Lipids and Fatty Acids of Nudibranch Mollusks: Potential Sources of Bioactive Compounds
Abstract
:1. Introduction
2. Results and Discussion
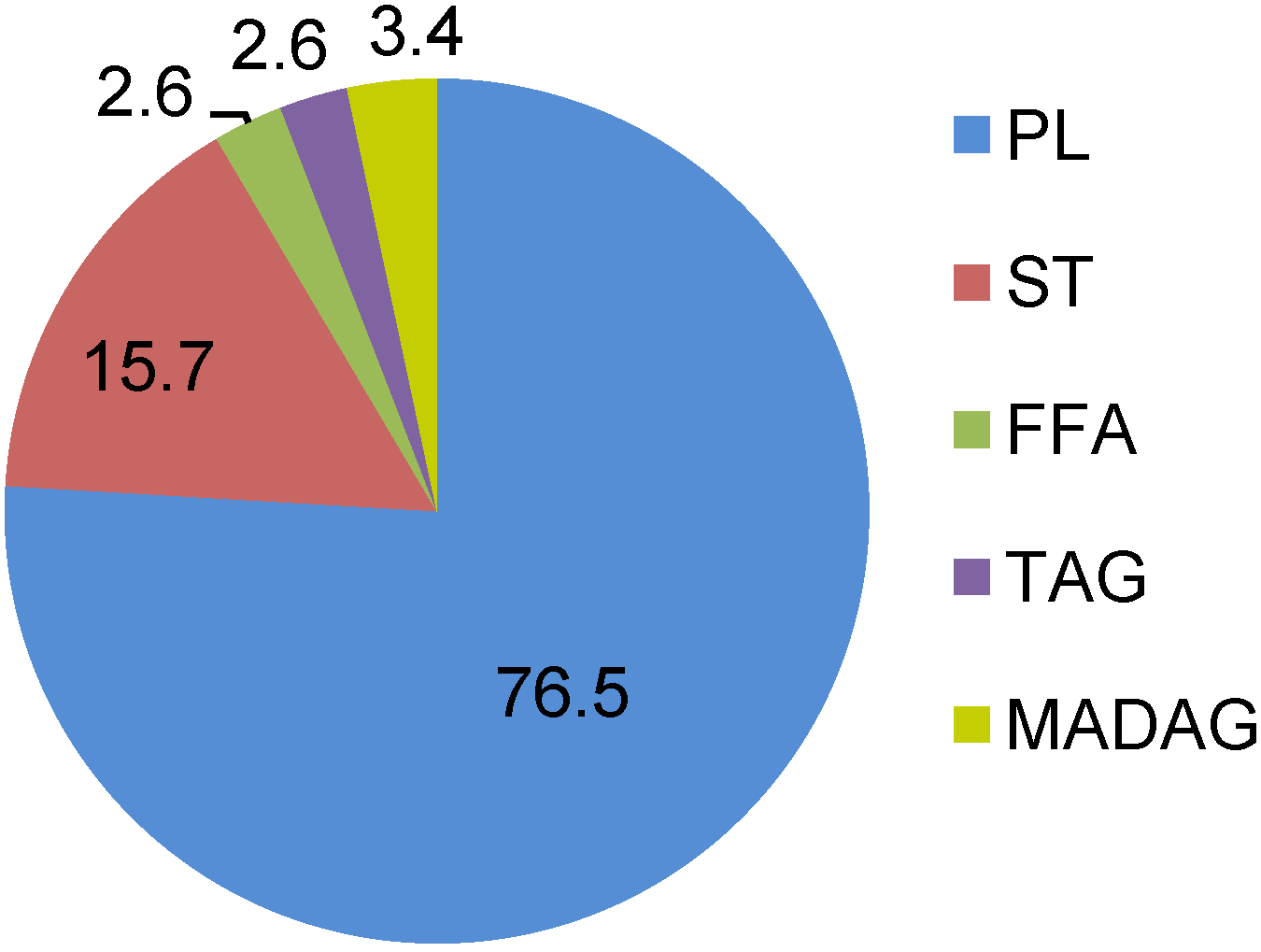
2.1. Lipids and Phospholipids
| PC | PE | PS | CAEP | PI | DPG | |
|---|---|---|---|---|---|---|
| Chromodoris tinctoria | 60.9 ± 2.1 | 11.7 ± 0.9 | 12.5 ± 1.1 | 5.1 ± 0.4 | 6.5 ± 0.8 | 2.0 ± 0.6 |
| C. michaeli | 53.1 ± 2.1 | 21.4 ± 1.1 | 13.4 ± 0.8 | 5.6 ± 0.5 | 4.9 ± 0.6 | 1.7 ± 0.4 |
| C. geometrica | 53.8 ± 1.5 | 15.4 ± 0.9 | 12.6 ± 0.7 | 12.2 ± 1.1 | 3.7 ± 1.1 | 1.2 ± 0.3 |
| Chromodoris sp. | 51.2 ± 1.1 | 17.4 ± 1.3 | 14.5 ± 1.2 | 9.1 ± 1.9 | 5.0 ± 0.6 | 1.8 ± 0.4 |
| Glossodoris cincta | 56.1 ± 0.6 | 16.4 ± 1.4 | 15.4 ± 1.3 | 5.1 ± 0.7 | 4.2 ± 0.6 | 1.8 ± 0.3 |
| G. atromarginata | 53.5 ± 2.3 | 18.2 ± 1.9 | 12.2 ± 1.4 | 9.9 ± 1.7 | 5.1 ± 0.6 | 1.1 ± 0.2 |
| Risbecia tryoni | 49.6 ± 0.4 | 18.2 ± 1.1 | 13.8 ± 0.8 | 10.6 ± 1.1 | 4.6 ± 1.0 | 3.2 ± 0.8 |
| Platydoris sp. | 50.9 ± 2.0 | 21.2 ± 1.5 | 10.2 ± 0.8 | 8.0 ± 1.7 | 5.4 ± 0.5 | 2.7 ± 0.5 |
| 1-Alkenyl-2-acyl-PE | 1-Alkenyl-2-acyl-PS | |
|---|---|---|
| Chromodoris tinctoria | 60.4 ± 1.6 | 47.1 ± 2.1 |
| Chromodoris michaeli | 52.9 ± 2.4 | 51.1 ± 2.4 |
| Chromodoris geometrica | 58.8 ± 2.1 | 50.6 ± 1.7 |
| Chromodoris sp. | 59.2 ± 1.8 | 48.7 ± 1.1 |
| Glossodoris cincta | 61.1 ± 1.1 | 47.8 ± 1.5 |
| Glossodoris atromarginata | 60.7 ± 2.1 | 48.6 ± 1.2 |
| Risbecia tryoni | 65.1 ± 1.6 | 56.5 ± 1.0 |
| Platydoris sp. | 50.3 ± 1.8 | 61.3 ± 1.5 |
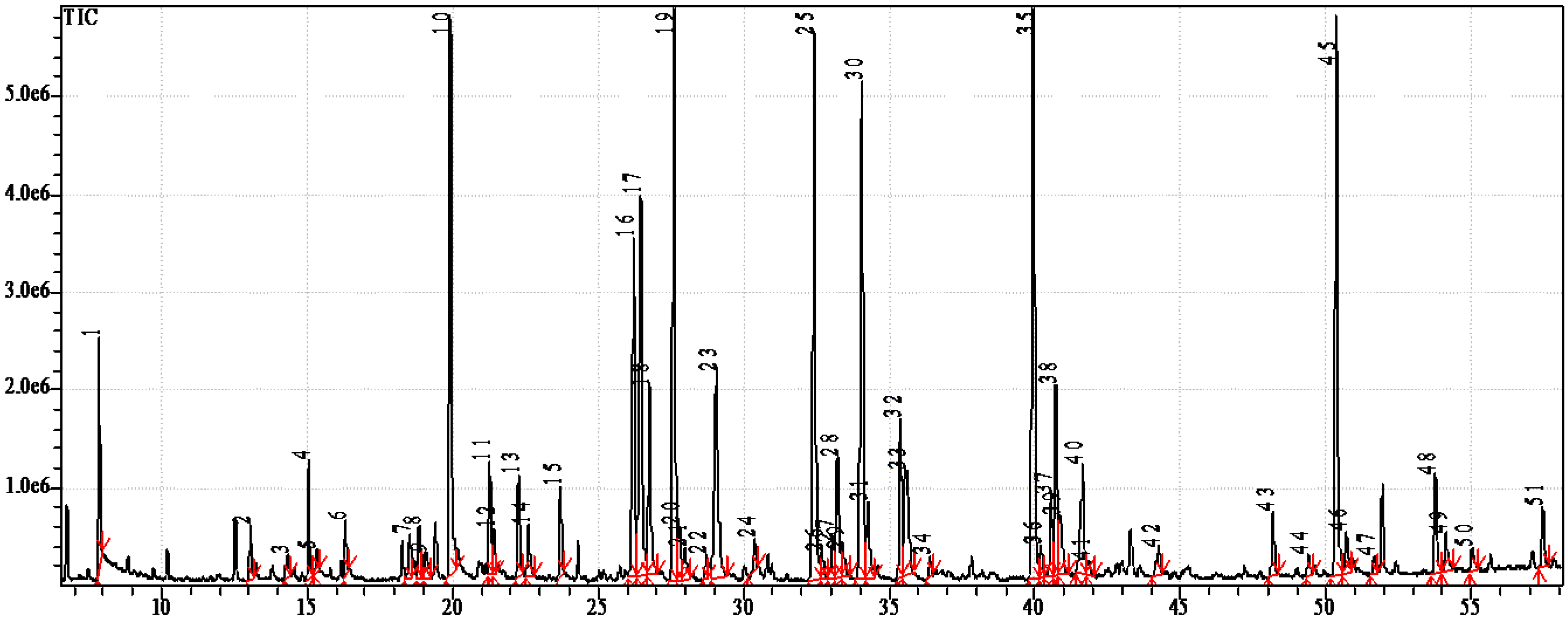
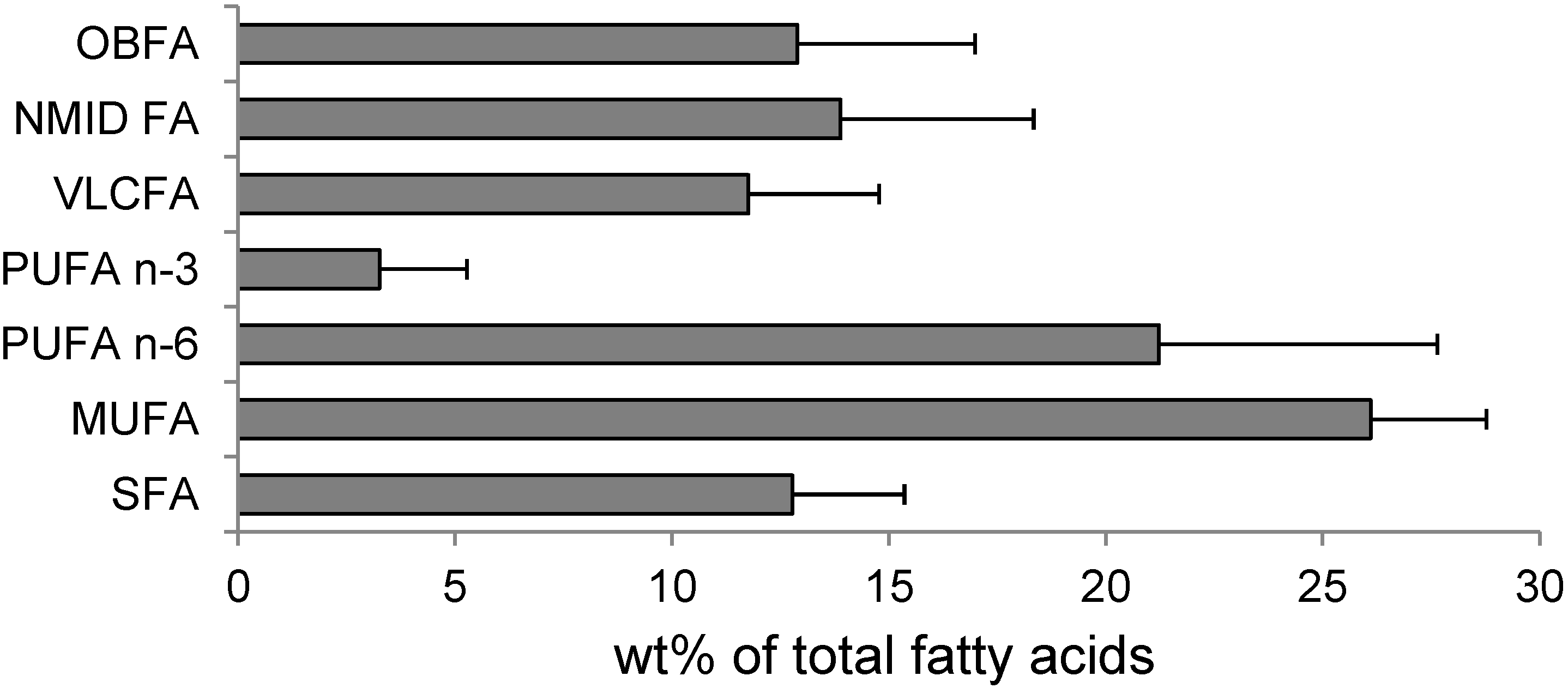
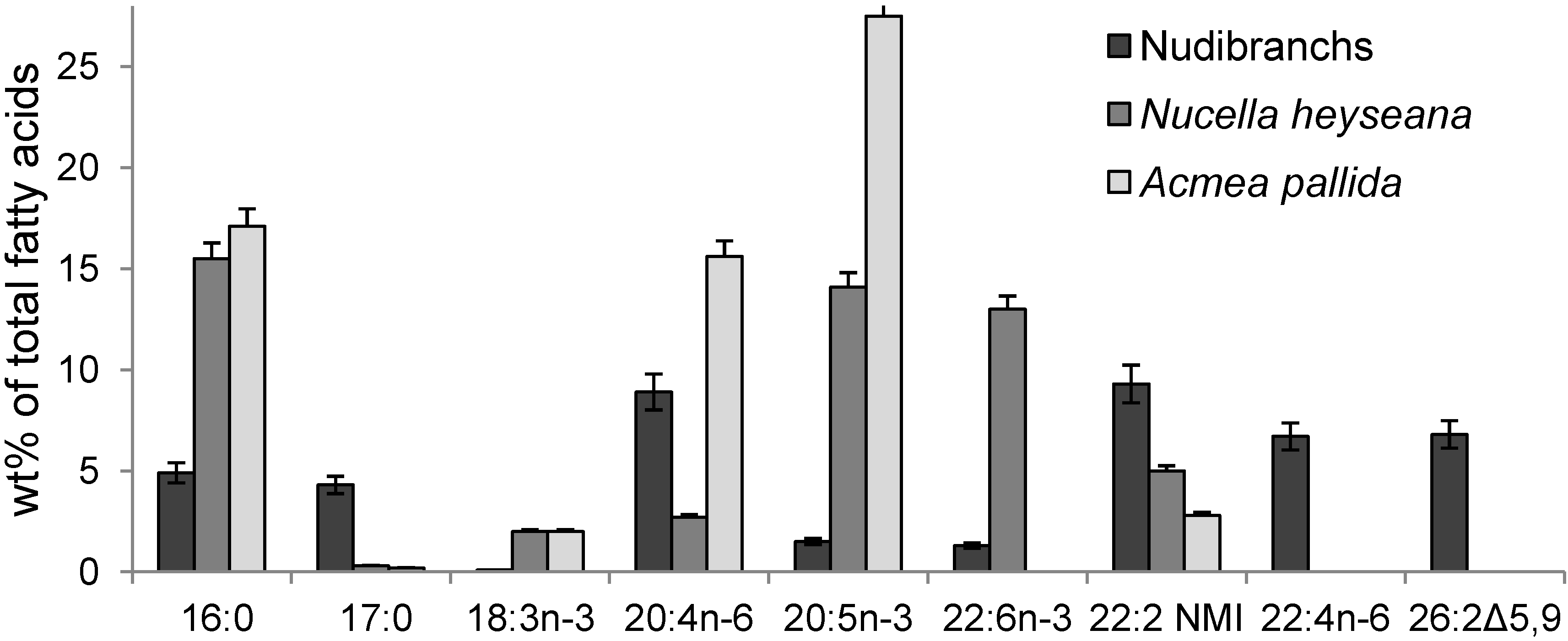
2.2. Fatty Acids
| FA | Molecular Ion (m/z) | % of Total FA | FA | Molecular Ion (m/z) | % of Total FA |
|---|---|---|---|---|---|
| 12:0 | 253 | 0.4 ± 0.1 | 20:5n-3 | 355 | 0.2 ± 0.1 |
| 14:0 | 281 | 0.9 ± 0.3 | 20:2Δ5,11 | 355 | 1.8 ± 0.5 |
| iso-15:0 | 295 | 1.9 ± 0.5 | 20:2Δ5,13 | 355 | 1.3 ± 0.4 |
| anteiso-15:0 | 295 | 0.3 ± 0.1 | 20:3n-6 | 359 | 0.7 ± 0.3 |
| 15:0 | 295 | 1.1 ± 0.2 | 20:1n-11 | 363 | 5.6 ± 0.7 |
| iso-16:0 | 309 | 0.5 ± 0.1 | 20:1n-9 | 363 | 0.2 ±0.1 |
| anteiso-16:0 | 309 | 0.7 ± 0.1 | 20:1n-7 | 363 | 2.5 ± 0.6 |
| 16:1n-7 | 307 | 1.7 ± 0.6 | 21:2Δ7,13 | 375 | 0.1 ± 0.0 |
| 16:0 | 309 | 5.9 ± 0.8 | iso-21:1 | 377 | 0.4 ± 0.1 |
| iso-17:0 | 323 | 1.4 ± 0.3 | 21:1n-7 | 377 | 2.0 ± 0.1 |
| anteiso-17:0 | 323 | 0.9 ± 0.1 | 21:1n-5 | 377 | 1.3 ± 0.5 |
| 17:1n-8 | 321 | 1.4 ± 0.4 | 22:5n-6 | 383 | 0.4 ± 0.1 |
| 17:1n-6 | 321 | 0.4 ± 0.1 | 22:6n-3 | 381 | 0.7 ± 0.2 |
| 17:0 | 323 | 1.4 ±0.1 | 22:4n-6 | 385 | 10.2 ± 1.3 |
| 18:3n-6 | 331 | 0.2 ± 0.1 | 22:5n-3 | 383 | 0.2 ± 0.1 |
| iso-18:0 | 337 | 0.4 ± 0.2 | 22:3n-6 | 387 | 0.2 ± 0.1 |
| anteiso-18:0 | 337 | 0.4 ± 0.2 | 22:2Δ7,13 | 389 | 3.6 ± 0.7 |
| 18:2n-6 | 333 | 7.0 ± 0.9 | 22:2Δ7,15 | 289 | 1.2 ± 0.3 |
| 18:1n-9 | 335 | 5.4 ± 0.4 | 22:1n-9 | 391 | 0.4 ± 0.2 |
| 18:1n-7 | 335 | 3.4 ± 1.0 | 22:1n-7 | 391 | 0.1 ± 0.1 |
| 18:0 | 337 | 7.4 ± 1.3 | iso-24:2Δ5,9 | 417 | 0.2 ± 0.1 |
| iso-19:1 | 349 | 0.1 ± 0.0 | 24:2Δ5,9 | 417 | 3.1 ± 0.5 |
| anteiso-19:1 | 349 | 0.2 ± 0.1 | iso-25:2Δ5,9 | 431 | 4.0 ± 1.0 |
| iso-19:0 | 351 | 0.2 ± 0.1 | anteiso-25:2Δ5,9 | 431 | 0.6 ± 0.2 |
| anteiso-19:0 | 351 | 0.2 ± 0.1 | 25:2Δ5,9 | 431 | 0.1 ± 0.1 |
| 19:1n-8 | 349 | 0.1 ± 0.0 | iso-26:2Δ5,9 | 445 | 0.8 ± 0.3 |
| 19:1n-12 | 349 | 2.6 ± 0.8 | anteiso-26:2Δ5,9 | 445 | 0.1 ± 0.0 |
| 19:0 | 351 | 0.3 ± 0.1 | 26:2Δ5,9 | 445 | 0.8 ± 0.3 |
| 20:4n-6 | 357 | 10.5 ± 1.2 | anteiso-27:2Δ5,9 | 459 | 0.2 ± 0.1 |
| VLCFA | Chromodoris sp. | C. geometrica | C. tinctoria | C. michaeli | Glossodoris atromarginata | G. cincta | Risbecia tryoni | Platydoris sp. |
|---|---|---|---|---|---|---|---|---|
| i-24:2∆5,9 | - | - | - | 0.2 | 0.2 | - | - | - |
| 24:2∆5,9 | 1.0 | 4.7 | 0.4 | 3.1 | 1.1 | 1.2 | 1.4 | - |
| 24:1 | - | 0.3 | 0.1 | - | - | - | - | - |
| i-25:2∆5,9 | - | - | - | 4.0 | - | - | - | - |
| 25:2∆5,9 | 4.0 | 1.6 | 0.7 | 0.2 | 2.3 | 2.6 | 0.5 | - |
| 25:3∆5,9 | 0.5 | 0.1 | - | - | - | 1.3 | - | - |
| 26:0 | - | 0.1 | - | - | - | 0.3 | - | - |
| i-26:2∆5,9 | 0.8 | 0.1 | 0.8 | 0.8 | - | 0.7 | - | |
| 26:2∆5,9 | 6.0 | 3.0 | 13.6 | 0.8 | 6.3 | 8.4 | 5.8 | 10.5 |
| i-26:3∆5,9,19 | - | 0.2 | - | - | 1.2 | 2.3 | - | - |
| ai-26:3∆5,9,19 | - | - | - | - | 1.1 | - | - | |
| ai-27:2∆5,9 | - | - | - | 0.2 | - | - | - | - |
3. Experimental Section
3.1. Site and Samples
3.2. Lipid Analysis
3.3. Fatty Acid Analysis
3.4. Statistical Analysis
4. Conclusions
Abbreviations
| CAEP | ceramide-aminoethylphosphonate |
| DPG | diphosphatidylglycerol |
| FA | fatty acid |
| FAME | fatty acid methyl ester |
| FFA | free fatty acids |
| GC-MS | gas chromatography-mass spectrometry |
| MADAG | monoalkyldiacylglycerol |
| MUFA | monounsaturated fatty acid |
| NMID FA | non-methylene-interrupted dienoic fatty acids |
| OBFA | odd-chain and branched fatty acids |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PI | phosphatidylinositol |
| PL | phospholipids |
| PS | phosphatidylserine |
| PUFA | polyunsaturated fatty acid |
| SFA | saturated fatty acid |
| ST | sterols |
| TLC | thin-layer chromatography |
| VLCFA | very long chain fatty acid |
Acknowledgments
Conflicts of Interest
References
- Evans-Illidge, E.A.; Logan, M.; Doyle, J.; Fromont, J.; Battershill, C.N.; Ericson, G.; Wolff, C.W.; Muirhead, A.; Kearns, P.; Abdo, D.; et al. Phylogeny drives large scale patterns in australian marine bioactivity and provides a new chemical ecology rationale for future biodiscovery. PLoS One 2013, 8, e73800. [Google Scholar] [CrossRef]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar]
- Cimino, G.; Gavagnin, M. Molluscs: Progress in Molecular and Subcellular Biology Subseries Marine Molecular Biochemistry; Springer-Verlag: Berlin, Heidelburg, Germany, 2006; p. 387. [Google Scholar]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2000, 17, 7–55. [Google Scholar] [CrossRef]
- Gavagnin, M.; Fontana, A. Diterpenes from marine opisthobranch mollusks. Curr. Org. Chem. 2000, 4, 1201–1248. [Google Scholar] [CrossRef]
- Avila, C. Natural products of opisthobranch molluscs: A biological review. Oceanogr. Mar. Biol. 1995, 33, 487–559. [Google Scholar]
- Fontana, A. Biogenetical proposals and biosynthetic studies on secondary metabolites of opisthobranch molluscs. In Molluscs: Progress in Molecular and Subcellular Biology Subseries Marine Molecular Biochemistry; Cimino, G., Gavagnin, M., Eds.; Springer-Verlag: Berlin, Heidelburg, Germany, 2006; pp. 303–328. [Google Scholar]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Wendel, M.; Heller, A.R. Anticancer actions of omega-3 fatty acids—Current state and future perspectives. Anticancer Agents Med. Chem. 2009, 9, 457–470. [Google Scholar] [CrossRef]
- Candela, C.G.; Lopez, L.; Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Banskota, A.H.; Stefanova, R.; Gallant, P.; McGinn, P. Mono- and digalactosyldiacylglycerols: Potent nitric oxide inhibitors from the marine microalga Nannochloropsis granulata. J. Appl. Phycol. 2013, 25, 349–357. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New diacylglyceryltrimethyl-homoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- De Souza, L.M.; Sassaki, G.L.; Villela, R.; Maria, T.; Barreto-Bergter, E. Structural Characterization and anti-HSV-1 and HSV-2 activity of glycolipids from the marine algae Osmundaria obtusiloba isolated from Southeastern Brazilian coast. Mar. Drugs 2012, 10, 918–931. [Google Scholar] [CrossRef]
- Plouguerne, E.; de Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Romanos, M.T.V.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Antiviral Sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef]
- Cantillo-Ciau, Z.; Moo-Puc, R.; Quijano, L.; Freile-Pelegrin, Y. The tropical brown alga Lobophora variegata: A source of antiprotozoal compounds. Mar. Drugs 2013, 8, 1292–1304. [Google Scholar]
- Farokhi, F.; Grellier, P.; Clement, M.; Roussakis, C.; Loiseau, P.M.; Genin-Seward, E.; Kornprobst, J.-M.; Barnathan, G.; Wielgosz-Collin, G. Antimalarial activity of axidjiferosides, new beta-galactosylceramides from the African sponge Axinyssa djiferi. Mar. Drugs 2013, 11, 1304–1315. [Google Scholar] [CrossRef]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates: Mollusca. Prog. Lipid. Res. 1982, 21, 109–153. [Google Scholar] [CrossRef]
- Zhukova, N.V. Lipid classes and fatty acid composition of the tropical nudibranch mollusks Chomodoris sp. and Phyllidia coelestis. Lipids 2007, 42, 1169–1175. [Google Scholar] [CrossRef]
- Gannefors, C.; Boer, M.; Kattner, G.; Graeve, M.; Eiane, K.; Gulliksen, B.; Hop, H.; Falk-Petersen, S. The Arctic sea butterfly Limacina helicina: Lipids and life strategy. Mar. Biol. 2005, 147, 169–177. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Sargent, J.R. Ether-Linked Glycerides in Marine Animals. In Marine Biogenic Lipids, Fats, and Oils; Ackman, R.G., Ed.; CRC Press: Boca Raton, FL, USA, 1987; pp. 176–193. [Google Scholar]
- Magnusson, C.D.; Haraldsson, G.G. Ether lipids. Chem. Phys. Lipids 2011, 164, 315–340. [Google Scholar]
- Dembitsky, V.M.; Rezanka, T.; Kashin, A.G. Fatty acid and phospholipid composition of freshwater molluscs Anodonta piscinalis and Limnaea fragilis from the river Volga. Comp. Biochem. Physiol. 1993, 105B, 597–601. [Google Scholar]
- Kraffe, E.; Sounant, P.; Marty, A. Fatty acids of Serine, ethanolamine, and choline plasmalogens in some marine bivalves. Lipids 2004, 39, 59–66. [Google Scholar] [CrossRef]
- Brosche, T.; Brueckmann, M.; Haase, K.K.; Sieber, C.; Bertsch, T. Decreased plasmalogen concentration as a surrogate marker of oxidative stress in patients presenting with acute coronary syndromes or supraventricular tachycardias. Clin. Chem. Lab. Med. 2007, 45, 689–691. [Google Scholar]
- Latorre, E.; Collado, M.P.; Fernandez, I.; Aragones, M.D.; Catalan, R.E. Signaling events mediating activation of brain ethanolamine plasmalogen hydrolysis by ceramide. Eur. J. Biochem. 2003, 270, 36–46. [Google Scholar]
- Hartmann, T.; Kuchenbecker, J.; Grimm, M.O. Alzheimer’s disease: The lipid connection. J. Neurochem. 2007, 103, 159–170. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Svetashev, V.I. Non-methylene-interrupted dienoic fatty acids in mollusks from the Sea of Japan. Comp. Biochem. Physiol. 1986, 83B, 643–646. [Google Scholar]
- Bergquist, P.R.; Lawson, M.P.; Lavis, A.; Cambie, R.C. Fatty acid composition and the classification of the Porifera. Biochem. Syst. Ecol. 1984, 12, 63–84. [Google Scholar] [CrossRef]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Reyes, E.D.; Sostre, A.; Rodriguez, A.D.; Rodriguez, J.L.; Gonzales, F.A. Identification of the novel antimicrobial fatty acid (5Z,9Z)-14-methyl-5,9-pentadecadienoic acid in Eunicea succinea. J. Nat. Prod. 1997, 60, 502–504. [Google Scholar] [CrossRef]
- Tasdemir, D.; Topaloglu, B.; Perozzo, R.; Brun, R.; O’Neill, R.; Carballeira, N.M.; Zhang, X.J.; Tonge, P.J.; Linden, A.; Ruedi, P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductase from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg. Med. Chem. 2007, 15, 6834–6845. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Santalova, E.A.; Gorshkova, I.A.; Dmitrenok, A.S.; Guzi, A.G.; Gorbach, V.I.; Svetashev, V.I.; Stonok, V.A. A new cytotoxic fatty acid (5Z,9Z)-22-methyl-5,9-tetrecosadienoic acid and the sterols from the far eastern sponge Geodinella robusta. Lipids 2002, 37, 75–80. [Google Scholar] [CrossRef]
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar] [CrossRef]
- Kawashima, H.; Ohnishi, M. Identification of minor fatty acids and various nonmethylene-interrupted diene isomers in mantle, muscle, and viscera of the marine bivalve Megangulus zyonoensis. Lipids 2004, 39, 265–271. [Google Scholar] [CrossRef]
- Kawashima, H. Unusual minor nonmethylene-interrupted di-, tri-, and tetraenoic fatty acids in limpet gonads. Lipids 2005, 40, 627–630. [Google Scholar] [CrossRef]
- Monroig, O.; Navarro, J.C.; Dick, J.R.; Alemany, F.; Tocher, D.R. Identification of a Δ5-like fatty acyl desaturase from the cephalopod Octopus vulgaris (Cuvier 1797) involved in the biosynthesis of essential fatty acids. Mar. Biotechnol. 2012, 14, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Houtsmuller, U.M.T. Columbinic acid, a new type of essential fatty acid. Prog. Lipid Res. 1981, 20, 889–896. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Eliseikina, M.G. Symbiotic bacteria in the nudibranch mollusk Dendrodoris nigra: Fatty acid composition and ultrastructure analysis. Mar. Biol. 2012, 159, 1783–1794. [Google Scholar] [CrossRef]
- Vasskog, T.; Andersen, J.H.; Hansen, E.; Svenson, J. Characterization and cytotoxicity studies of the rare 21:4n-7 acid and other polyunsaturated fatty acids from the marine opisthobranch Scaphander lignaris, isolated using bioassay guided fractionation. Mar. Drugs 2012, 10, 2676–2690. [Google Scholar] [CrossRef]
- Proksch, P.; Edrada, R.A.; Ebel, R. Drugs from the seas—Current status and microbiological implications. Appl. Microbiol. Biot. 2002, 59, 125–134. [Google Scholar] [CrossRef]
- Schmidt, E.W.; Donia, M.S. Life in cellulose houses: Symbiotic bacterial biosynthesis of ascidian drugs and drug leads. Curr. Opin. Biotechnol. 2010, 21, 827–833. [Google Scholar] [CrossRef]
- Lindquist, N.; Barber, P.H.; Weisz, J.B. Episymbiotic microbes as food and defence for marine isopods, unique symbioses in a hostile environment. Proc. Biol. Sci. 2005, 272, 1209–1216. [Google Scholar] [CrossRef]
- Haine, E.R. Symbiont-mediated protection. Proc. Biol. Sci. 2008, 275, 353–361. [Google Scholar] [CrossRef]
- Wagele, H.; Ballesteros, M.; Avila, C. Defensive glandular structures in Opisthobranch mollusks—From histology to ecology. Oceanogr. Mar. Biol. 2006, 44, 197–276. [Google Scholar]
- Schuett, C.; Doepke, H. Endobacterial morphotypes in nudibranch cerata tips: A SEM analysis. Helgol. Mar. Res. 2013, 67, 219–227. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Eliseikina, M.G.; Balakirev, E.S.; Ayala, F.J. A novel symbiotic association of the nudibranch mollusk Rostanga alisae with bacteria. Unpublished work. 2014. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–918. [Google Scholar] [CrossRef]
- Vaskovsky, V.E.; Kostetsky, E.Y.; Vasendin, I.M. A universal reagent for phospholipids analysis. J. Chromatogr. 1975, 114, 129–142. [Google Scholar] [CrossRef]
- Carreau, J.P.; Dubacq, J.P. Adaptation of the macroscale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J. Chromatogr. 1979, 151, 384–390. [Google Scholar] [CrossRef]
- Svetashev, V.I. Mild method for preparation of 4,4-dimethyloxazoline derivatives of polyunsaturated fatty acids for GC-MS. Lipids 2011, 46, 463–467. [Google Scholar] [CrossRef]
- Christie, W.W. Equivalent chain lengths of methyl ester derivatives of fatty acids on gas chromatography—A reappraisal. J. Chromatogr. 1988, 447, 305–314. [Google Scholar] [CrossRef]
- Christie, W.W. 2013, Mass Spectrometry of Fatty Acid Derivatives. Available online: http://lipidlibrary.aocs.org/ms/masspec.html (accessed on 21 January 2014).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhukova, N.V. Lipids and Fatty Acids of Nudibranch Mollusks: Potential Sources of Bioactive Compounds. Mar. Drugs 2014, 12, 4578-4592. https://doi.org/10.3390/md12084578
Zhukova NV. Lipids and Fatty Acids of Nudibranch Mollusks: Potential Sources of Bioactive Compounds. Marine Drugs. 2014; 12(8):4578-4592. https://doi.org/10.3390/md12084578
Chicago/Turabian StyleZhukova, Natalia V. 2014. "Lipids and Fatty Acids of Nudibranch Mollusks: Potential Sources of Bioactive Compounds" Marine Drugs 12, no. 8: 4578-4592. https://doi.org/10.3390/md12084578





