1. Introduction
Angiogenesis is the formation of blood vessels from pre-existing vasculature. The process of angiogenesis is very complex and tightly controlled under physiological conditions. Excessive or uncontrolled angiogenesis is associated with various pathological conditions including cancer, diabetic retinopathy, and rheumatoid arthritis [
1]. Thus, development of novel anti-angiogenesis could greatly benefit human health. For example, cancer is one of the most devastating diseases, and can be a great burden on modern society, while anti-angiogenic therapy is becoming a powerful treatment approach in cancer therapy.
Traditionally, great efforts have been placed on developing drugs that attack and kill cancer cells. During past years, chemotherapy based on cytotoxic agents has been the main treatment for most cancer patients. Although cytotoxic agents have significantly improved cancer survival, these conventional anti-cancer drugs often cause serious side effects, chemo- and radio-resistance, disease relapse and metastases. Therefore, the alternative chemotherapeutic regimens with minimal side effects are currently the priority consideration for the development of cancer treatment. Angiogenesis plays a critical role in the progression of cancer. Through stimulating blood vessel growth from nearby pre-existing capillaries, tumors achieve sufficient blood supply for cancer cell progression and metastasis [
2]. It has been shown that increased angiogenesis is associated with poor prognosis and relapse of cancer whereas blocking the formation of new blood vessels prevents the growth of cancer. Thus, the development of inhibitors of angiogenesis is increasingly receiving attention in the field of cancer. In the U.S., there are currently thirteen approved anti-angiogenesis therapies in oncology. However, as current anti-angiogenesis therapy is still in the infant stage of the drug development, there are lots of limitations of anti-angiogenesis therapy such as low efficacy, and development of resistance during the long term use of these therapeutic agents. Therefore, intensive efforts are needed to develop novel anti-angiogenic agents, especially small molecule targeting the tumor vasculature [
3].
Vascular endothelial growth factor (VEGF) is a very important stimulator for tumor angiogenesis. VEGF is regarded as the prognostic indicator in cancers because of its significant association with tumor size and progress in several cancers. Thus, VEGF signaling pathway is an attractive target for the development of anti-angiogenesis inhibitors.
The marine environment is a rich source of novel and unusual secondary metabolites for drug discovery. Catunaregin was first isolated from the stem bark of
catunaregam spinosa, a Chinese mangrove associate and the extraction and isolation of catunaregin was described in the literature [
4]. Our previous study demonstrated that catunaregin may have anti-cancer property. In this study, we investigated the potential inhibitory action of catunaregin on angiogenesis in human umbilical vein endothelial cells (HUVECs) and transgenic zebrafish (Danio rerio; fli1:EGFP). We further explored the mechanism by which catunaregin inhibited angiogenesis in HUVECs. We found that catunaregin exerted anti-angiogenic activity in both HUVECs and zebrafish. In addition, catunaregin significantly reduced the phosphorylation of Akt and eNOS and the inhibition was in parallel to its anti-angiogenic effect, suggesting that catunaregin inhibited angiogenesis possibly through the modulation of the Akt and eNOS signaling pathways. Since angiogenesis plays an important pathological role in the progress of a wide range of diseases, our findings provide a rationale for future development of this compound as a potential drug or chemopreventive supplement to target diseases with excessive angiogenesis.
3. Experimental Section
3.1. Preparation of Catunaregin
Reagents and solvents were commercial quality and used without further purification, except for re-purified methanol used in HPLC system. NMR spectra were recorded on Bruker DRX-500 spectrometer (Bruker, Bremen, Germany) with SiMe4 (Cambridge Isctope Laboratories, Tewksbury, MA, USA) as internal standard. HRESI-MS was recorded on VG Auto Spec-3000 MS spectrometer (Bruker, Bremen, Germany). Thin layer chromatography (TLC) was carried out on precoated silica gel G plates (Qingdao Haiyang Chemical Plant, Qingdao, China) and spots were visualized by spraying the plates with 50% H2SO4 solution, followed by heating. Semi-preparative RP-HPLC was carried out on ODS columns (YMC-Pack ODS-5-A, 250 × 10 mm, 5 μm, YMC, Kyoto, Japan) with the CH3OH–H2O solvent system as eluents. Waters 600 HPLC system (Waters, Voorhees, NJ, USA) was equipped with a Waters 996 photodiode array detector (Waters, Voorhees, NJ, USA) to check the fraction and purity of target compound.
3.2. The Isolation of Catunaregin
Catunaregin was isolated and purified by CAS Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China. The purity of the compound was >98%. Catunaregin was dissolved in dimethyl sufoxide (DMSO) and stored at −20 °C until use. The solution form of catunaregin was then diluted by PBS to the concentration needed. All reagents were purchased from Sigma (Sigma, Shanghai, China) unless otherwise stated.
3.3. Preparation of Reagents and Cell Culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Sciencell, Carlsbad, NM, USA. HUVECs (Passages 4 to 7) were cultured at 37 °C in M199 media (Invitrogen, Carlsbad, NM, USA) supplemented with 10% FBS. Cells were maintained in a humidified atmosphere of 5% CO2. The anti-angiogenic effect of catunaregin on HUVECs was evaluated using a stock solution of catunaregin (1 mg/mL) prepared in PBS containing 10% DMSO (GIBCO, Langley, VA, USA). The anti-angiogenic effect on zebrafish was evaluated using a stock solution of catunaregin (1 mg/mL) prepared in sterilized Milli-Q water containing 0.5% DMSO. Vascular endothelial growth factor (VEGF) was obtained from Sigma, St. Louis, MO, USA and prepared as a stock solution of 100 μg/mL in sterilized Milli-Q water.
3.5. Western Immunoblot Analysis
HUVECs were plated in 60-mm dishes (16,700 cells per centimeter square) and cultured for 2 days. The culture medium was then replaced with fresh medium containing 2% FBS, and the cells were treated with indicated concentration of the compound of catunaregin for 24 h. Whole-cell protein extracts (20 μg) were separated on 10%–12.5% sodium dodecylsulfate polyacrylamide gels, and transferred to nitrocellulose membranes (Amersham, Pittsburgh, PA, USA). The blotted membranes were incubated with antibodies against Akt, phosphorylated Akt (Ser473), endothelial nitric oxide synthase (eNOS), phosphorylated eNOS (Ser1177) and β-actin. Densitometrical results were calculated by NIH image software. The primary antibodies used were antiphospho-ERK1/2 (Cell Signaling Technology, Danvers, MA, USA), antibody to total ERK1/2 (Cell Signaling), anti-eNOS (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphorylation of eNOS at S1177 (P-eNOS S1177) (Cell Signaling), anti-phospho-Akt (Cell Signaling), anti-Akt (Cell Signaling), and anti-β-actin (Biosynthesis Biotechnology, Beijing, China).
3.6. Zebrafish Embryo Assay
The transgenic zebrafish cell line TG (fli1:EGFP), in which endothelial cells expressed eGFP, was kindly provided by ZFIN (Eugene, OR, USA) and maintained as described [
11,
12]. Zebrafish embryos were generated by natural pair-wise mating of fish that were between 3 and 12 months old. Embryos were collected as described. Healthy embryos were harvested at the 1–4 cell stage, plated in a 96-well microplate and incubated with 100 μL of the indicated concentrations of the tested compounds at 28 °C for 24 h. DMSO was used as both carrier of drugs and control. After incubation, fish embryos were anesthetized with tricaine (0.02%), placed on slides and examined under an OLYMPUS IX71 fluorescence inverted microscope (Olympus, Tokyo, Japan). Phenotypic changes were evaluated by two different observers.
3.7. Zebrafish Caudal Fin Regeneration Assay
Adult TG (Fli-EGFP) transgenic fishes were anesthetized with tricaine (0.02%), and their caudal fin was partially amputated. Then, fishes were maintained for 3 days at 28 °C in 100 μL water containing the indicated concentration of the compound. After three days, fishes were anesthetized and examined and photographed under an OLYMPUS IX71 fluorescence inverted microscope.
3.8. Statistical Analysis
All data were presented as mean ± SEM. Differences among test groups were analyzed by ANOVA, using Newman-Keuls multiple comparison test (Prism 4.0, GraphPad Software, Inc., San Diego, CA, USA). A p value <0.05 was considered statistically significant.
4. Conclusions
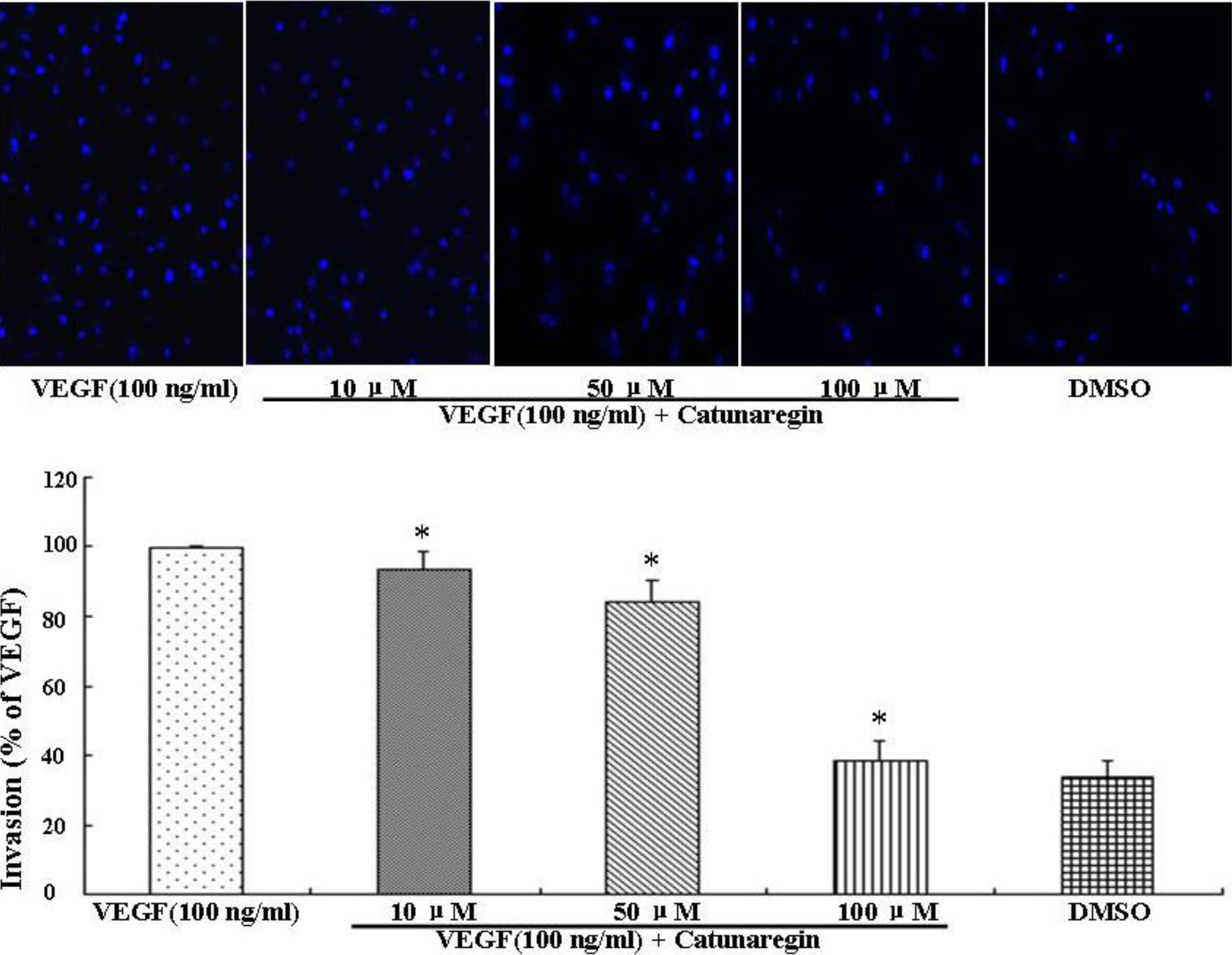
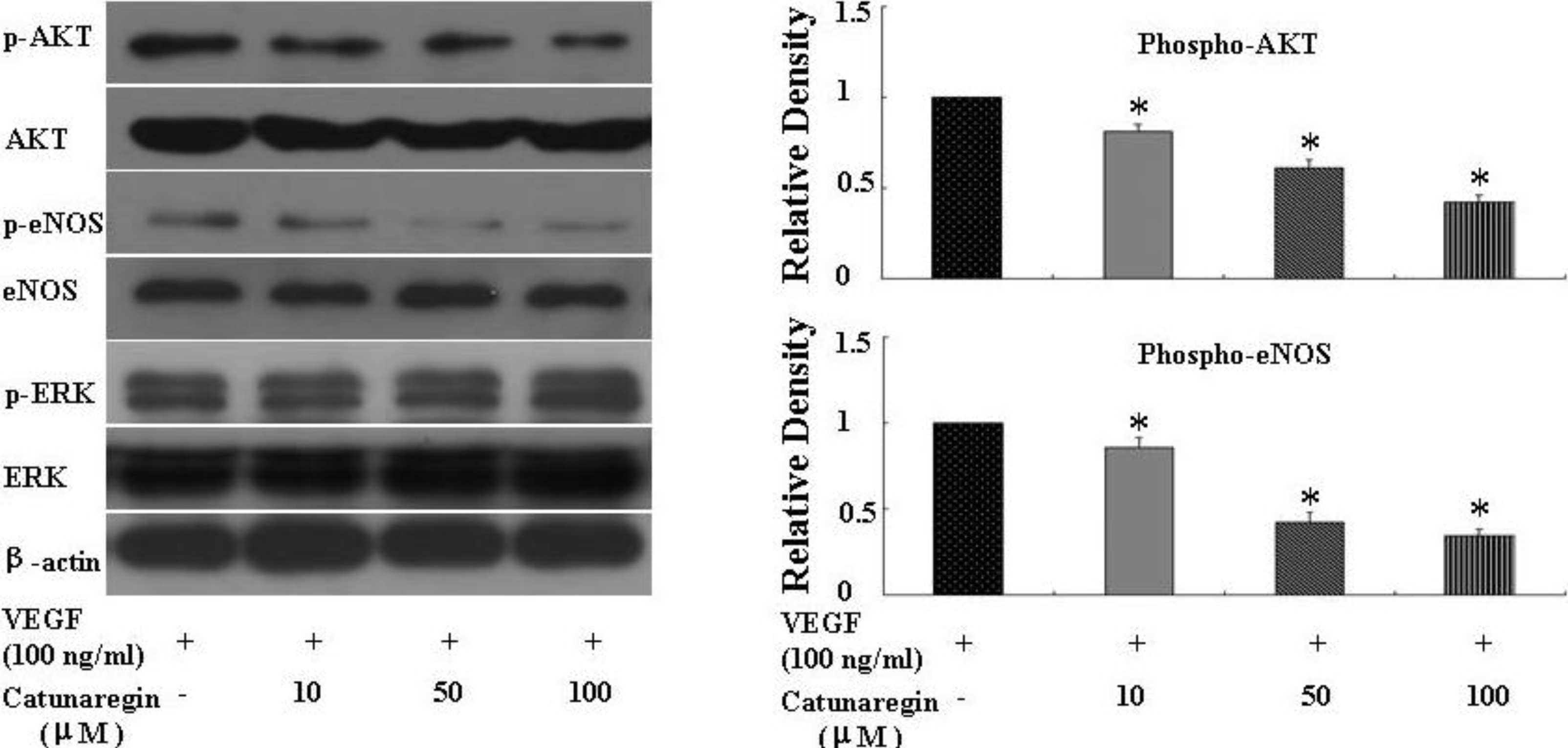
This is the first study concerning the anti-angiogenic action of catunaregin. Our experimental results demonstrated that the catunaregin could significantly inhibit VEGF-induced different angiogenic phenotypes of HUVECs in vitro and the vessel formation in transgenic zebrafish. To further investigate the underlying mechanisms behind the anti-angiogenic property of catunaregin, we examined angiogenesis-related signaling pathways in HUVECs. Our results demonstrated that catunaregin inhibited the phosphorylation of Akt and eNOS in HUVECs, indicating a new mode of biological activity for catunaregin. Interestingly, catunaregin inhibited regenerative angiogenesis without severely affecting tissue regrowth. Thus, catunaregin may primarily inhibit the formation of new blood vessels through blockade of angiogenic process of endothelial cells. Given the critical role of Akt and eNOS signaling pathways in tumorigenesis and tumor metastasis, our results provide an impetus for further investigation and development of catunaregin as a novel anti-angiogenesis candidate for the treatment of diseases with increased angiogenesis.
Catunaregin is a novel norneolignan with a unique O-bridged furopyran ring. It can produce hemiacetal compound when 7-position is cleaved via hydrolysis. Moreover, hemiacetal compound can be open loop at 1-position and 5-position through acetal hydrolysis, thereby affording diketone intermediate. Thus, unsubstituted hydroxyl group or easily activating hydroxyl group like hemiacetal at 6-position and 8-position of compound may be important for its anti-angiogenic action.