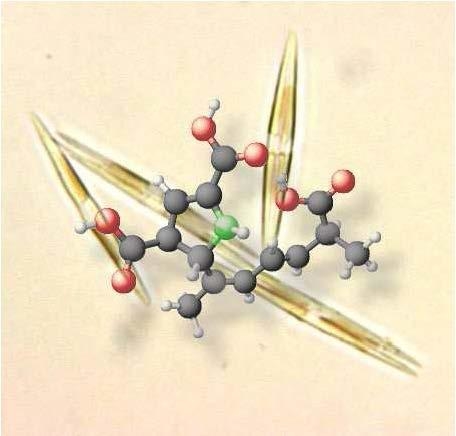
Effects of Organic and Inorganic Nitrogen on the Growth and Production of Domoic Acid by Pseudo-nitzschia multiseries and P. australis (Bacillariophyceae) in Culture
Abstract
:1. Introduction
2. Results
2.1. Growth
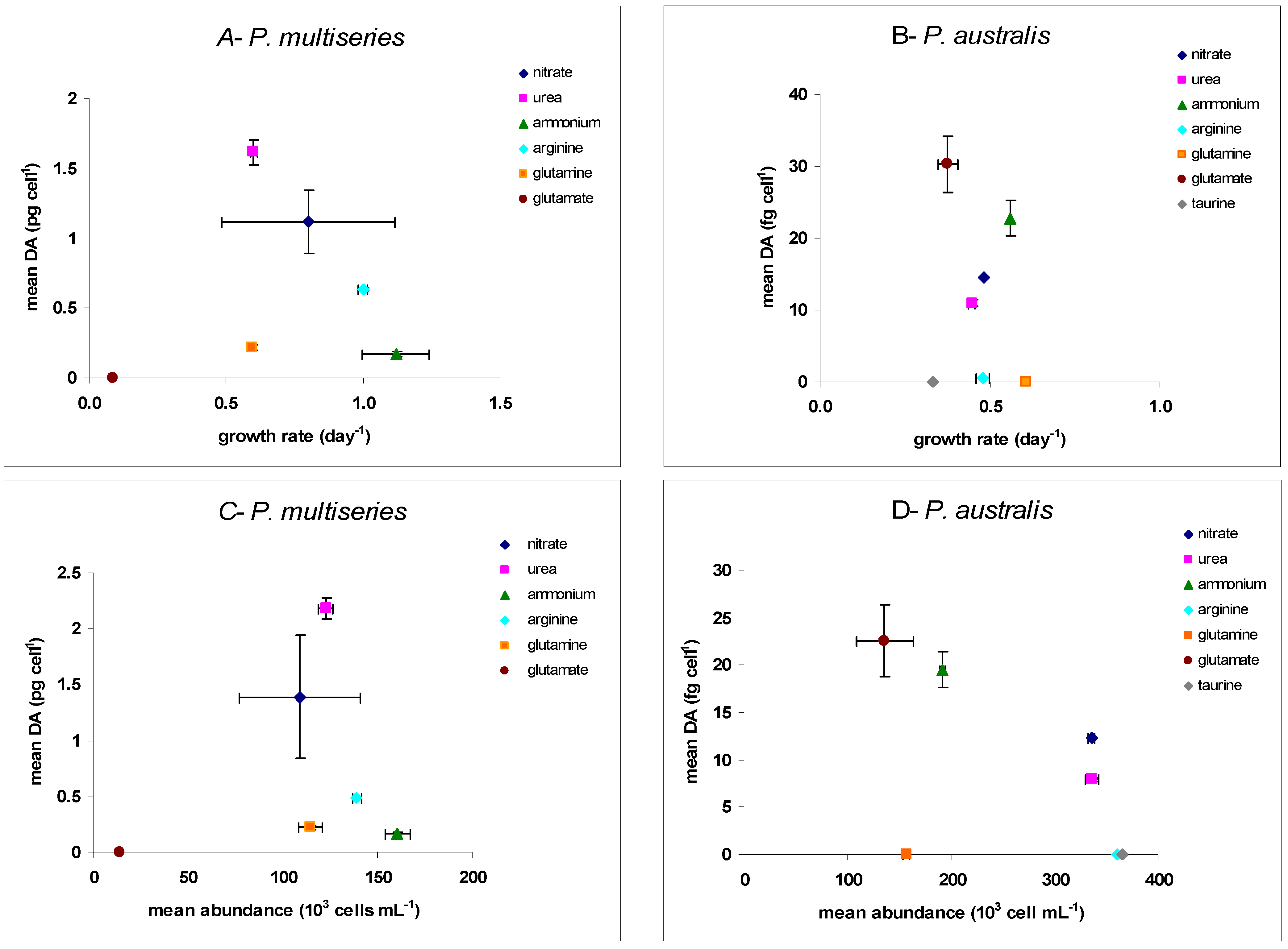
| P. multiseries CCL70 | P. australis PNC1 | |||||
|---|---|---|---|---|---|---|
| Specific Growth Rate µ (day−1) | Mean Biomass in Stationary Phase (103 Cells mL−1) | Mean DA (pg·Cell−1) | Specific Growth Rate µ (day−1) | Mean Biomass in Stationary Phase (103 Cells mL−1) | Mean DA (fg·Cell−1) | |
| Nitrate | 0.80 ± 0.32 | 109.0 ± 32.0 | 1.12 ± 0.23 | 0.48 ± 0.003 | 336.0 ± 3.2 | 14.5 ± 0.1 |
| Urea | 0.60 ± 0.01 | 123.0 ± 4.1 | 1.62 ± 0.09 | 0.44 ± 0.01 | 336.0 ± 6.8 | 10.9 ± 0.5 |
| Ammonium | 1.12 ± 0.12 | 161.0 ± 6.5 | 0.17 ± 0.02 | 0.56 ± 0.003 | 191.0 ± 2.6 | 22.7 ± 2.4 |
| Arginine | 0.63 ± 0.01 | 139.0 ± 2.4 | 0.49 ± 0.01 | 0.47 ± 0.02 | 360.0 ± 9.4 | nd |
| Glutamine | 0.59 ± 0.01 | 114.0 ± 6.3 | 0.22 ± 0.02 | 0.60 ± 0.01 | 157.0 ± 3.5 | nd |
| Glutamate | 0.08 ± 0.03 | 14.0 ± 3.7 | nd | 0.37 ± 0.03 | 136.0 ± 28.0 | 30.3 ± 3.9 |
| Taurine | - | - | - | 0.33 ± 0.02 | 365.0 ± 25.0 | nd |
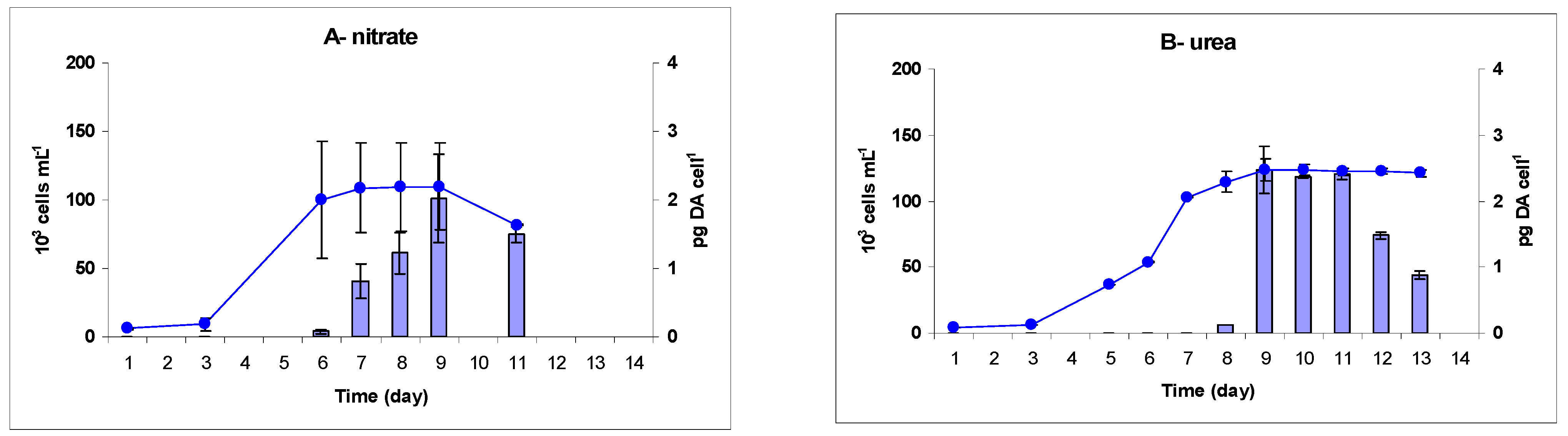
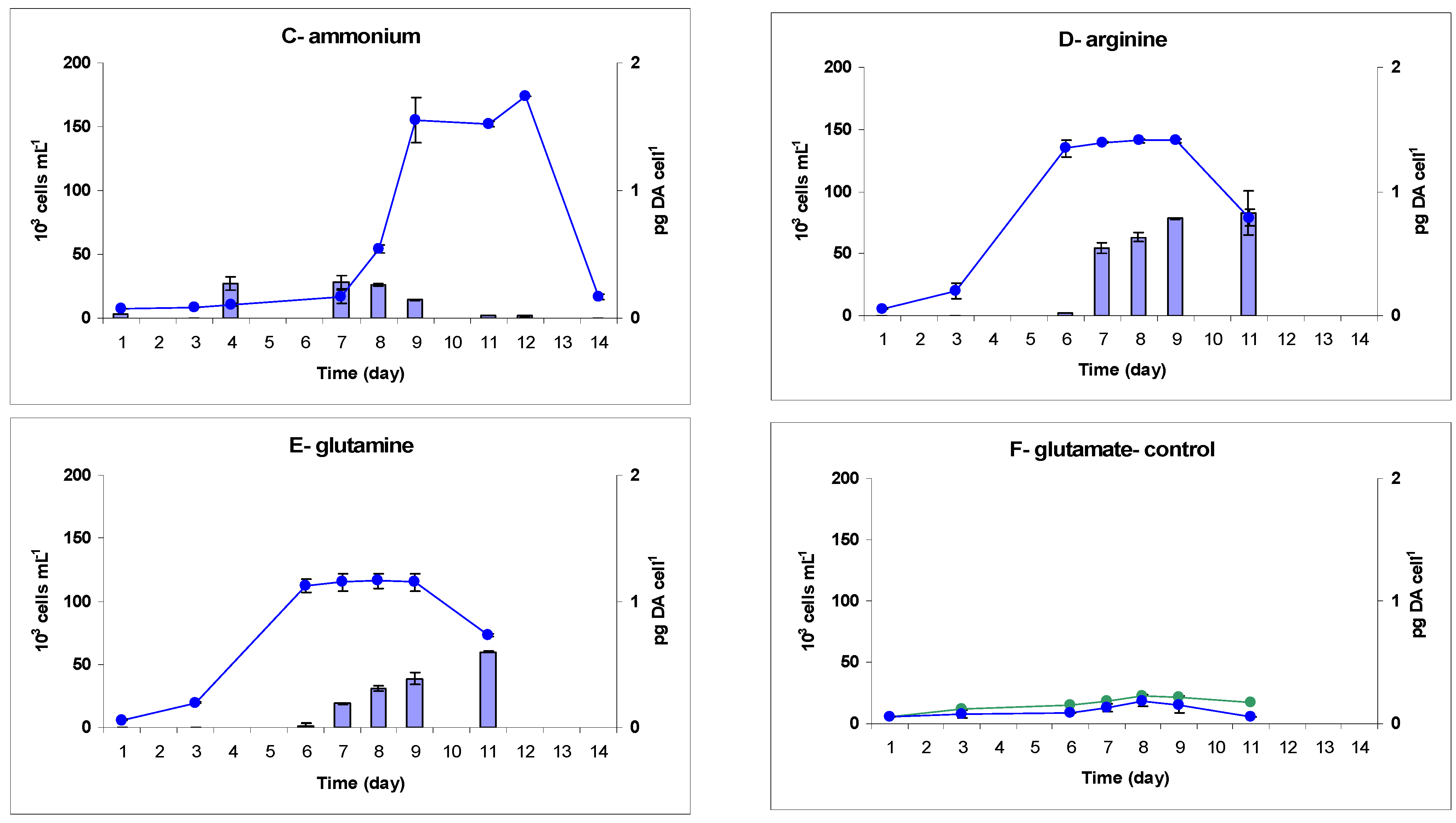
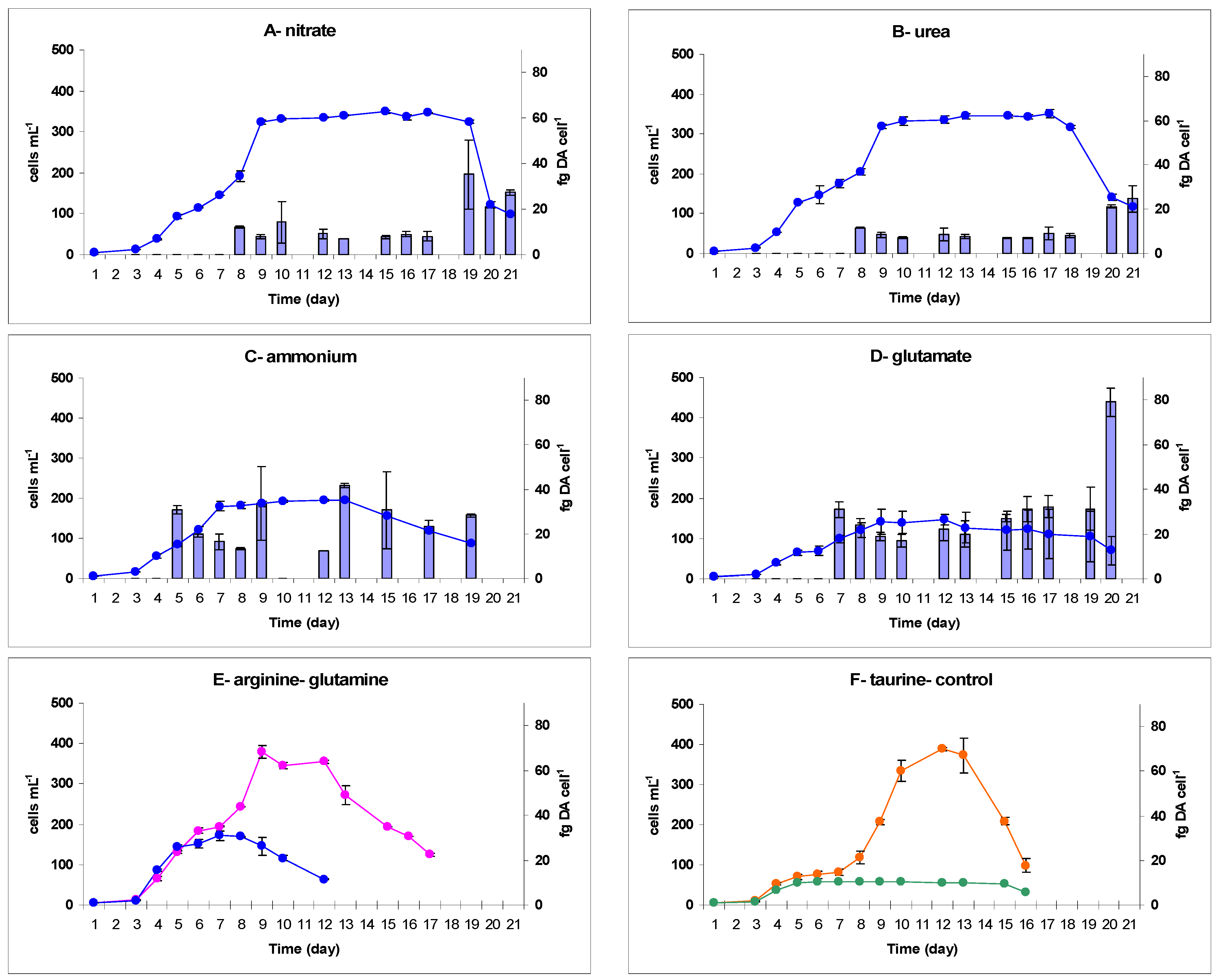
2.2. Toxicity
3. Discussion
3.1. Growth and Biomass
3.1.1. Nitrate, Ammonium, Urea
3.1.2. Amino Acids
3.2. Toxicity
3.2.1. P. multiseries and P. australis
3.2.2. Timing of DA Production
3.2.3. DA Production with Respect to Growth and Biomass
3.2.4. DA Production with Respect to Nitrogen Sources and Energetics
4. Experimental Section
4.1. Cell Cultures
4.2. Experimental Procedures
4.3. Cell Quantification
4.4. Domoic Acid Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCarthy, J.J. Nitrogen. In The Physiological Ecology of Phytoplankton; Morris, L., Ed.; Blackwell Scientific Publication: London, UK, 1980; pp. 191–233. [Google Scholar]
- Syrett, P.J. Nitrogen metabolism of microalgae. In Physiological Bases of Phytoplankton Ecology; Platt, T., Ed.; Canadian Bulletin of Fisheries and Aquatic Sciences: Ottawa, ON, Canada, 1981; Volume 210, pp. 182–210. [Google Scholar]
- Antia, N.J.; Harrison, P.J.; Oliveira, L. The role of dissolved organic nitrogen in phytoplankton nutrition, cell biology and ecology. Phycologia 1991, 30. [Google Scholar] [CrossRef]
- Smil, V. Enriching the Earth: Fritz Haber, Carl Bosh, and the Transformation of World Food; The MIT Press: Cambridge, UK, 2001. [Google Scholar]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating worldwide use of urea—A global change contributing to coastal eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Glibert, P.M.; Seitzinger, S.; Heil, C.A.; Burkolder, J.M.; Parrow, M.W.; Codispoti, L.A.; Kelly, V. The role of eutrophization in the global proliferation of harmful algae blooms. Oceanography 2005, 18, 198–208. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Bates, S.S.; Trainer, V. The ecology of harmful diatoms. In Ecology of Harmful Algae; Granéli, E., Turner, J., Eds.; Springer-Verlag: Heidelberg, Germany, 2006; Volume 189, pp. 81–93. [Google Scholar]
- Sahraoui, I.; Bates, S.S.; Bouchouichaa, D.; Hadj-Mabrouk, H.; Sakka-Hlaili, A. Toxicity of Pseudo-nitzschia populations from Bizerte Lagoon, Tunisia, southwest Mediterranean, and first report of domoic acid production by P. brasiliana. Diatom Res. 2011, 26, 293–303. [Google Scholar] [CrossRef]
- Dao, H.V.; Lim, P.T.; Ky, P.X.; Takata, Y.; Te’g, S.T.; Omura, T.; Fukuyo, Y.; Kodama, M. Diatom Pseudo-nitzschia cf. caciantha (Bacillariophyceae), the most likely source of domoic acid contamination in the thorny oyster Spondylus versicolor Schreibers 1793 in Nha Phu Bay, Khanh Hoa Province, Vietnam. Asian Fish. Sci. 2014, 27, 16–29. [Google Scholar]
- Dao, H.V.; Phan, V.B.; Teng, S.T.; Uchida, H.; Leaw, C.P.; Lim, P.T.; Suzuki, T.; Pham, K.X. Pseudo-nitzschia fukuyoi (Bacillariophyceae), a domoic acid-producing species from Nha Phu Bay, Khanh Hoa Province, Vietnam. Fish. Sci. 2015, 81, 533–539. [Google Scholar] [CrossRef]
- Teng, S.T.; Lim, H.C.; Lim, P.T.; Dao, V.H.; Bates, S.S.; Leaw, C.P. Pseudo-nitzschia kodamae sp. nov. (Bacillariophyceae), a toxigenic species from the Strait of Malacca, Malaysia. Harmful Algae 2014, 34, 17–28. [Google Scholar] [CrossRef]
- Orive, E.; Pérez-Aicua, L.; David, H.; Garcia-Etxebarria, K.; Laza-Martinez, A.; Seoane, S.; Miguel, I. The genus Pseudo-nitzschia (Bacillariophyceae) in a temperate estuary with description of two new species: Pseudo-nitzschia plurisecta sp. nov. and Pseudo-nitzschia abrensis sp. nov. J. Phycol. 2013, 49, 1192–1206. [Google Scholar] [CrossRef]
- Fernandes, L.F.; Hubbard, K.A.; Richlen, M.L.; Smith, J.; Bates, S.S.; Ehrman, J.; Léger, C.; Mafra, L.L., Jr.; Kulis, D.; Quilliam, M.; et al. Diversity and toxicity of the diatom Pseudo-nitzschia Peragallo in the Gulf of Maine, Northwestern Atlantic Ocean. Deep Sea Res. II 2014, 103, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, Y.; Lundholm, N.; Onodera, H.; Kobayashi, K.; Bajarias, A.F.F.; Furio, E.; Iwataki, M.; Fukuyo, Y.; Kodoma, M. Wide distribution of Nitzschia navis-varingica, a new domoic acid-producing benthic diatom found in Vietnam. Fish. Sci. 2004, 70, 28–32. [Google Scholar] [CrossRef]
- Hasle, G.R. Are most of the domoic acid producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae 2002, 1, 137–146. [Google Scholar] [CrossRef]
- Bates, S.S. Ecophysiology and metabolism of ASP toxin production. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer-Verlag: Heidelberg, Germany, 1998; pp. 405–426. [Google Scholar]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom dynamics and physiology of domoic-acid-producing Pseudo-nitzschia species. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer-Verlag: Heidelberg, Germany, 1998; pp. 267–292. [Google Scholar]
- Parsons, M.L.; Dortch, Q.; Turner, R.E. Sedimentological evidence of an increase in Pseudo-nitzschia (Bacillariophyceae) abundance in response to coastal eutrophication. Limnol. Oceanogr. 2002, 47, 551–558. [Google Scholar] [CrossRef]
- Kudela, R.M.; Lane, J.Q.; Cochlan, W.P. The potential role of anthropogenically derived nitrogen in the growth of harmful algae in California, USA. Harmful Algae 2008, 8, 103–110. [Google Scholar] [CrossRef]
- Antia, N.J.; Berland, B.R.; Bonin, D.J.; Maestrini, S.Y. Comparative evaluation of certain organic and inorganic sources of nitrogen for phototrophic growth of marine microalgae. J. Mar. Biol. Assoc. UK 1975, 55, 519–539. [Google Scholar] [CrossRef]
- Bates, S.S.; Worms, J.; Smith, J.C. Effects of ammonium and nitrate on domoic acid production by Pseudo-nitzschia pungens in batch culture. Can. J. Fish. Aquat. Sci. 1993, 50, 1248–1254. [Google Scholar] [CrossRef]
- Bates, S.S.; de Freitas, A.S.W.; Milley, J.E.; Pocklington, R.; Quilliam, M.A.; Smith, J.C.; Worms, J. Controls on domoic acid production by the diatom Nitzschia pungens f. multiseries in culture: Nutrients and irradiance. Can. J. Fish. Aquat. Sci. 1991, 48, 1136–1144. [Google Scholar] [CrossRef]
- Subba-Rao, D.V.; Quilliam, M.A.; Pocklington, R. Domoic acid—A neurotoxic amino acid produced by the marine diatom Nitzschia pungens in culture. Can. J. Fish. Aquat. Sci. 1988, 45, 2076–2079. [Google Scholar]
- Thessen, A.E.; Bowers, H.A.; Stoecker, D.K. Intra- and interspecies differences in growth and toxicity of Pseudo-nitzschia while using different nitrogen sources. Harmful Algae 2009, 8, 792–810. [Google Scholar] [CrossRef]
- Auro, M.E.; Cochlan, W.P. Nitrogen utilization and toxin production by two diatoms of the Pseudo-nitzschia pseudodelicatissima complex: P. cuspidata and P. fryxelliana. J. Phycol. 2013, 49, 156–169. [Google Scholar] [CrossRef]
- Hillebrand, H.; Sommer, U. Nitrogenous nutrition of the potentially toxic diatom Pseudonitzschia pungens f. multiseries Hasle. J. Plankton Res. 1996, 18, 295–301. [Google Scholar] [CrossRef]
- Howard, M.D.A.; Cochlan, W.P.; Ladizinsky, N.; Kudela, R.M. Nitrogenous preference of toxigenic Pseudo-nitzschia australis (Bacillariophyceae) from field and laboratory experiments. Harmful Algae 2007, 6, 206–217. [Google Scholar] [CrossRef]
- Cochlan, W.P.; Herndon, J.; Kudela, R.M. Inorganic and organic nitrogen uptake by the toxigenic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 2008, 8, 111–118. [Google Scholar] [CrossRef]
- Calu, G.; Martin-Jézéquel, V.; Lefaux, E.; Séchet, V.; Lassus, P.; Weigel, P.; Amzil, Z. The influence of nitrogen speciation on growth and toxicity of Pseudo-nitzschia multiseries and P. pungens in batch and continuous cultures. In Proceedings of 7th International Conference on Molluscan Shellfish Safety; Lassus, P., Ed.; Ifremer: Nantes, France, 2009; pp. 157–162. [Google Scholar]
- Allen, J.A.; Garett, M.R. Taurine in invertebrates. In Advances in Marine Biology; Russel, F.S., Yonge, M., Eds.; Academic Press: London, UK, 1971; Volume 9, pp. 205–253. [Google Scholar]
- Martin-Jézéquel, V.; Camus, P.; Poulet, S.A. The partitioning of free amino acids between phytoplankton, zooplankton and ichthyoplankton in the Ushant region. Sci. Mar. 1989, 53, 259–268. [Google Scholar]
- Vincendeau, M.-L. Étude Expérimentale de la Fertilité des Eaux des Milieux Conchylicoles: Influence de l’Excrétion des Huitres et des Palourdes sur la Production des Diatomées Dominantes. Ph.D. Thesis, University of Nantes, Nantes, France, 1987. [Google Scholar]
- Robert, J.-M. Fertilité des Eaux des Claires Ostréicoles et Verdissement: Utilisation de l’Azote par les Diatomées Dominantes. Ph.D. Thesis, University of Nantes, Nantes, France, 1983. [Google Scholar]
- Jackson, A.E.; Ayer, S.W.; Laycock, M.V. The effect of salinity on growth and amino acid composition in the marine diatom Nitzschia pungens. Can. J. Bot. 1992, 70, 2198–2201. [Google Scholar] [CrossRef]
- Bates, S.S.; Bird, C.J.; de Freitas, A.S.W.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the Primary source of domoic acid, a toxin in shellfish from Eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Douglas, D.J.; Bates, S.S. Production of domoic acid, a neurotoxic amino acid, by an axenic culture of the marine diatom Nitzschia pungens f. multiseries Hasle. Can. J. Fish. Aquat. Sci. 1992, 49, 85–90. [Google Scholar] [CrossRef]
- Pan, Y.; Subba Rao, D.V.; Mann, K.H.; Brown, R.G.; Pocklington, R. Effect of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. I. Batch culture studies. Mar. Ecol. Prog. Ser. 1996, 131, 225–233. [Google Scholar] [CrossRef]
- Lyons, D. Effect of Organic Enrichment on Growth and Domoic Acid Production by Axenic Cultures of the Pennate Diatom Pseudo-nitzschia multiseries. Bachelor’s Thesis, Mount Allison University, Sackville, New Brunswick, NB, Canada, 2002. [Google Scholar]
- Kudela, R.; Roberts, A.; Armstrong, M. Laboratory analyses of nutrient stress and toxin production in Pseudo-nitzschia spp. from Monterey Bay, California. In Harmful Algae; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2002; pp. 136–138. [Google Scholar]
- Lundholm, N.; Hansen, P.J.; Koyaki, Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar. Ecol. Prog. Ser. 2004, 273, 1–15. [Google Scholar] [CrossRef]
- Trimborn, S.; Lundholm, N.; Thoms, S.; Richter, K.; Krock, B.; Hansen, P.J.; Rost, B. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry. Physiol. Plant. 2007, 133, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, W.F. The daily pattern of nitrogen uptake by phytoplankton in dynamic mixed layer environments. Hydrobiologia 1992, 238, 37–52. [Google Scholar] [CrossRef]
- Garrison, D.L.; Conrad, S.M.; Eilers, P.P.; Waldron, E.M. Confirmation of domoic acid production by Pseudonitzschia australis (Bacillariophyceae) cultures. J. Phycol. 1992, 28, 604–607. [Google Scholar] [CrossRef]
- Cusack, C.K.; Bates, S.S.; Quillliam, M.A.; Patching, J.W.; Raine, R. Confirmation of domoic acid production by Pseudo-nitzschia australis (Bacillariophyceae) isolated from Irish waters. J. Phycol. 2002, 38, 1106–1112. [Google Scholar] [CrossRef]
- Fehling, J.; Green, D.H.; Davidson, K.; Bolch, C.J.; Bates, S.S. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) in Scottish waters. J. Phycol. 2004, 40, 622–630. [Google Scholar] [CrossRef]
- Álvarez, G.; Uribe, E.; Quijano-Scheggia, S.; López-Rivera, A.; Mariño, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar]
- Santiago-Morales, I.S.; García-Mendoza, E.G. Growth and domoic acid content of Pseudo-nitzschia australis isolated from northwestern Baja California, Mexico, cultured under batch conditions at different temperatures and two Si:NO3 ratios. Harmful Algae 2011, 12, 82–94. [Google Scholar] [CrossRef]
- Thorel, M.; Fauchot, J.; Morelle, J.; Raimbault, V.; le Roy, B.; Miossec, C.; Kientz-Bouchart, V.; Claquin, P. Interactive effects of irradiance and temperature on growth and domoic acid production of the toxic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 2014, 39, 232–241. [Google Scholar] [CrossRef]
- Eppley, R.W.; Renger, E.H. Nitrogen assimilation of an oceanic diatom in nitrogen limited continuous culture. J. Phycol. 1974, 10, 15–23. [Google Scholar] [CrossRef]
- Conway, H.L.; Harrison, P.J. Marine diatoms grown in chemostats under silicate or ammonium limitation. IV Transient response of Chaetoceros debilis, Skeletonema costatum and Thalassiosira gravida to a single addition of the limiting nutrient. Mar. Biol. 1977, 43, 33–43. [Google Scholar] [CrossRef]
- Dortch, Q. Effect of growth conditions on accumulation of internal nitrate, ammonium, amino acids and protein in three marine diatoms. J. Exp. Mar. Biol. Ecol. 1982, 61, 243–264. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Interactions between NH4 and NO3 uptake and assimilation: Comparison of diatoms and dinoflagellates at several growth temperatures. Mar. Biol. 1999, 133, 541–551. [Google Scholar] [CrossRef]
- Loureiro, S.; Jauzein, C.; Garcés, E.; Collos, Y.; Camp, J.; Vaqué, D. The significance of organic nutrients in the nutrition of Pseudo-nitzschia delicatissima (Bacillariophyceae). J. Plankton Res. 2009, 31, 399–410. [Google Scholar] [CrossRef]
- Osada, M.; Stewart, J.E. Gluconic acid/gluconolactone: Physiological influences on domoic acid production by bacteria associated with Pseudo-nitzschia multiseries. Aquat. Microb. Ecol. 1997, 12, 203–209. [Google Scholar] [CrossRef]
- Stewart, J.E.; Marks, L.J.; Wood, C.R.; Risser, S.M.; Gray, S. Symbiotic relations between bacteria and the domoic acid producing diatom Pseudo-nitzschia multiseries and the capacity of these bacteria for gluconic acid/gluconolactone formation. Aquat. Microb. Ecol. 1997, 12, 211–221. [Google Scholar] [CrossRef]
- Mengelt, C.; Prézelin, B.B. Dark survival and subsequent light recovery for Pseudo-nitzschia multiseries. In Harmful Algae; Steidinger, K.A., Landsberg, J.H., Tomas, C.R., Vargo, G.A., Eds.; Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and UNESCO: Paris, France, 2002; pp. 388–390. [Google Scholar]
- Admiraal, W.; Laane, R.W.P.M.; Peletier, H. Participation of diatoms in the amino acid cycle of coastal waters; uptake and excretion in cultures. Mar. Ecol. Prog. Ser. 1984, 15, 303–306. [Google Scholar] [CrossRef]
- Admiraal, W.; Riaux-Gobin, C.; Laane, R.W.P.M. Interactions of ammonium, nitrate, and d- and l-amino acids in the nitrogen assimilation of two species of estuarine benthic diatoms. Mar. Ecol. Prog. Ser. 1987, 40, 267–273. [Google Scholar] [CrossRef]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.E.; Dupont, C.L.; Obornik, M.; Horák, A.; Nunes-Nesi, A.; McCrow, J.P.; Zheng, H.; Johnson, D.A.; Hu, H.; Fernie, A.R.; et al. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 2011, 473, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, P.A.; North, B.B.; Stephens, G.C. Amino acid uptake by marine phytoplankters. Limnol. Oceanogr. 1974, 19, 249–259. [Google Scholar] [CrossRef]
- Fischer, N.S.; Cowdell, R.A. Growth of marine planktonic diatoms on inorganic and organic nitrogen. Mar. Biol. 1982, 72, 147–155. [Google Scholar] [CrossRef]
- Neilson, A.H.; Larsson, T. The utilization of organic nitrogen for growth of algae: Physiological aspects. Physiol. Plant. 1980, 48, 542–553. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Putt, M. Heterotrophy and photoheterotrophy by Antarctic microalgae: Light-dependent incorporation of amino acids and glucose. J. Phycol. 1987, 23, 442–452. [Google Scholar] [CrossRef]
- Lewin, J.; Hellebust, J.A. Heterotrophic nutrition of the marine pennate diatom Navicula pavillardi Hustedt. Can. J. Microbiol. 1975, 21, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.H.; Gillmor, G.G. Utilization of glutamine and glutamic acid by Chlorella pyrenoidosa. Biochem. Biophys. Acta 1966, 115, 253–259. [Google Scholar] [CrossRef]
- Smith, S.R.; Abbriano, R.M.; Hildebrand, M. Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res. 2012, 1. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bolch, C.J.; Bates, S.S. Growth and domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) under phosphate and silicate limitation. J. Phycol. 2004, 40, 674–683. [Google Scholar] [CrossRef]
- Pan, Y.; Subba Rao, D.V.; Mann, K.H.; Brown, R.G.; Pocklington, R. Effect of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. II Continuous culture studies. Mar. Ecol. Prog. Ser. 1996, 131, 235–243. [Google Scholar] [CrossRef]
- Pan, Y.; Parsons, M.L.; Busman, M.; Moeller, P.D.R.; Dortch, Q.; Powell, C.L.; Doucette, G.J. Pseudo-nitzschia sp. cf. pseudodelicatissima—A confirmed producer of domoic acid from the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2001, 220, 83–92. [Google Scholar] [CrossRef]
- Pan, Y.; Bates, S.S.; Cembella, A.D. Environmental stress and domoic acid production by Pseudo-nitzschia: A physiological perspective. Nat. Toxins 1998, 6, 127–135. [Google Scholar] [CrossRef]
- Terseleer, N.; Gypens, N.; Lancelot, C. Factors controlling the production of domoic acid by Pseudo-nitzschia (Bacillariophyceae): A model study. Harmful Algae 2013, 24, 45–53. [Google Scholar] [CrossRef]
- Bates, S.S.; Léger, C.; Smith, K.M. Domoic acid production by the diatom Pseudo-nitzschia multiseries as a function of division rate in silicate-limited chemostat cultures. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commmission of UNESCO: Paris, France, 1996; pp. 163–166. [Google Scholar]
- Pan, Y.; Subba Rao, D.V.; Mann, K.H. Changes in domoic acid production and cellular chemical composition of the toxigenic diatom Pseudo-nitzschia multiseries under phosphate limitation. J. Phycol. 1996, 32, 371–381. [Google Scholar] [CrossRef]
- Hagström, J.A.; Granéli, E.; Moreira, M.O.P.; Odebrecht, C. Domoic acid production and elemental composition of two Pseudo-nitzschia strains, from the NW and SW Atlantic Ocean, growing in phosphorus- or nitrogen-limited chemostat cultures. J. Plankton Res. 2011, 33, 297–308. [Google Scholar] [CrossRef]
- Flynn, K.J.; Syrett, P.J. Development of the ability to take up L-lysine by the diatom Phaeodactylum tricornutum. Mar. Biol. 1985, 89, 317–325. [Google Scholar] [CrossRef]
- Ramsey, U.P.; Douglas, D.J.; Walter, J.A.; Wright, J.L.C. Biosynthesis of domoic acid by the diatom Pseudo-nitzschia multiseries. Nat. Toxins 1998, 6, 137–146. [Google Scholar] [CrossRef]
- US Department of Energy, Joint Genome Institute; Armbrust, E.V.; Parker, M.S.; Rocap, G.; Jenkins, B.; Bates, S.S. Pseudo-nitzschia multiseries CLN-47 Genome Sequence; Assembly Volume 2011. Available online: http://genome.jgi.doe.gov/Psemu1/Psemu1.home.html (accessed on 20 November 2015).
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Lund, J.W.G.; Kipling, C.; le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Amzil, Z.; Fresnel, J.; le Gal, D.; Billard, C. Domoic acid accumulation in French shellfish in relation to toxic species of Pseudo-nitzschia multiseries and P. pseudodelicatissima. Toxicon 2001, 39, 1245–1251. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid extraction and cleanup for liquid chromatographic determination of domoic acid in unsalted seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Jézéquel, V.; Calu, G.; Candela, L.; Amzil, Z.; Jauffrais, T.; Séchet, V.; Weigel, P. Effects of Organic and Inorganic Nitrogen on the Growth and Production of Domoic Acid by Pseudo-nitzschia multiseries and P. australis (Bacillariophyceae) in Culture. Mar. Drugs 2015, 13, 7067-7086. https://doi.org/10.3390/md13127055
Martin-Jézéquel V, Calu G, Candela L, Amzil Z, Jauffrais T, Séchet V, Weigel P. Effects of Organic and Inorganic Nitrogen on the Growth and Production of Domoic Acid by Pseudo-nitzschia multiseries and P. australis (Bacillariophyceae) in Culture. Marine Drugs. 2015; 13(12):7067-7086. https://doi.org/10.3390/md13127055
Chicago/Turabian StyleMartin-Jézéquel, Véronique, Guillaume Calu, Leo Candela, Zouher Amzil, Thierry Jauffrais, Véronique Séchet, and Pierre Weigel. 2015. "Effects of Organic and Inorganic Nitrogen on the Growth and Production of Domoic Acid by Pseudo-nitzschia multiseries and P. australis (Bacillariophyceae) in Culture" Marine Drugs 13, no. 12: 7067-7086. https://doi.org/10.3390/md13127055