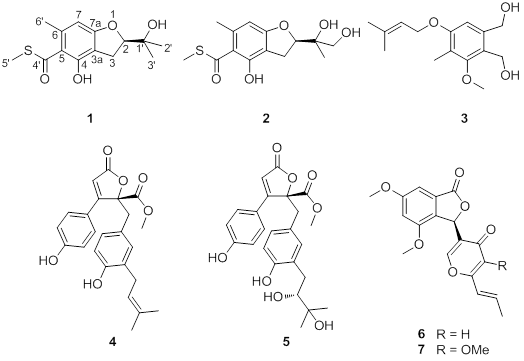
Eurothiocin A and B, Sulfur-Containing Benzofurans from a Soft Coral-Derived Fungus Eurotium rubrum SH-823
Abstract
:1. Introduction

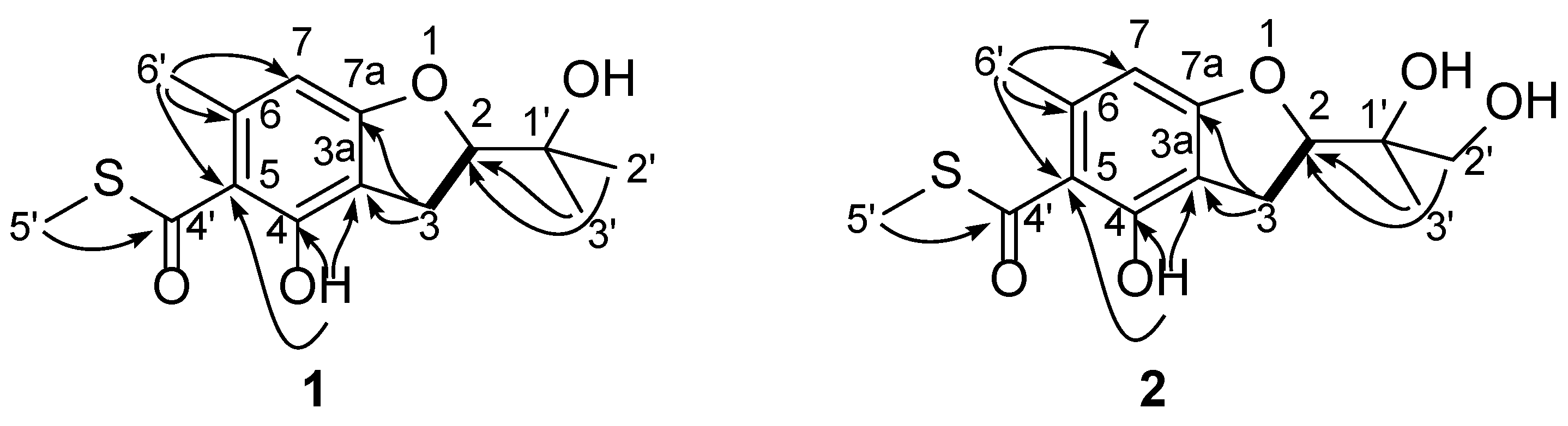
2. Results and Discussion
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH | δC | δH | |
| 2 | 91.3, C | 4.71, dd (9.5, 8.4) | 87.2, C | 4.90, t (8.8) |
| 3 | 27.7, CH2 | 3.05, dd (15.6, 8.4) | 27.2, CH2 | 3.17, d (8.8) |
| 3.14, dd (15.6, 9.5) | ||||
| 3a | 111.5, C | - | 111.3, C | - |
| 4 | 158.2, C | - | 158.2, C | - |
| 5 | 116.1, C | - | 116.2, C | - |
| 6 | 141.8, C | - | 141.9, C | - |
| 7 | 105.7, CH | 6.26, s | 105.7, CH | 6.24, s |
| 7a | 164.3, C | - | 164.1, C | - |
| 1' | 71.9, C | - | 73.7, C | - |
| 2' | 25.8, CH3 | 1.33, s | 66.9, CH2 | 3.53, d (11.0) |
| 3.75, d (11.0) | ||||
| 3' | 23.8, CH3 | 1.22, s | 19.1, CH3 | 1.20, s |
| 4' | 197.7, C | - | 197.8, C | - |
| 5' | 13.0, CH3 | 2.47, s | 13.1, CH3 | 2.46, s |
| 6' | 25.0, CH3 | 2.69, s | 25.1, CH3 | 2.68, s |
| 4-OH | - | 11.83, brs | - | 11.83, brs |
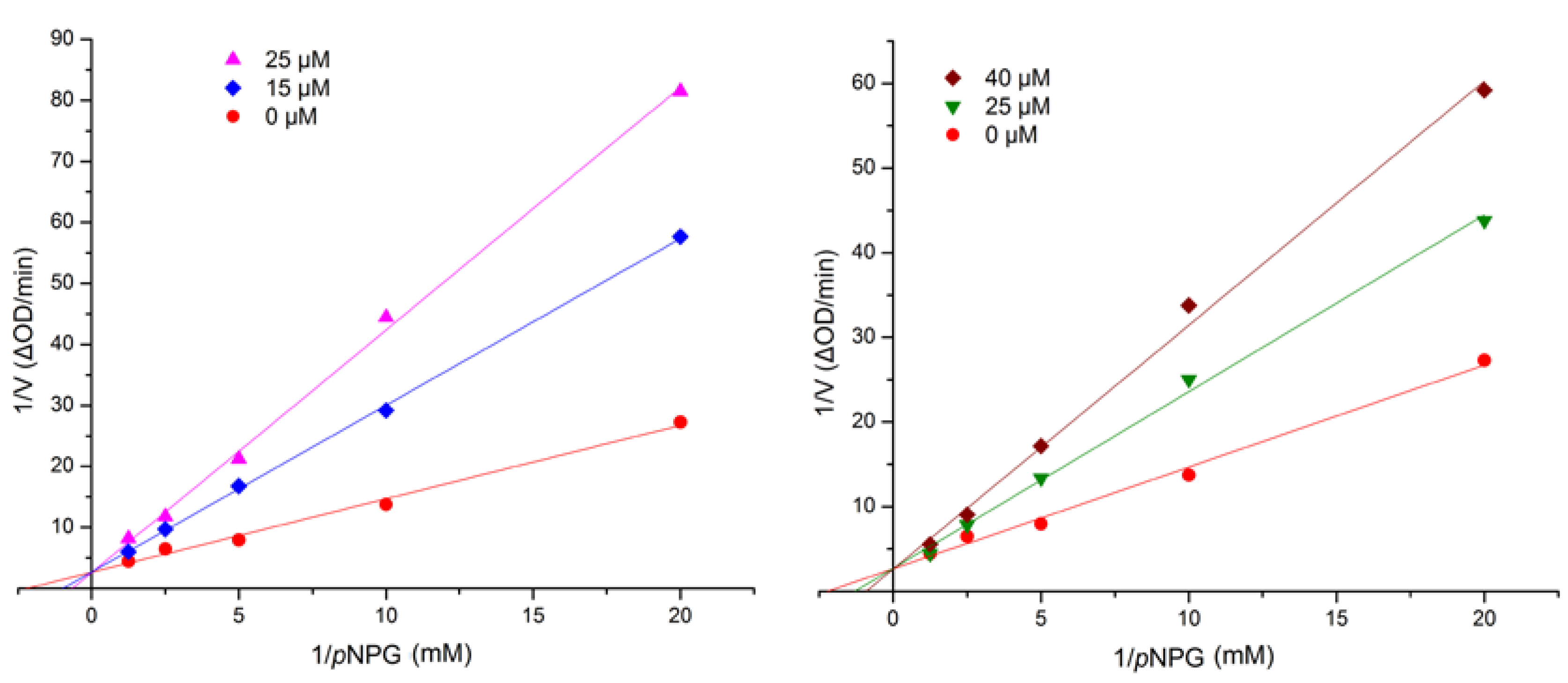
| Compounds | 1 | 2 | 4 | 5 | 6 | 7 | Acarbose b |
|---|---|---|---|---|---|---|---|
| IC50 (μM) | 17.1 ± 0.7 | 42.6 ± 1.4 | 98.5 ± 3.3 | 110.8 ± 1.7 | 107.1 ± 2.6 | 236 ± 4.2 | 376.7 ± 5.2 |
3. Experimental Section
3.1. General
3.2. Fungal Material
3.3. Extraction and Isolation
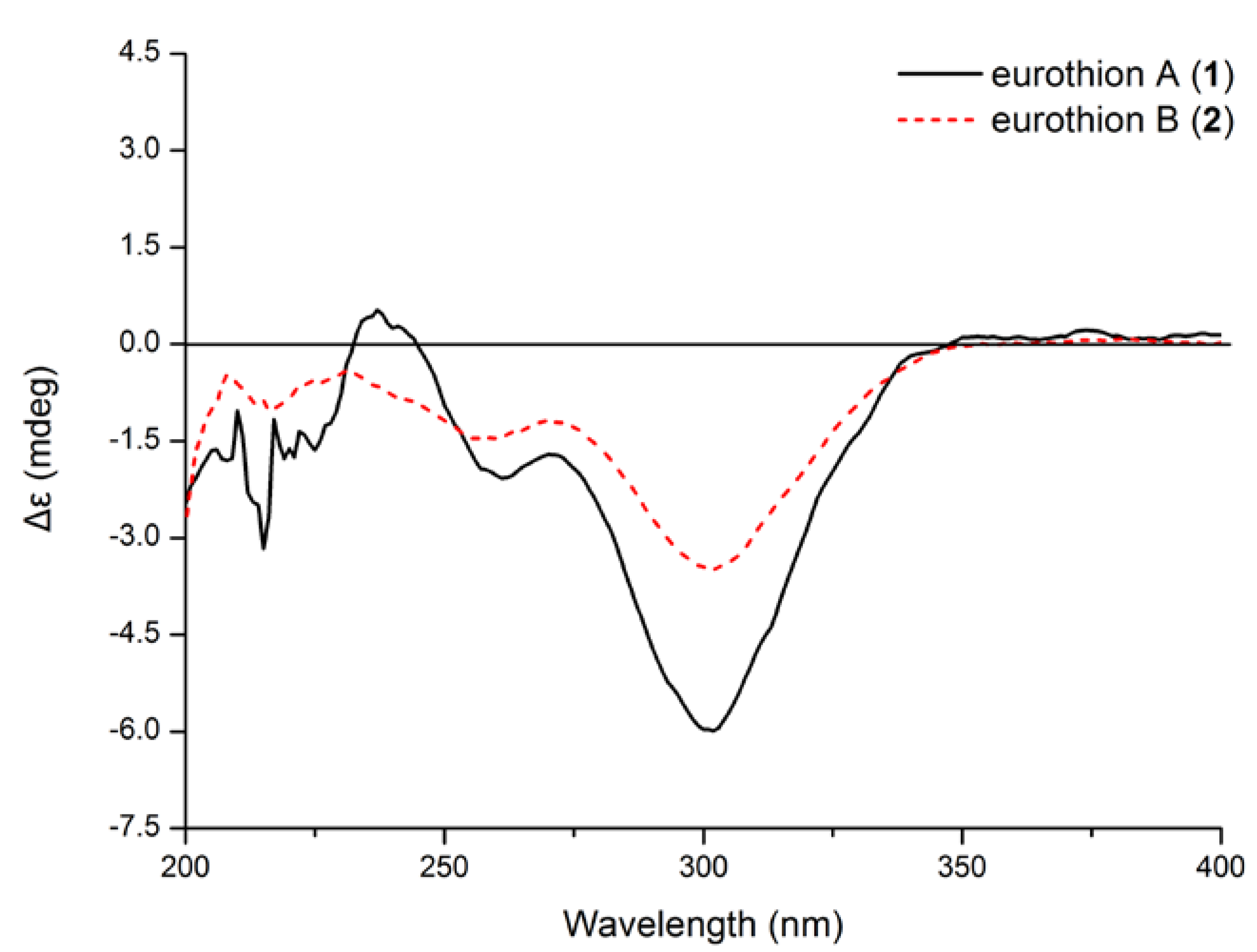
 −135 (c 0.20, MeCN); UV (MeOH) (λmax) (log ε) 239 (sh), 302 (3.97) nm; 1H and 13C NMR spectroscopic data, see Table 1; EIMS m/z 282 [M]+, HREIMS m/z 282.1621 ([M]+, C14H18O4S, calcd 282.1618).
−135 (c 0.20, MeCN); UV (MeOH) (λmax) (log ε) 239 (sh), 302 (3.97) nm; 1H and 13C NMR spectroscopic data, see Table 1; EIMS m/z 282 [M]+, HREIMS m/z 282.1621 ([M]+, C14H18O4S, calcd 282.1618). −69 (c 0.29, MeCN); UV (MeOH) (λmax) (log ε) 243 (sh), 301 (3.95) nm; 1H and 13C NMR spectroscopic data, see Table 1; EIMS m/z 298 [M]+, HREIMS m/z 298.0942 [M]+ (C14H18O5S, calcd 298.0941).
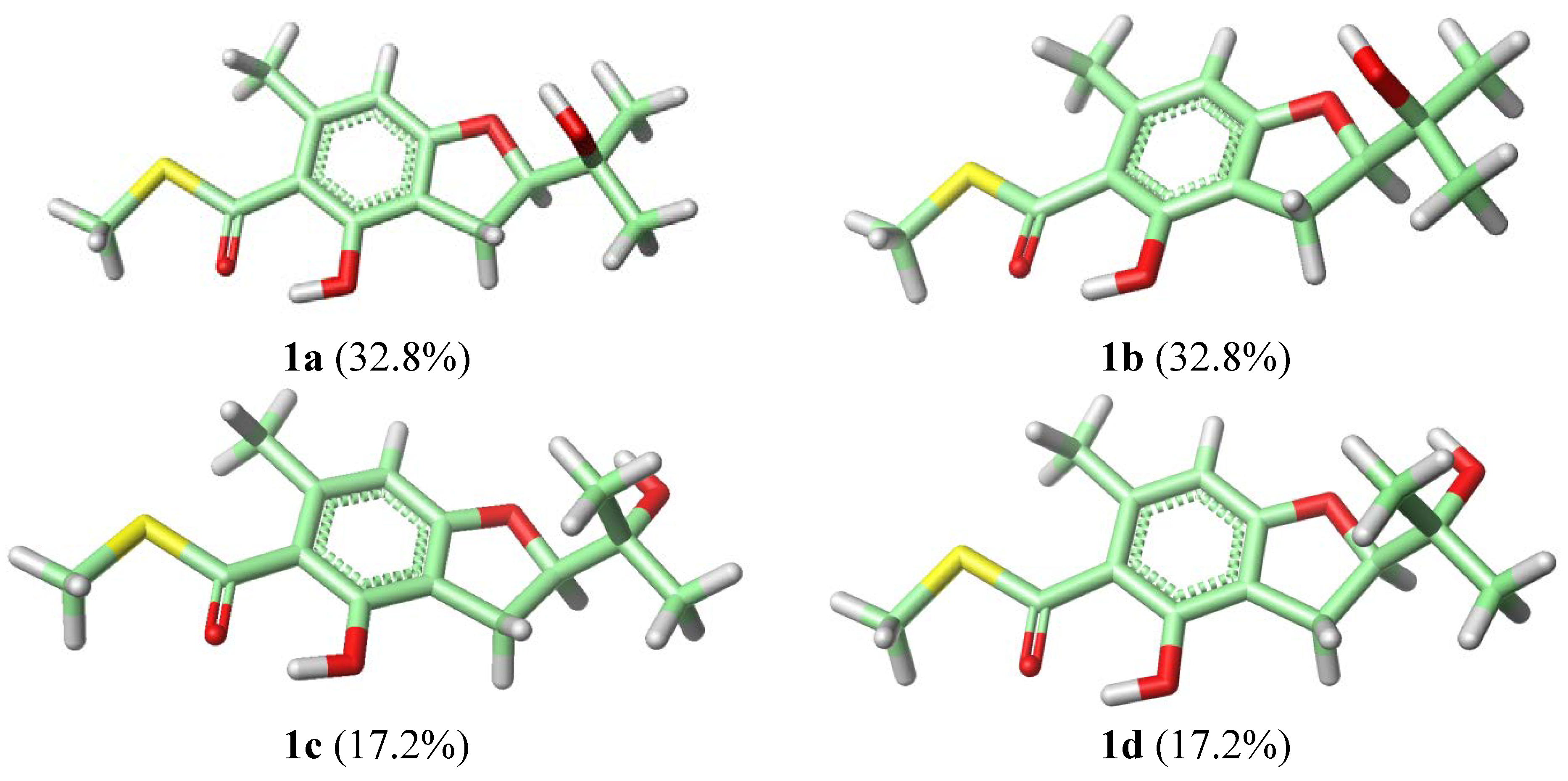
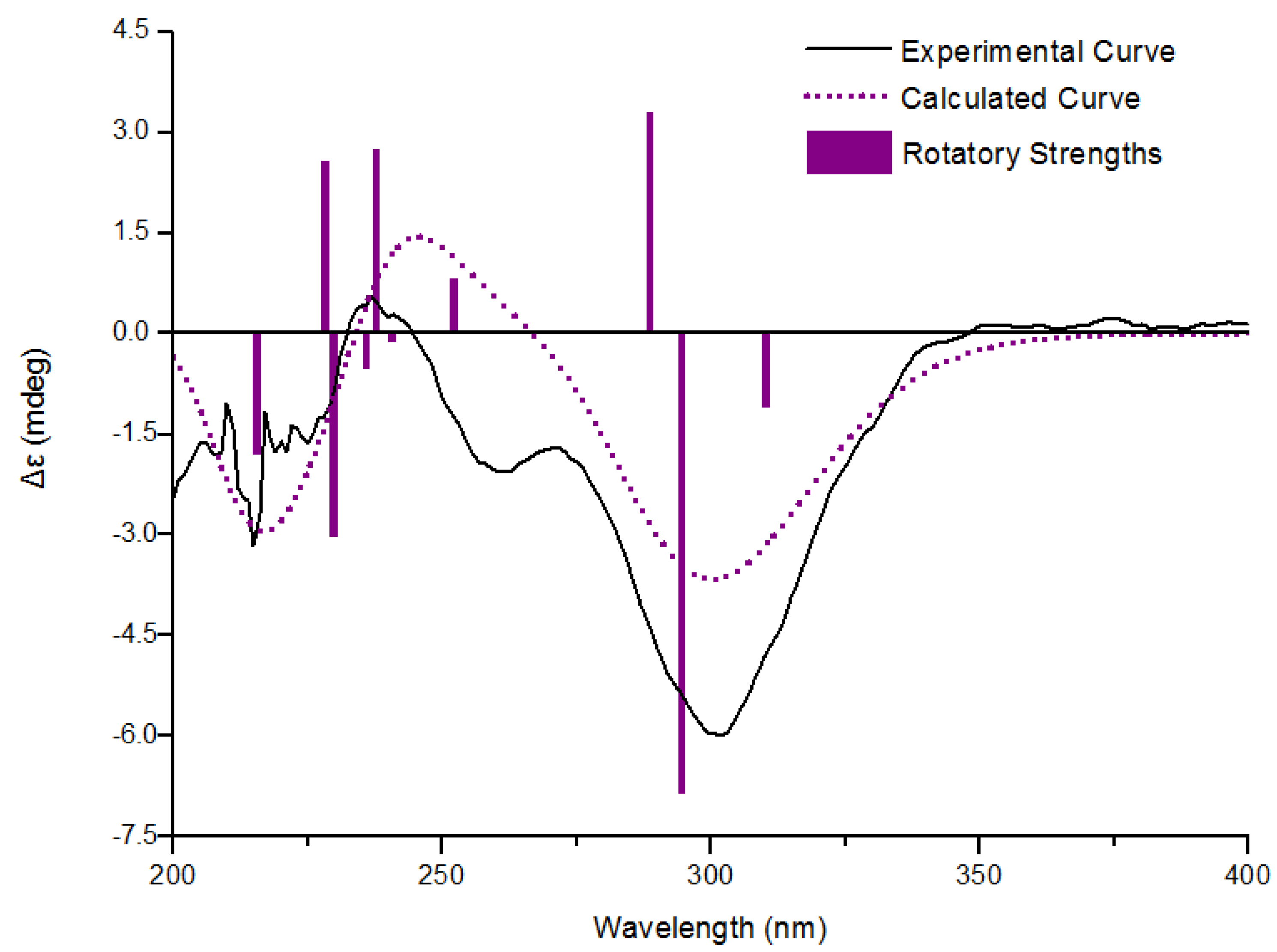
−69 (c 0.29, MeCN); UV (MeOH) (λmax) (log ε) 243 (sh), 301 (3.95) nm; 1H and 13C NMR spectroscopic data, see Table 1; EIMS m/z 298 [M]+, HREIMS m/z 298.0942 [M]+ (C14H18O5S, calcd 298.0941).3.4. Calculation of ECD Spectra
3.5. Assay for α-Glucosidase Inhibitory Activity
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef]
- König, G.M.; Kehraus, S.; Seibert, S.F.; Abdel-Lateff, A.; Müller, D. Natural products from marine organisms and their associated microbes. ChemBioChem 2006, 7, 229–238. [Google Scholar] [CrossRef]
- Watts, K.R.; Loveridge, S.T.; Tenney, K.; Media, J.; Valeriote, F.A.; Crews, P. Utilizing DART mass spectrometry to pinpoint halogenated metabolites from a marine invertebrate-derived fungus. J. Org. Chem. 2011, 76, 6201–6208. [Google Scholar] [CrossRef]
- Menezes, C.B.; Bonugli-Santos, R.C.; Miqueletto, P.B.; Passarini, M.R.; Silva, C.H.; Justo, M.R.; Leal, R.R.; Fantinatti-Garboggini, F.; Oliveira, V.M.; Berlinck, R.G.; et al. Microbial diversity associated with algae, ascidians and sponges from the north coast of São Paulo state, Brazil. Microbiol. Res. 2010, 165, 466–482. [Google Scholar] [CrossRef]
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of marine invertebrate-derived biosynthetic products: their biomedical potential and possible production by microbial associants. Bioorg. Med. Chem. 2011, 19, 6658–6674. [Google Scholar]
- Unson, M.D.; Holland, N.D.; Faulkner, D.J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar. Biol. 1994, 119, 1–11. [Google Scholar] [CrossRef]
- Unson, M.D.; Faulkner, D.J. Cyanobacterial symbiont biosynthesis of chlorinated metabolites from Dysidea herbacea (Porifera). Experientia 1993, 49, 349–353. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, B.G.; Brauers, G.; Guan, H.S.; Proksch, P.; Ebel, R. Microsphaerones A and B, two novel γ-pyrone derivatives from the sponge-derived fungus Microsphaeropsis. sp. J. Nat. Prod. 2002, 65, 772–775. [Google Scholar] [CrossRef]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar]
- Suciati Fraser, J.A.; Lambert, L.K.; Pierens, G.K.; Bernhardt, P.V.; Garson, M.J. Secondary metabolites of the sponge-derived fungus Acremonium persicinum. J. Nat. Prod. 2013, 76, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Amend, A.S.; Barshis, D.J.; Oliver, T.A. Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J. 2012, 6, 1291–1301. [Google Scholar]
- Knowlton, N.; Rohwer, F. Multispecies microbial mutualisms on coral reefs: The host as a habitat. Am. Nat. 2003, 162, S51–S62. [Google Scholar] [CrossRef]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Wegley, L.; Edwards, R.; Rodriguez-Brito, B.; Liu, H.; Rohwer, F. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environ. Microbiol. 2007, 9, 2707–2719. [Google Scholar] [CrossRef]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Parvatkar, R.R.; D’Souza, C.; Tripathi, A.; Naik, C.G. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. [Google Scholar] [CrossRef]
- Li, H.J.; Lan, W.J.; Lam, C.K.; Yang, F.; Zhu, X.F. Hirsutane sesquiterpenoids from the marine-derived fungus Chondrostereum sp. Chem. Biodivers. 2011, 8, 317–324. [Google Scholar] [CrossRef]
- Zheng, C.J.; Shao, C.L.; Wu, L.Y.; Chen, M.; Wang, K.L.; Zhao, D.L.; Sun, X.P.; Chen, G.Y.; Wang, C.Y. Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Mar. Drugs 2013, 11, 2054–2068. [Google Scholar]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Wang, H.; Li, J.; Li, G.; Zhu, W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron 2011, 67, 7085–7089. [Google Scholar] [CrossRef]
- Huang, X.S.; Huang, H.B.; Li, H.X.; Sun, X.F.; Huang, H.R.; Lu, Y.J.; Lin, Y.C.; Long, Y.H.; She, Z.G. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus. sp. 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar]
- Li, H.; Jiang, J.; Liu, Z.; Lin, S.; Xia, G.; Xia, X.; Ding, B.; He, L.; Lu, Y.; She, Z. Peniphenones A–D from the Mangrove Fungus Penicillium dipodomyicola HN4-3A as Inhibitors of Mycobacterium tuberculosis Phosphatase MptpB. J. Nat. Prod. 2014, 77, 800–806. [Google Scholar]
- Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu., Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org. Lett. 2013, 15, 2522–2525. [Google Scholar]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef]
- Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W.L.; Vrijmoed, L.L.P.; Jones, E.B.G.; Lin, Y. Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem. 2009, 74, 1093–1098. [Google Scholar]
- Donatella, M.C.; Ronaldo, P.F. α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism 2006, 55, 832–841. [Google Scholar] [CrossRef]
- Mohan, S.; Eskandari, R.; Pinto, B.M. Naturally occurring sulfonium-ion glucosidase inhibitors and their derivatives: A promising class of potential antidiabetic agents. Acc. Chem. Res. 2014, 47, 211–225. [Google Scholar] [CrossRef]
- Calis, I.; Heilmann, J.; Tasdemir, D.; Linden, A.; Ireland, C.M.; Sticher, O. Flavonoid, iridoid, and lignan glycosides from Putoria calabric. J. Nat. Prod. 2001, 64, 961–964. [Google Scholar] [CrossRef]
- Soman, A.G.; Gloer, J.B.; Wicklow, D.T. Antifungal and antibacterial metabolites from a sclerotium-colonizing isolate of Mortierella vinacea. J. Nat. Prod. 1999, 62, 386–388. [Google Scholar] [CrossRef]
- Yu, Z.; Smanski, M.J.; Peterson, R.M.; Marchillo, K.; Andes, D.; Rajski, S.R.; Shen, B. Engineering of Streptomyces platensis MA7339 for overproduction of platencin and congeners. Org. Lett. 2010, 12, 1744–1747. [Google Scholar] [CrossRef]
- Martin, J.A.; Vogel, E. The synthesis of zinniol. Tetrahedron 1980, 36, 791–794. [Google Scholar] [CrossRef]
- Rao, K.V.; Sadhukhan, A.K.; Veerender, M.; Ravikumar, V.; Mohan, E.V.; Dhanvantri, S.D.; Sitaramkumar, M.; Babu, J.M.; Vyas, K.; Reddy, G.O. Butyrolactones from Aspergillus terreus. Chem. Pharm. Bull. 2000, 48, 559–562. [Google Scholar] [CrossRef]
- Nuclear, P.; Sommit, D.; Boonyuen, N.; Pudhom, K. Butenolide and furandione from an endophytic Aspergillus terreus. Chem. Pharm. Bull. 2010, 58, 1221–1223. [Google Scholar] [CrossRef]
- Fuska, J.; Uhrín, D.; Proksa, B.; Votický, Z.; Ruppeldt, J. The structure of vermistatin, a new metabolite from Penicillium vermiculatum. J. Antibiot. 1986, 39, 1605–1608. [Google Scholar] [CrossRef]
- Xia, X.; Huang, H.; She, Z.; Cai, J.; Liu, F.; Zhang, J.; Fu, L.; Vrijimoed, L.L.P.; Lin, Y.C. Structural and biological properties of vermistatin and two new vermistatin derivatives isolated from the marine-Mangrove endophytic fungus Guignardia sp. No. 4382. Helv. Chim. Acta 2007, 90, 1925–1931. [Google Scholar]
- Brindis, F.; Rodríguez, R.; Bye, R.; González-Andrade, M.; Mata, R. (Z)-3-butylidenephthalide from Ligusticum porteri, an α-glucosidase inhibitor. J. Nat. Prod. 2011, 74, 314–320. [Google Scholar] [CrossRef]
- Omar, R.; Li, L.; Yuan, T.; Seeram, N.P. α-Glucosidase inhibitory hydrolyzable tannins from Eugenia jambolana seeds. J. Nat. Prod. 2012, 75, 1505–1509. [Google Scholar] [CrossRef]
- Suzuki, H.; Tahara, M.; Takahashi, M.; Matsumura, F.; Okabe, T.; Shimazu, A.; Hirata, A.; Yamaki, H.; Yamaguchi, H.; Tanaka, N.; et al. Resorthiomycin, a novel antitumor antibiotic. I. Taxonomy, isolation and biological activity. J. Antibiot. 1990, 43, 129–134. [Google Scholar]
- Tahara, M.; Okabe, T.; Furihata, K.; Tanaka, N.; Yamaguchi, H.; Nishimura, T.; Suzuki, H. Revised structure of resorthiomycin. J. Antibiot. 1991, 44, 255. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, Sulfur-Containing Benzofurans from a Soft Coral-Derived Fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669-3680. https://doi.org/10.3390/md12063669
Liu Z, Xia G, Chen S, Liu Y, Li H, She Z. Eurothiocin A and B, Sulfur-Containing Benzofurans from a Soft Coral-Derived Fungus Eurotium rubrum SH-823. Marine Drugs. 2014; 12(6):3669-3680. https://doi.org/10.3390/md12063669
Chicago/Turabian StyleLiu, Zhaoming, Guoping Xia, Senhua Chen, Yayue Liu, Hanxiang Li, and Zhigang She. 2014. "Eurothiocin A and B, Sulfur-Containing Benzofurans from a Soft Coral-Derived Fungus Eurotium rubrum SH-823" Marine Drugs 12, no. 6: 3669-3680. https://doi.org/10.3390/md12063669