pharmacol. 2014, 87, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006, 26, 3579–3583. [Google Scholar] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chien, Y.C.; Pan, C.H.; Sheu, J.H.; Chen, C.Y.; Wu, C.H. Inhibitory effect of dihydroaustrasulfone alcohol on the migration of human non-small cell lung carcinoma A549 cells and the antitumor effect on a Lewis lung carcinoma-bearing tumor model in C57BL/6J mice. Mar. Drugs 2014, 12, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; National Sun Yat-Sen University and Academia Sinica, Kaohsiung, Taiwan. Unpublished Work. 2015.
- Bee, Y.S.; Sheu, S.J.; Ma, Y.L.; Lin, H.C.; Weng, W.T.; Kuo, H.M.; Hsu, H.C.; Tang, C.H.; Liou, J.C.; Tai, M.H. Topical application of recombinant calreticulin peptide, vasostatin 48, alleviates laser-induced choroidal neovascularization in rats. Mol. Vis. 2010, 16, 756–767. [Google Scholar] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.T.; Huang, S.C.; Ma, Y.L.; Chan, H.H.; Lin, S.W.; Wu, J.C.; Wu, C.Y.; Wen, Z.H.; Wang, E.M.; Wu, C.L.; et al. alpha-Melanocyte-stimulating hormone inhibits angiogenesis through attenuation of VEGF/VEGFR2 signaling pathway. Biochim. Biophys. Acta 2014, 1840, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish; Institute of Neuroscience, University of Oregon: Eugene, OR, USA, 1995. [Google Scholar]
- Yang, X.; Cui, W.; Yu, S.; Xu, C.; Chen, G.; Gu, A.; Li, T.; Cui, Y.; Zhang, X.; Bian, X. A synthetic dl-nordihydroguaiaretic acid (Nordy), inhibits angiogenesis, invasion and proliferation of glioma stem cells within a zebrafish xenotransplantation model. PLoS One 2014, 9, e85759. [Google Scholar] [CrossRef] [PubMed]
- Proulx, K.; Lu, A.; Sumanas, S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev. Biol. 2010, 348, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.L.; Pham, V.N.; Lawson, N.D.; Kulik, M.; Childs, S.; Lekven, A.C.; Garrity, D.M.; Moon, R.T.; Fishman, M.C.; Lechleider, R.J.; et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 2002, 129, 3009–3019. [Google Scholar] [PubMed]
- Ma, Y.L.; Lin, S.W.; Fang, H.C.; Chou, K.J.; Bee, Y.S.; Chu, T.H.; Chang, M.C.; Weng, W.T.; Wu, C.Y.; Cho, C.L.; et al. A novel poly-naphthol compound ST104P suppresses angiogenesis by attenuating matrix metalloproteinase-2 expression in endothelial cells. Int. J. Mol. Sci. 2014, 15, 16611–16627. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.H.; Kuo, S.M.; Liang, H.T.; Chiou, K.R.; Lam, H.C.; Hsu, C.M.; Pownall, H.J.; Chen, H.H.; Huang, M.T.; Yang, C.Y. Modulation of angiogenic processes in cultured endothelial cells by low density lipoproteins subfractions from patients with familial hypercholesterolemia. Atherosclerosis 2006, 186, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.M.; Lin, C.Y.; Lam, H.C.; Lin, P.R.; Chan, H.H.; Tseng, J.C.; Sun, C.K.; Hsu, T.F.; Wu, C.C.; Yang, C.Y.; et al. PTEN overexpression attenuates angiogenic processes of endothelial cells by blockade of endothelin-1/endothelin B receptor signaling. Atherosclerosis 2010, 221, 341–349. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-W.; Huang, S.-C.; Kuo, H.-M.; Chen, C.-H.; Ma, Y.-L.; Chu, T.-H.; Bee, Y.-S.; Wang, E.-M.; Wu, C.-Y.; Sung, P.-J.; et al. Coral-Derived Compound WA-25 Inhibits Angiogenesis by Attenuating the VEGF/VEGFR2 Signaling Pathway. Mar. Drugs 2015, 13, 861-878. https://doi.org/10.3390/md13020861
Lin S-W, Huang S-C, Kuo H-M, Chen C-H, Ma Y-L, Chu T-H, Bee Y-S, Wang E-M, Wu C-Y, Sung P-J, et al. Coral-Derived Compound WA-25 Inhibits Angiogenesis by Attenuating the VEGF/VEGFR2 Signaling Pathway. Marine Drugs. 2015; 13(2):861-878. https://doi.org/10.3390/md13020861
Chicago/Turabian StyleLin, Shih-Wei, Shih-Chung Huang, Hsiao-Mei Kuo, Chiu-Hua Chen, Yi-Ling Ma, Tian-Huei Chu, Youn-Shen Bee, E-Ming Wang, Chang-Yi Wu, Ping-Jyun Sung, and et al. 2015. "Coral-Derived Compound WA-25 Inhibits Angiogenesis by Attenuating the VEGF/VEGFR2 Signaling Pathway" Marine Drugs 13, no. 2: 861-878. https://doi.org/10.3390/md13020861





