Spiromastixones Inhibit Foam Cell Formation via Regulation of Cholesterol Efflux and Uptake in RAW264.7 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
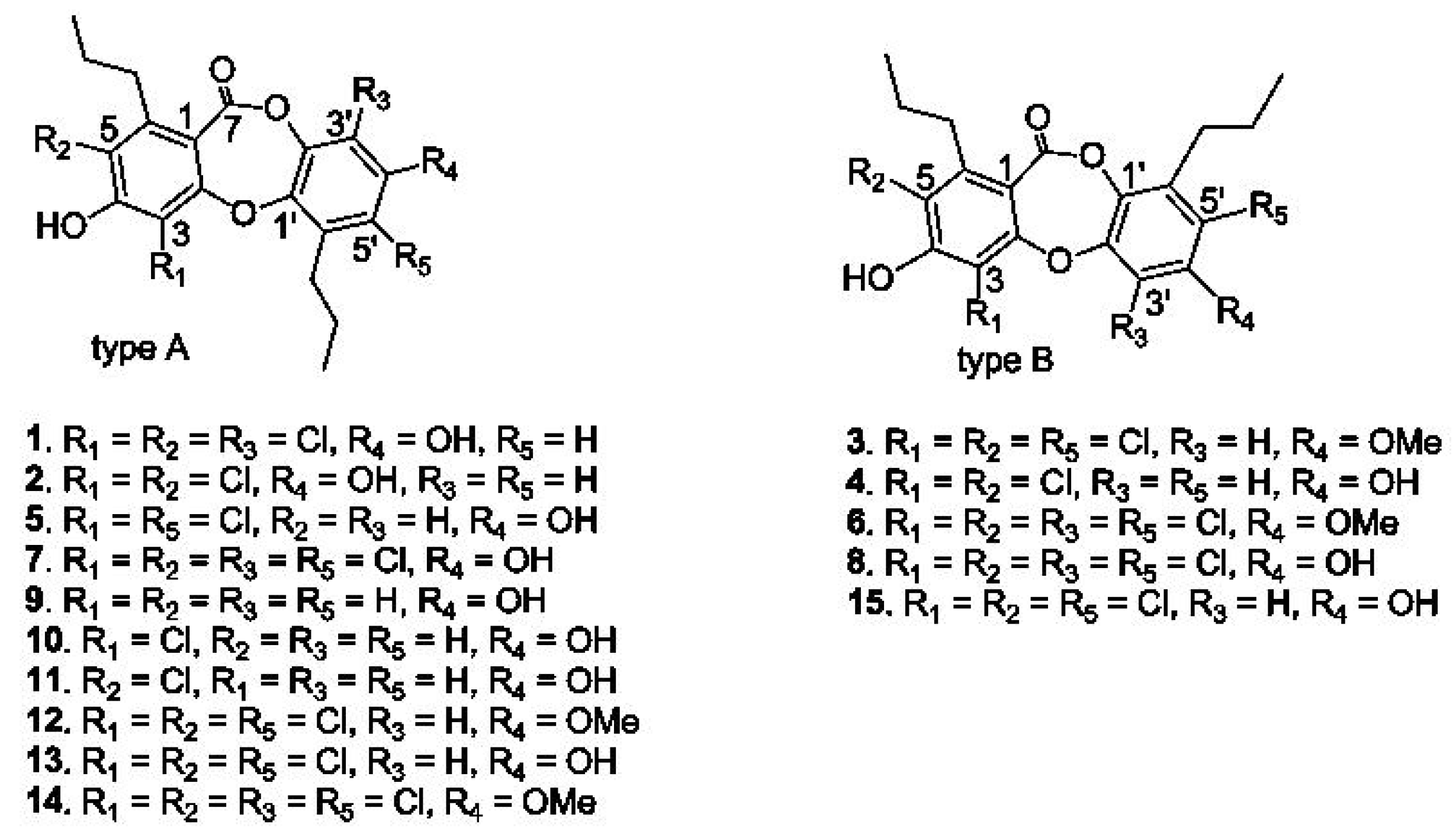
2.1. Structural Characterization of Spiromastixones
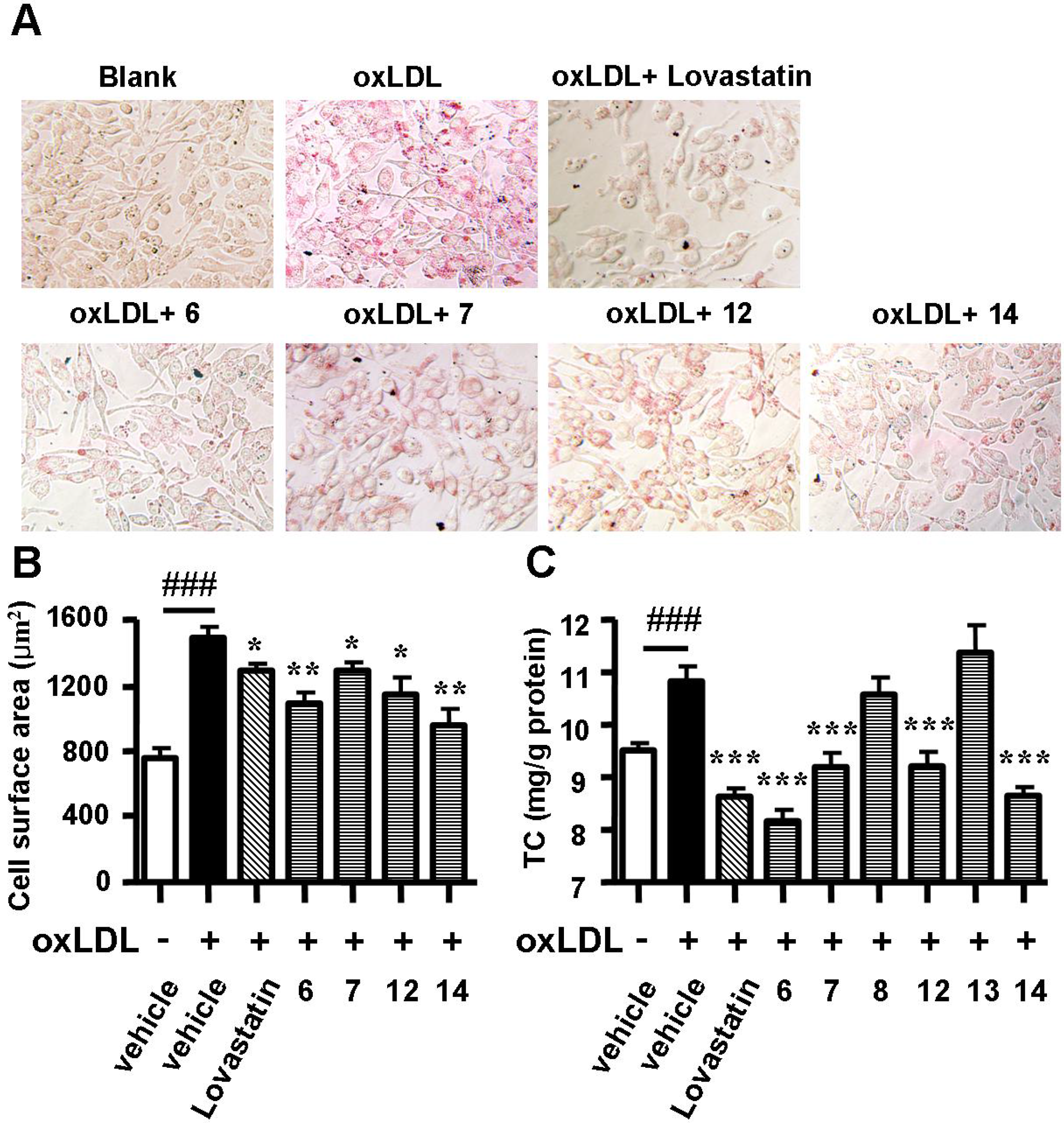
2.2. Spiromastixones Decrease oxLDL-Induced Lipid Overaccumulation in RAW264.7 Cells
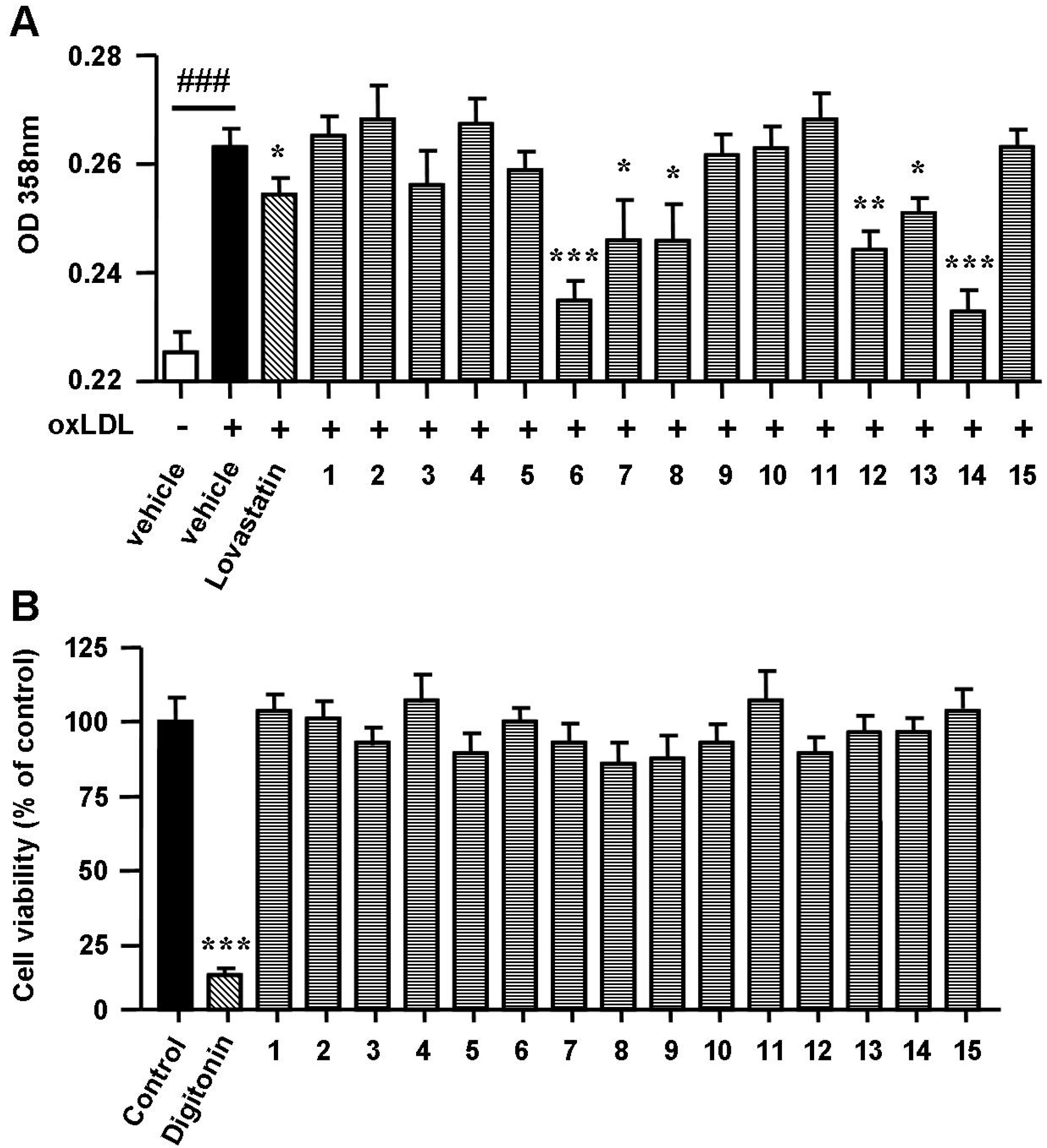
2.3. Spiromastixones Inhibit Cholesterol Uptake by RAW264.7 Macrophages
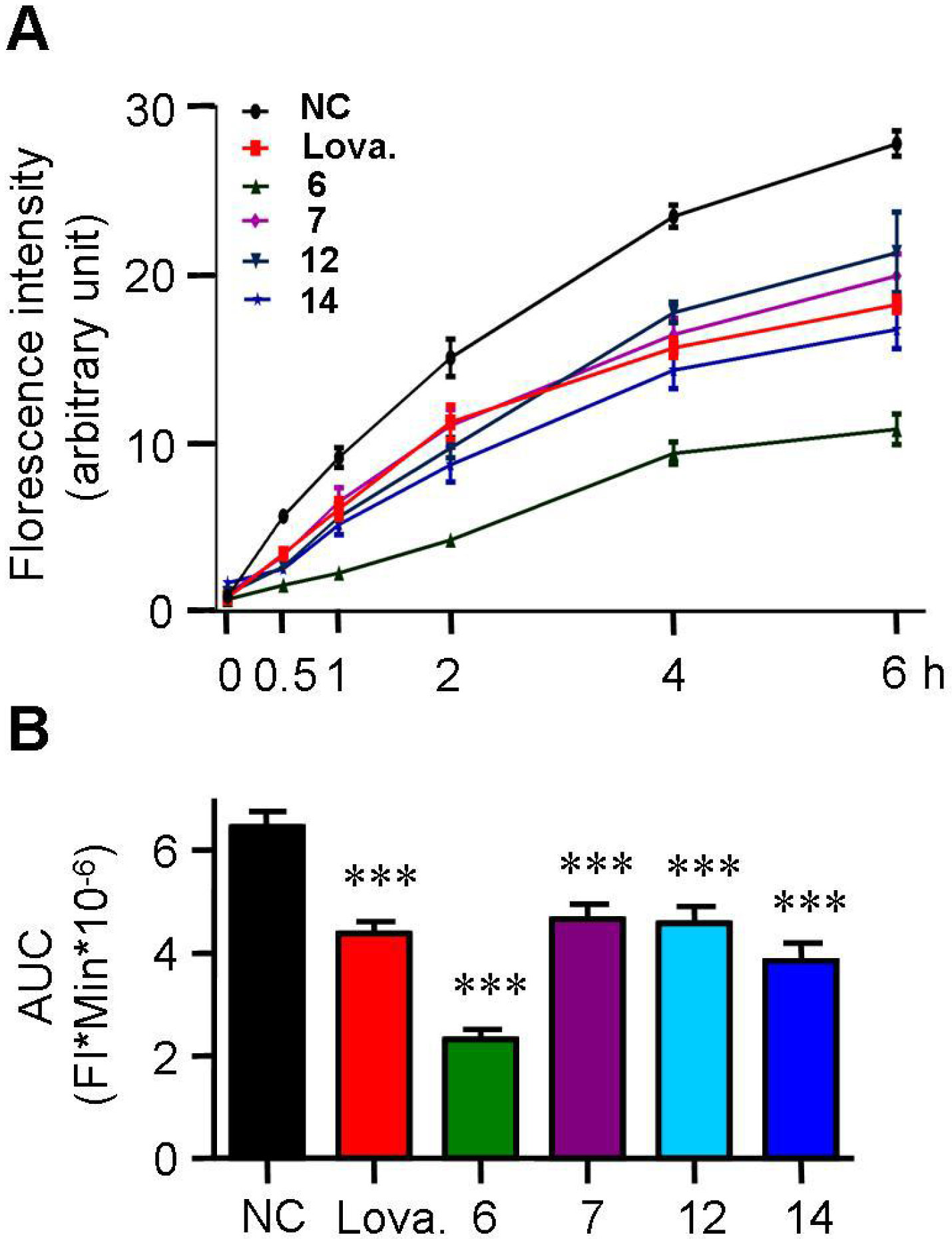
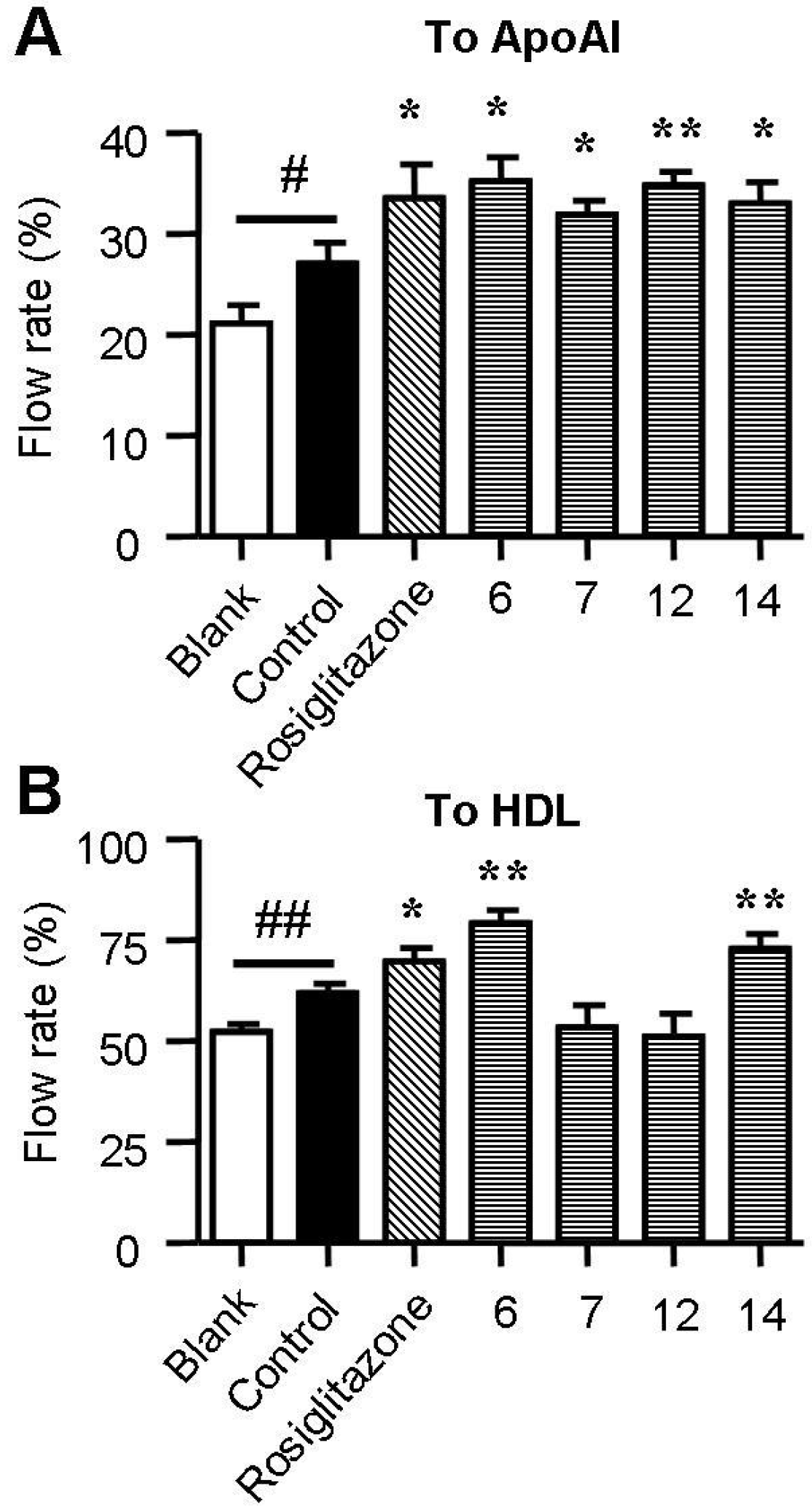
2.4. Spiromastixones Promote Cholesterol Efflux from RAW264.7 Macrophages
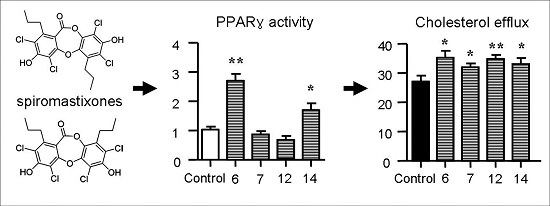
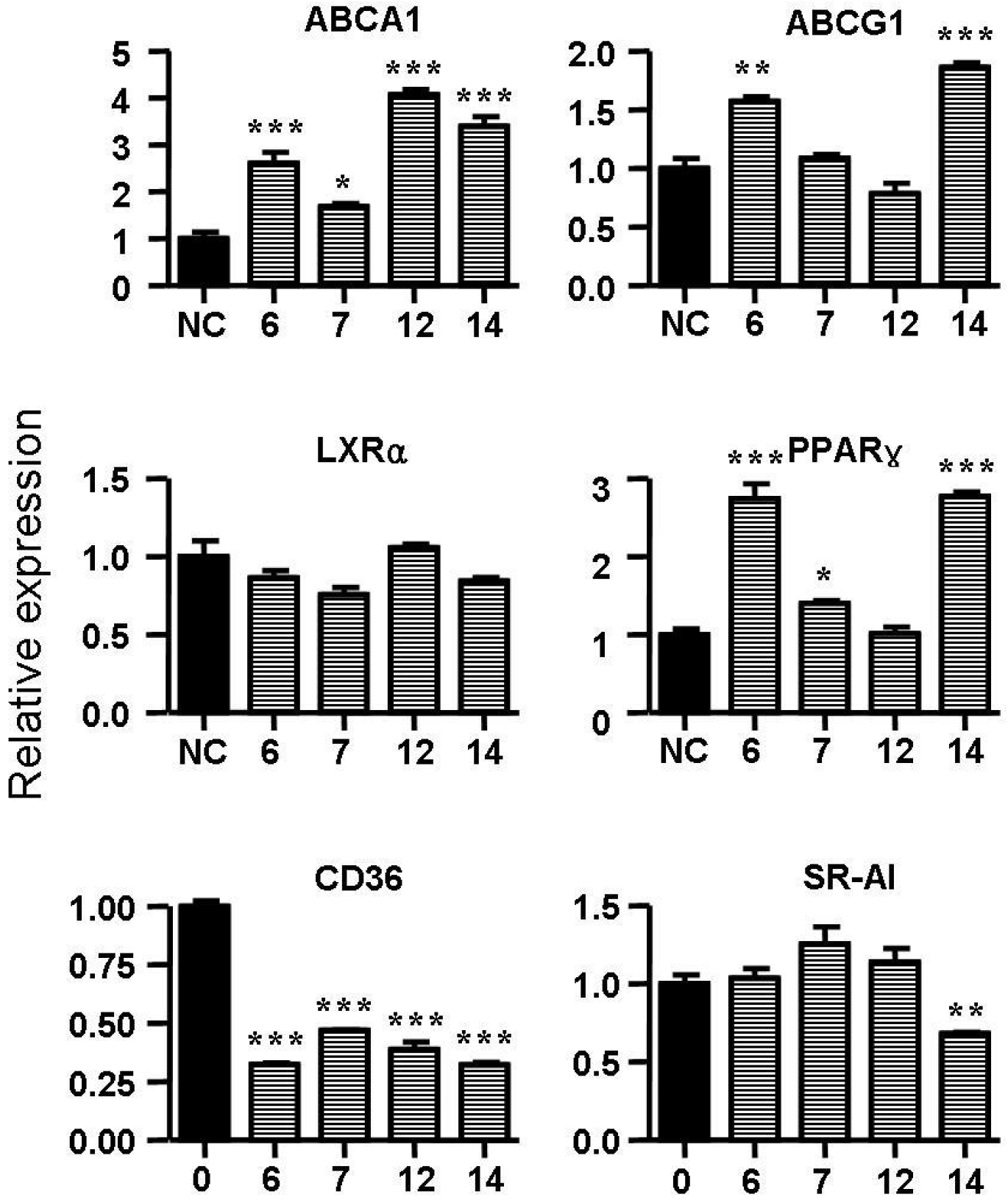
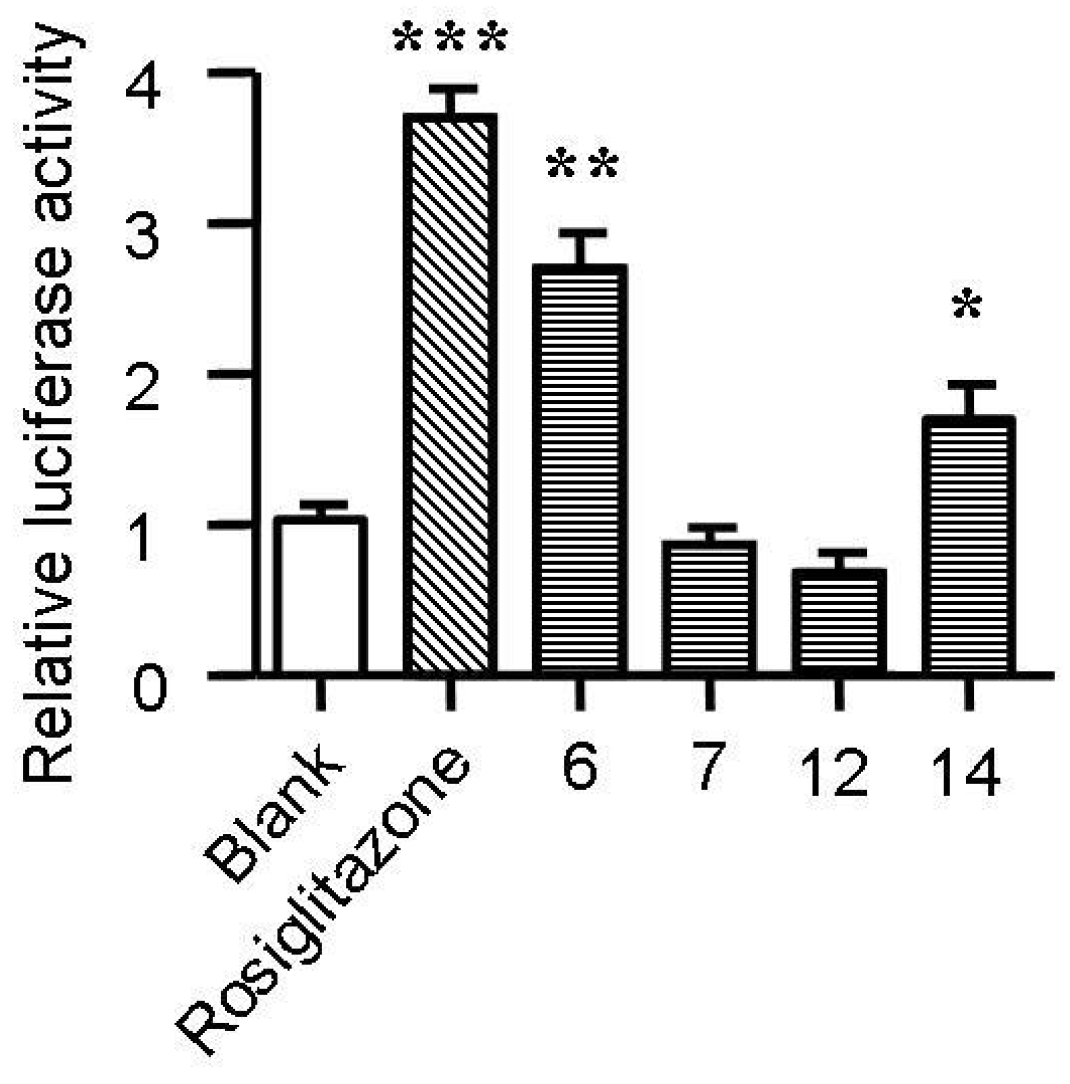
2.5. Spiromastixones Alter mRNA Levels of Cholesterol Efflux/Influx-Modulating Genes and PPARγ Transcriptional Activity
2.6. Structure-Activity Relationship of Spiromastixones
3. Experimental Section
3.1. Materials and Reagents
3.2. Cell Culture
3.3. Oil Red O Staining
3.4. Measurement of Cholesterol in Macrophages
3.5. 25-NBD Cholesterol Uptake Assay
3.6. Cholesterol Efflux Assay
3.7. Real-Time Quantitative PCR
| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| PPARγ | GCAGCTACTGCATGTGATCAAGA | GTCAGCGGGTGGGACTTTC |
| LXRα | AGGAGTGTCGACTTCGCAAA | CTCTTCTTGCCGCTTCAGTTT |
| ABCA1 | CCCAGAGCAAAAAGGGACTC | GGTCATCATCACTTTGGTCCTTG |
| ABCG1 | CAAGACCCTTTTGAAAGGGATCTC | GCCAGAATATTCATGAGTGTGGAC |
| CD36 | CAAGCTCCTTGGCATGGTAGA | TGGATTTGCAAGCACAATATGAA |
| SR-A1 | TTAAAGGTGATCGGGGACAAA | CAACCAGTCGAACTGTCTTAAG |
| β-actin | CCTGGCACCCAGCACAAT | GCCGATCCACACACGGAGTACT |
3.8. Measurement of PPARγ Promoter Activity
3.9. Cell Viability Assay
3.10. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernandez-Friera, L.; Penalvo, J.L.; Fernandez-Ortiz, A.; Ibanez, B.; Lopez-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martinez de Vega, V.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Agewall, S.; Tawakol, A.; Staels, B.; Stein, E.; Mentz, R.J.; Leite-Moreira, A.; Zannad, F.; Koenig, W. Atherosclerosis: Recent trials, new targets and future directions. Int. J. Cardiol. 2015, 192, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Mendis, S.; Chestnov, O. The global burden of cardiovascular diseases: A challenge to improve. Curr. Cardiol. Rep. 2014, 16, 486. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.M.; Strong, A.; Tohyama, J.; Jin, X.; Morales, C.R.; Billheimer, J.; Millar, J.; Kruth, H.; Rader, D.J. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ. Res. 2015, 116, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.N.; Ponnusamy, T.; Philip, S.; Mukhopadhyay, R.; Kakkar, V.V.; Mundkur, L. Hypercholesterolemia Induced Immune Response and Inflammation on Progression of Atherosclerosis in Apob Ldlr/J. Mice. Lipids 2015. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.O.; Swat, W.; Febbraio, M.; Silverstein, R.L. Vav family Rho guanine nucleotide exchange factors regulate CD36-mediated macrophage foam cell formation. J. Biol. Chem. 2011, 286, 7010–7017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Yin, K.; Fu, Y.C.; Tang, C.K. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol. Med. 2012, 18, 149–158. [Google Scholar] [PubMed]
- Lee, J.Y.; Karwatsky, J.; Ma, L.; Zha, X. ABCA1 increases extracellular ATP to mediate cholesterol efflux to ApoA-I. Am. J. Physiol. Cell Physiol. 2011, 301, C886–C894. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Wroblewski, J.M.; Cai, L.; de Beer, M.C.; Webb, N.R.; van der Westhuyzen, D.R. Nascent HDL formation in hepatocytes and role of ABCA1, ABCG1, and SR-BI. J. Lipid Res. 2012, 53, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [PubMed]
- Ravelli, A. Should children and adolescents with systemic lupus erythematosus be given statin therapy to prevent early atherosclerosis? Arthritis Rheumatol. 2012, 64, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Nozue, T.; Yamamoto, S.; Tohyama, S.; Fukui, K.; Umezawa, S.; Onishi, Y.; Kunishima, T.; Sato, A.; Nozato, T.; Miyake, S.; et al. Impacts of age on coronary atherosclerosis and vascular response to statin therapy. Heart Vessel. 2014, 29, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, W.; Zheng, X.; Liao, K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009, 19, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luan, H.; Zhang, X.; Wang, S.; Sun, X.; Guo, P. Chlorogenic acid protects against atherosclerosis in ApoE-/mice and promotes cholesterol efflux from RAW264.7 macrophages. PLoS ONE 2014, 9, e95452. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Gogaki, V.; Stamatakis, G.; Papaharisis, L.; Demopoulos, C.A.; Zabetakis, I. Evaluation of the in vitro anti-atherogenic properties of lipid fractions of olive pomace, olive pomace enriched fish feed and gilthead sea bream (Sparus aurata) fed with olive pomace enriched fish feed. Mar. Drugs 2013, 11, 3676–3688. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Liu, M.; Luan, H.; Ji, Y.; Guo, P.; Wu, C. Chrysin inhibits foam cell formation through promoting cholesterol efflux from RAW264.7 macrophages. Pharm. Biol. 2015, 53, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Efendy, J.L.; Smith, N.J.; Campbell, G.R. Molecular basis by which garlic suppresses atherosclerosis. J. Nutr. 2001, 131, 1006S–1009S. [Google Scholar] [PubMed]
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Zeb, I.; Flores, F.; Ebrahimi, R.; Budoff, M. Aged garlic extract with supplement is associated with increase in brown adipose, decrease in white adipose tissue and predict lack of progression in coronary atherosclerosis. Int. J. Cardiol. 2013, 168, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. Spiromastixones A–O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L.; Terasaka, N.; Pagler, T.; Wang, N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008, 7, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L. Inflammation, atherosclerosis, and arterial thrombosis: Role of the scavenger receptor CD36. Clevel. Clin. J. Med. 2009, 76, S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Kai, Q.; Gao, J.; Lian, Z.Q.; Wu, C.M.; Wu, C.A.; Zhu, H.B. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J. Pharmacol. Sci. 2010, 113, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, F.; Zhao, S.P. Ac-hE-18A-NH2, a novel dual-domain apolipoprotein mimetic peptide, inhibits apoptosis in macrophages by promoting cholesterol efflux. Mol. Med. Rep. 2014, 9, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Lee, G.Y.; Chung, J.J.; Ahn, Y.H.; Hong, S.H.; Kim, J.B. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 2006, 55, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Chen, R.; Liu, M.; Liu, D.; Li, X.; Wang, S.; Niu, S.; Guo, P.; Lin, W. Spiromastixones Inhibit Foam Cell Formation via Regulation of Cholesterol Efflux and Uptake in RAW264.7 Macrophages. Mar. Drugs 2015, 13, 6352-6365. https://doi.org/10.3390/md13106352
Wu C, Chen R, Liu M, Liu D, Li X, Wang S, Niu S, Guo P, Lin W. Spiromastixones Inhibit Foam Cell Formation via Regulation of Cholesterol Efflux and Uptake in RAW264.7 Macrophages. Marine Drugs. 2015; 13(10):6352-6365. https://doi.org/10.3390/md13106352
Chicago/Turabian StyleWu, Chongming, Ran Chen, Mingyue Liu, Dong Liu, Xin Li, Shuai Wang, Siwen Niu, Peng Guo, and Wenhan Lin. 2015. "Spiromastixones Inhibit Foam Cell Formation via Regulation of Cholesterol Efflux and Uptake in RAW264.7 Macrophages" Marine Drugs 13, no. 10: 6352-6365. https://doi.org/10.3390/md13106352