Marine Bromophenol Bis (2,3-Dibromo-4,5-dihydroxy-phenyl)-methane Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Modulating β1-Integrin/FAK Signaling
Abstract
:1. Introduction
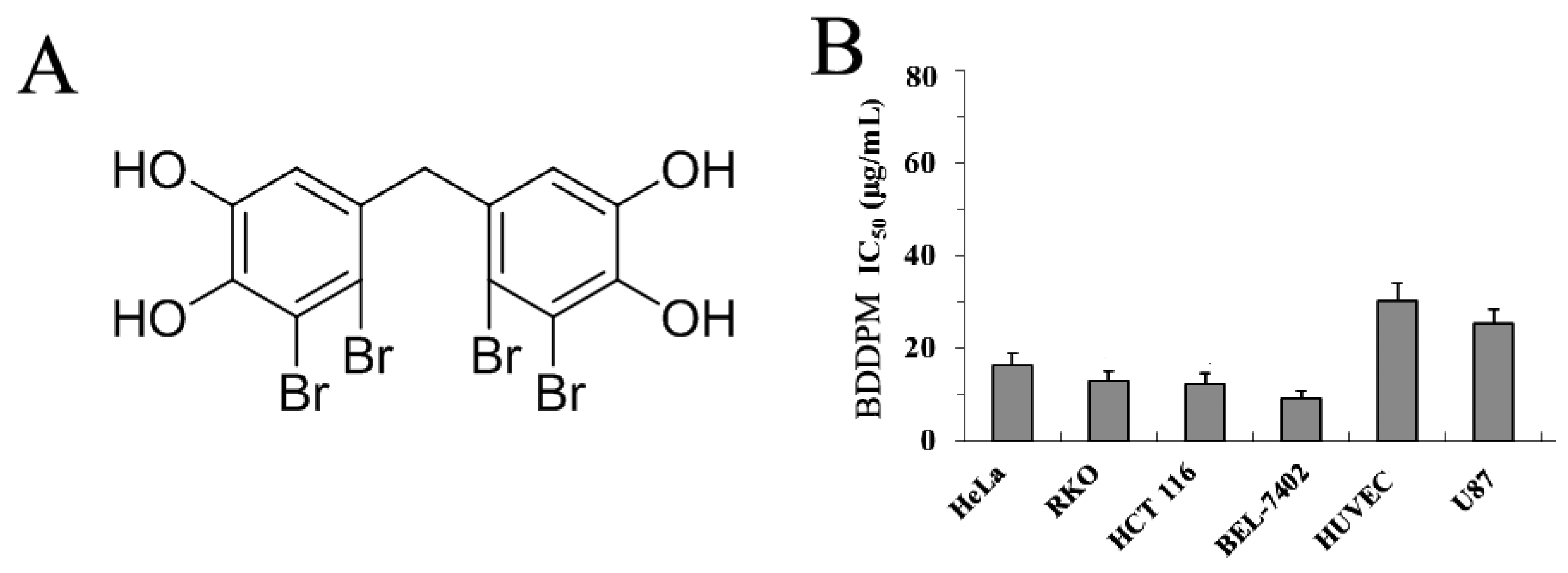
2. Results
2.1. BDDPM Inhibits Cancer Cell Proliferation
2.2. BDDPM Induces Morphological Changes and Apoptosis in BEL-7402 Cell
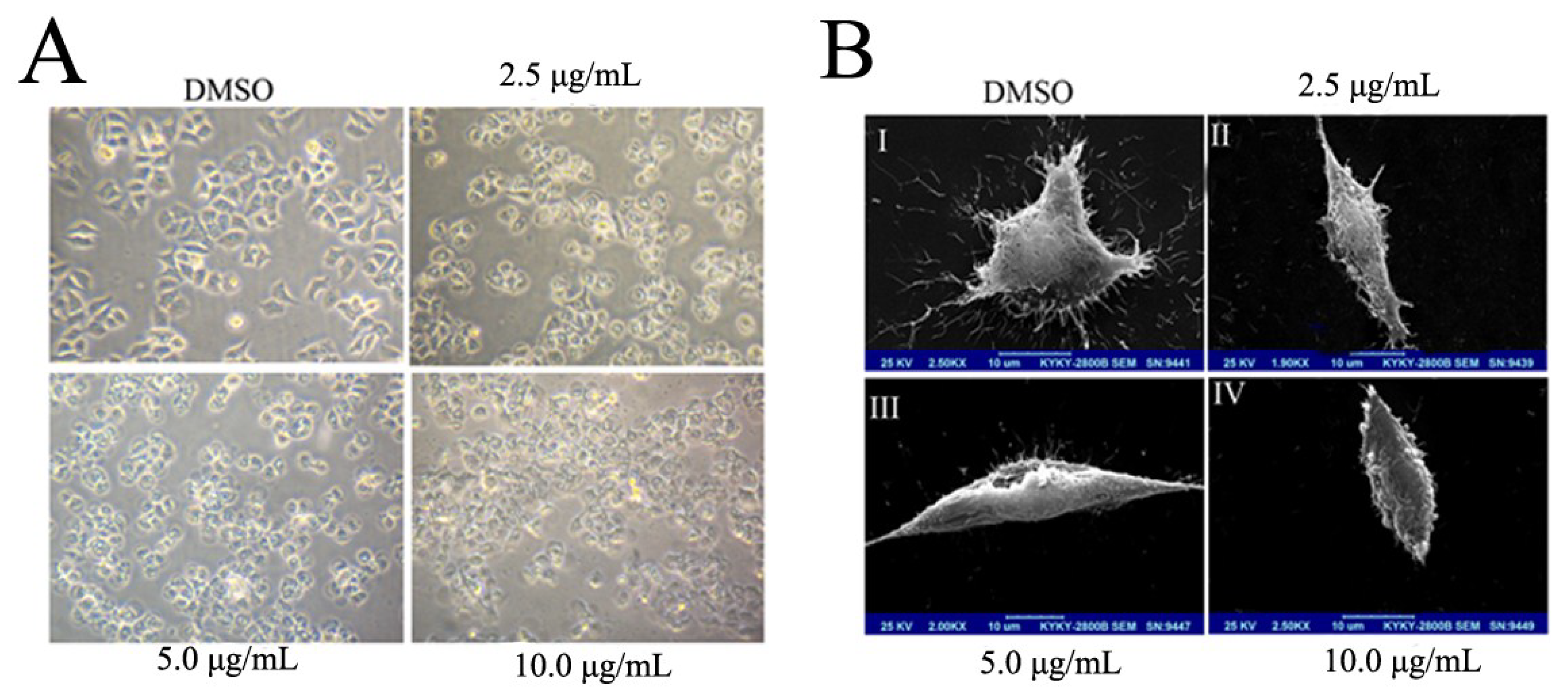
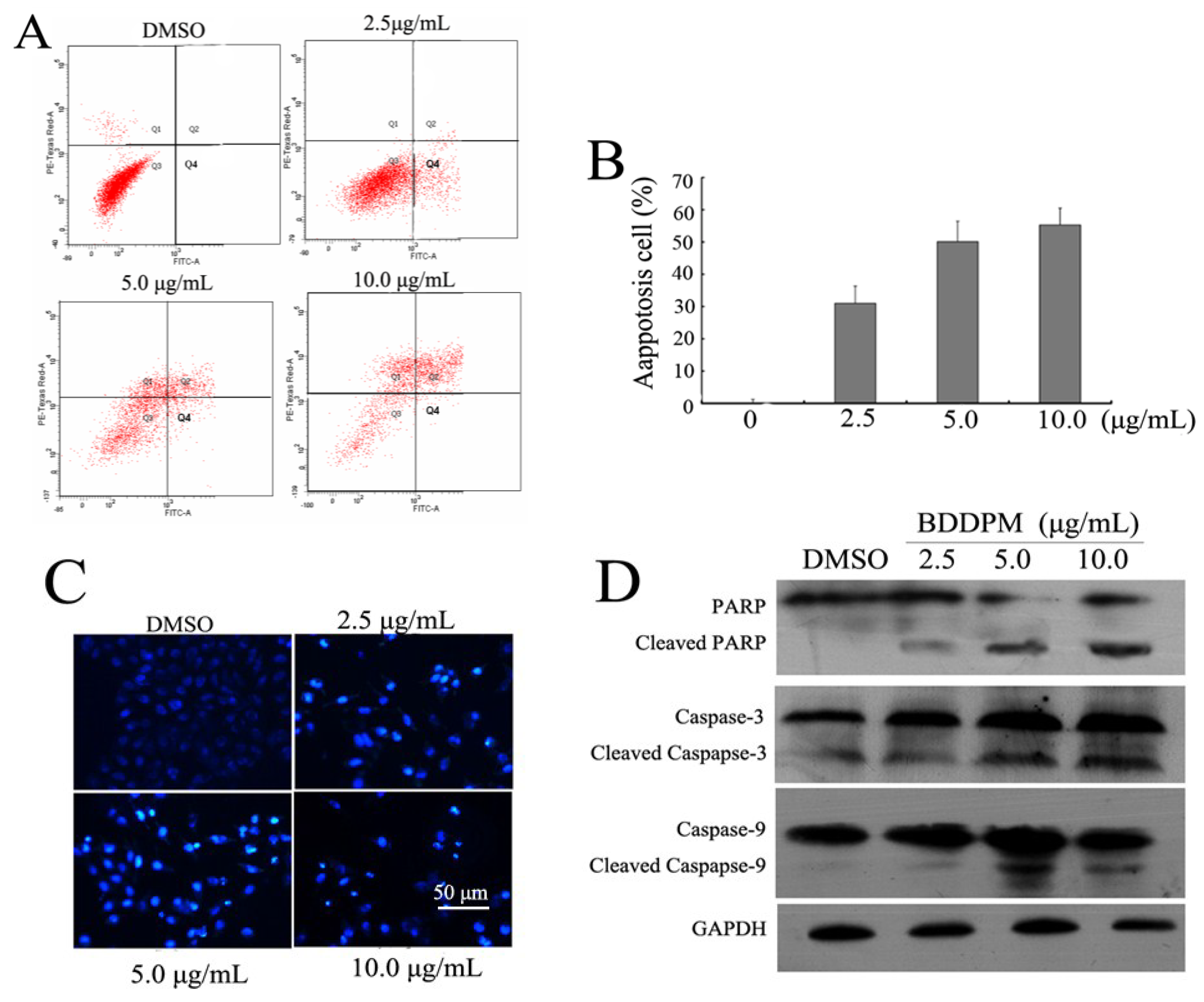
2.3. BDDPM Induces Apoptosis in BEL-7402 Cells
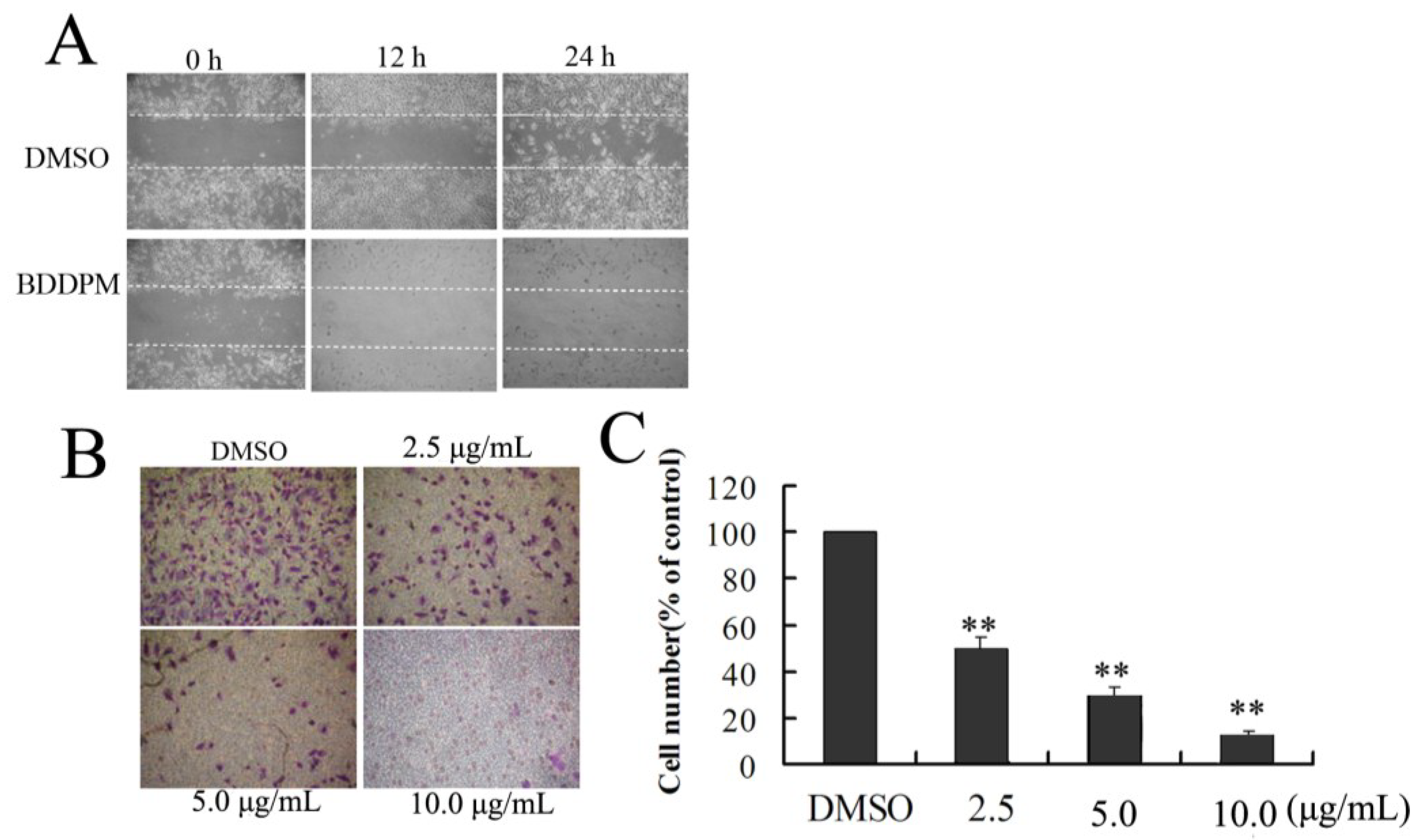
2.4. BDDPM Affects the Migration and Invasion of BEL-7402 Cells
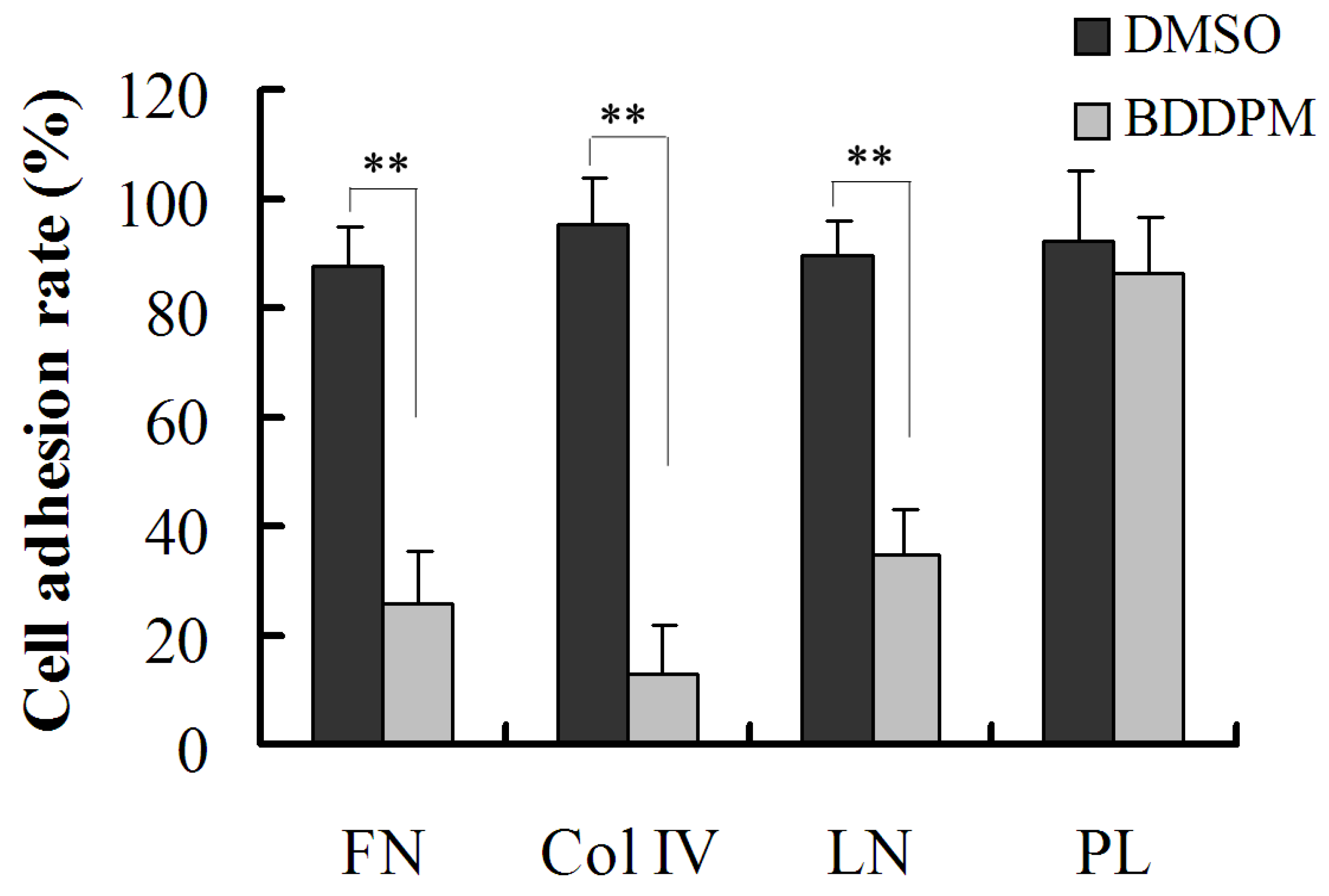
2.5. BDDPM Inhibits the Ability of BEL-7402 Cells to Adhere to ECM
2.6. BDDPM Disrupts the Cytoskeleton and Changes the Morphology of BEL-7402

2.7. BDDPM Inhibits the Expression of β1-Integrin and FAK
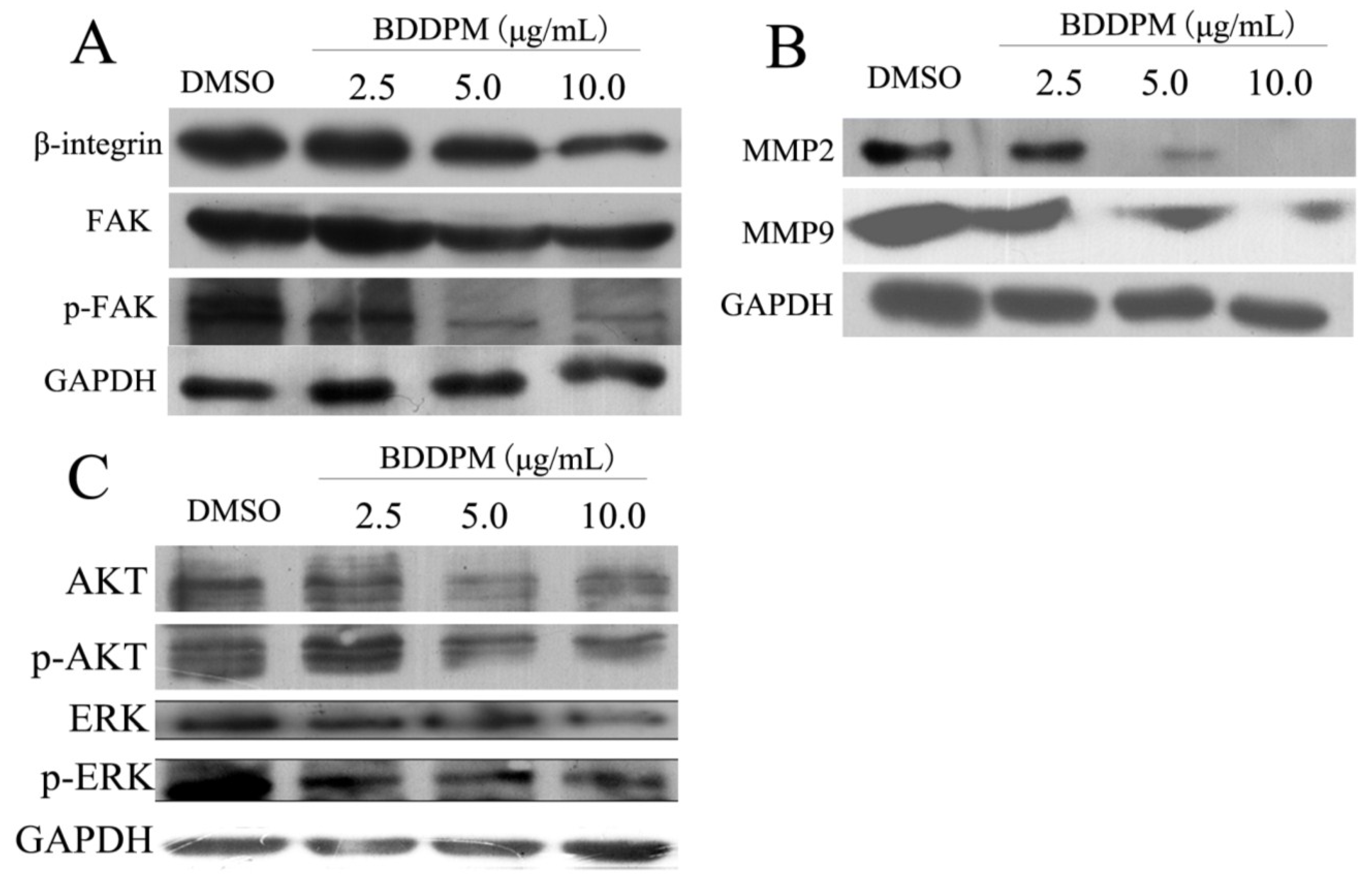
3. Discussion
4. Experimental Section
4.1. Materials
4.2. MTT Assay
4.3. Cell Morphological Observation
4.4. Hoechst33342/Propidium Iodide (PI) Dual Staining Assays
4.5. Apoptosis Assay
4.6. Cell Adhesion Assay
4.7. Cell Migration Assay
4.8. Cell Invasion Assay
4.9. Cytoskeleton Immunofluorescence
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in k562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J.; Fan, X.; He, L. Bromophenols coupled with methyl gamma-ureidobutyrate and bromophenol sulfates from the red alga rhodomela confervoides. J. Nat. Prod. 2006, 69, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wei, J.; Lin, X. Synthesis and alpha-glucosidase inhibitory mechanisms of bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a potential marine bromophenol alpha-glucosidase inhibitor. Mar. Drugs 2011, 9, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Benedetti, R.; Perez-Rodriguez, S.; Nebbioso, A.; Garcia-Rodriguez, J.; Carafa, V.; Stuhldreier, M.; Conte, M.; Rodriguez-Barrios, F.; Stunnenberg, H.G.; et al. Indole-derived psammaplin a analogues as epigenetic modulators with multiple inhibitory activities. J. Med. Chem. 2012, 55, 9467–9491. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.Y.; Li, J.; Guo, S.J.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Oceanol. Limn. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Oh, K.B.; Lee, J.H.; Lee, J.W.; Yoon, K.M.; Chung, S.C.; Jeon, H.B.; Shin, J.; Lee, H.S. Synthesis and antimicrobial activities of halogenated bis(hydroxyphenyl)methanes. Bioorg. Med. Chem. Lett. 2009, 19, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, S.J.; Su, H.; Han, L.J.; Shi, D.Y. Total synthesis of bis-(2,3-dibromo-4,5-dihydroxyphenyl)-methane as potent PTP1B inhibitor. Chin. Chem. Lett. 2008, 19, 1290–1292. [Google Scholar] [CrossRef]
- Bauvois, B. New facets of matrix metalloproteinases mmp-2 and mmp-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Bba-Rev. Cancer 2012, 1825, 29–36. [Google Scholar]
- Ma, W.L.; Jeng, L.B.; Lai, H.C.; Liao, P.Y.; Chang, C. Androgen receptor enhances cell adhesion and decreases cell migration via modulating beta 1-integrin-Akt signaling in hepatocellular carcinoma cells. Cancer Lett. 2014, 351, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.L.; Ko, B.S.; Liu, T.A.; Liang, S.M.; Liu, C.C.; Lu, Y.J.; Tzean, S.S.; Shen, T.L.; Liou, J.Y. Cordycepin suppresses integrin/FAK signaling and epithelial-mesenchymal transition in hepatocellular carcinoma. Anti-Cancer Agent Me 2014, 14, 29–34. [Google Scholar] [CrossRef]
- Eke, I.; Deuse, Y.; Hehlgans, S.; Gurtner, K.; Krause, M.; Baumann, M.; Shevchenko, A.; Sandfort, V.; Cordes, N. Beta(1) integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Invest. 2012, 122, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Li, J.M.; Yee, S.P.; Fellows, G.F.; Goodyer, C.G.; Wang, R.N. Beta 1 integrin/FAK/Erk signalling pathway is essential for human fetal islet cell differentiation and survival. J. Pathol. 2009, 219, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.; Harnois, C.; Demers, M.J.; Thibodeau, S.; Laquerre, V.; Gauthier, R.; Vezina, A.; Noel, D.; Fujita, N.; Tsuruo, T.; et al. Beta 1 integrin/FAK/Src signaling in intestinal epithelial crypt cell survival: Integration of complex regulatory mechanisms. Apoptosis 2008, 13, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Lee, H.J.; Bae, G.U.; Kang, J.S. Promyogenic function of integrin/FAK signaling is mediated by Cdo, Cdc42 and MyoD. Cell Signal. 2011, 23, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, A.; Sahai, E. Editorial overview: Cell adhesion and migration. Curr. Opin. Cell Biol. 2014, 30, V–Vi. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Wu, N.; Wei, J.T.; Liu, J.; Zhao, J.; Ji, A.G.; Lin, X.K. A novel protein from eupolyphaga sinensis inhibits adhesion, migration, and invasion of human lung cancer A549 cells. Biochem. Cell Biol. 2013, 91, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, E.C.; Chuaqui, R.F.; Liotta, L.A. General mechanisms of metastasis. Cancer 1997, 80, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Nam, K.T.; Lee, S.; Yu, D.; Choi, G.; Shin, S.K.; Lee, Y.C. Regulation of epithelial mesenchymal transition through protein kinase CK2 in helicobacter pylori infected gastric cancer cells. Gastroenterology 2012, 142, S515–S516. [Google Scholar] [CrossRef]
- Lauffenburger, D.A. Cell motility. Making connections count. Nature 1996, 383, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Beekman, K.W.; Colevas, A.D.; Cooney, K.; Dipaola, R.; Dunn, R.L.; Gross, M.; Keller, E.T.; Pienta, K.J.; Ryan, C.J.; Smith, D.; et al. Phase II evaluations of cilengitide in asymptomatic patients with androgen-independent prostate cancer: Scientific rationale and study design. Clin. Genitourin Cancer 2006, 4, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Manegold, C.; Vansteenkiste, J.; Cardenal, F.; Schuette, W.; Woll, P.J.; Ulsperger, E.; Kerber, A.; Eckmayr, J.; von Pawel, J. Randomized phase II study of three doses of the integrin inhibitor cilengitide vs. docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest. New Drugs 2013, 31, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B.; Prieto, V.; Joseph, R.W.; Diwan, A.H.; Gallick, G.E.; Papadopoulos, N.E.; Bedikian, A.Y.; Camacho, L.H.; Hwu, P.; Ng, C.S.; et al. A randomized phase II study of cilengitide (EMD 121974) in patients with metastatic melanoma. Melanoma Res. 2012, 22, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Fink, K.L.; Mikkelsen, T.; Cloughesy, T.F.; O’Neill, A.; Plotkin, S.; Glantz, M.; Ravin, P.; Raizer, J.J.; Rich, K.M.; et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 2008, 26, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Besse, B.; Tsao, L.C.; Chao, D.T.; Fang, Y.; Soria, J.C.; Almokadem, S.; Belani, C.P. Phase IB safety and pharmacokinetic study of volociximab, an anti-alpha5beta1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006, 66, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Zha, R.; Guo, W.; Zhang, Z.; Qiu, Z.; Wang, Q.; Ding, J.; Huang, S.; Chen, T.; Gu, J.; Yao, M.; et al. Genome-wide screening identified that miR-134 acts as a metastasis suppressor by targeting integrin beta1 in hepatocellular carcinoma. PLoS One 2014, 9, e87665. [Google Scholar] [CrossRef] [PubMed]
- Speicher, T.; Siegenthaler, B.; Bogorad, R.L.; Ruppert, R.; Petzold, T.; Padrissa-Altes, S.; Bachofner, M.; Anderson, D.G.; Koteliansky, V.; Fassler, R.; et al. Knockdown and knockout of beta1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat. Commun. 2014, 5, 3862. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.A.; Mitra, S.K.; Hauck, C.R.; Streblow, D.N.; Nelson, J.A.; Ilic, D.; Huang, S.; Li, E.; Nemerow, G.R.; Leng, J.; et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003, 160, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gan, B.; Yoo, Y.; Guan, J.L. Fak-mediated src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev. Cell 2005, 9, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Lin, X.; Zhao, X.; Zheng, L.; Xiao, L.; Liu, J.; Ge, L.; Cao, S. miR-125b acts as an oncogene in glioblastoma cells and inhibits cell apoptosis through p53 and p38MAPK-independent pathways. Br. J. Cancer 2013, 109, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine Bromophenol Bis (2,3-Dibromo-4,5-dihydroxy-phenyl)-methane Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Modulating β1-Integrin/FAK Signaling. Mar. Drugs 2015, 13, 1010-1025. https://doi.org/10.3390/md13021010
Wu N, Luo J, Jiang B, Wang L, Wang S, Wang C, Fu C, Li J, Shi D. Marine Bromophenol Bis (2,3-Dibromo-4,5-dihydroxy-phenyl)-methane Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Modulating β1-Integrin/FAK Signaling. Marine Drugs. 2015; 13(2):1010-1025. https://doi.org/10.3390/md13021010
Chicago/Turabian StyleWu, Ning, Jiao Luo, Bo Jiang, Lijun Wang, Shuaiyu Wang, Changhui Wang, Changqing Fu, Jian Li, and Dayong Shi. 2015. "Marine Bromophenol Bis (2,3-Dibromo-4,5-dihydroxy-phenyl)-methane Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Modulating β1-Integrin/FAK Signaling" Marine Drugs 13, no. 2: 1010-1025. https://doi.org/10.3390/md13021010





