The Influence of 1-Butanol and Trisodium Citrate Ion on Morphology and Chemical Properties of Chitosan-Based Microcapsules during Rigidification by Alkali Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Step by Step Microencapsulation Process and Alkali Treatment of Microcapsules
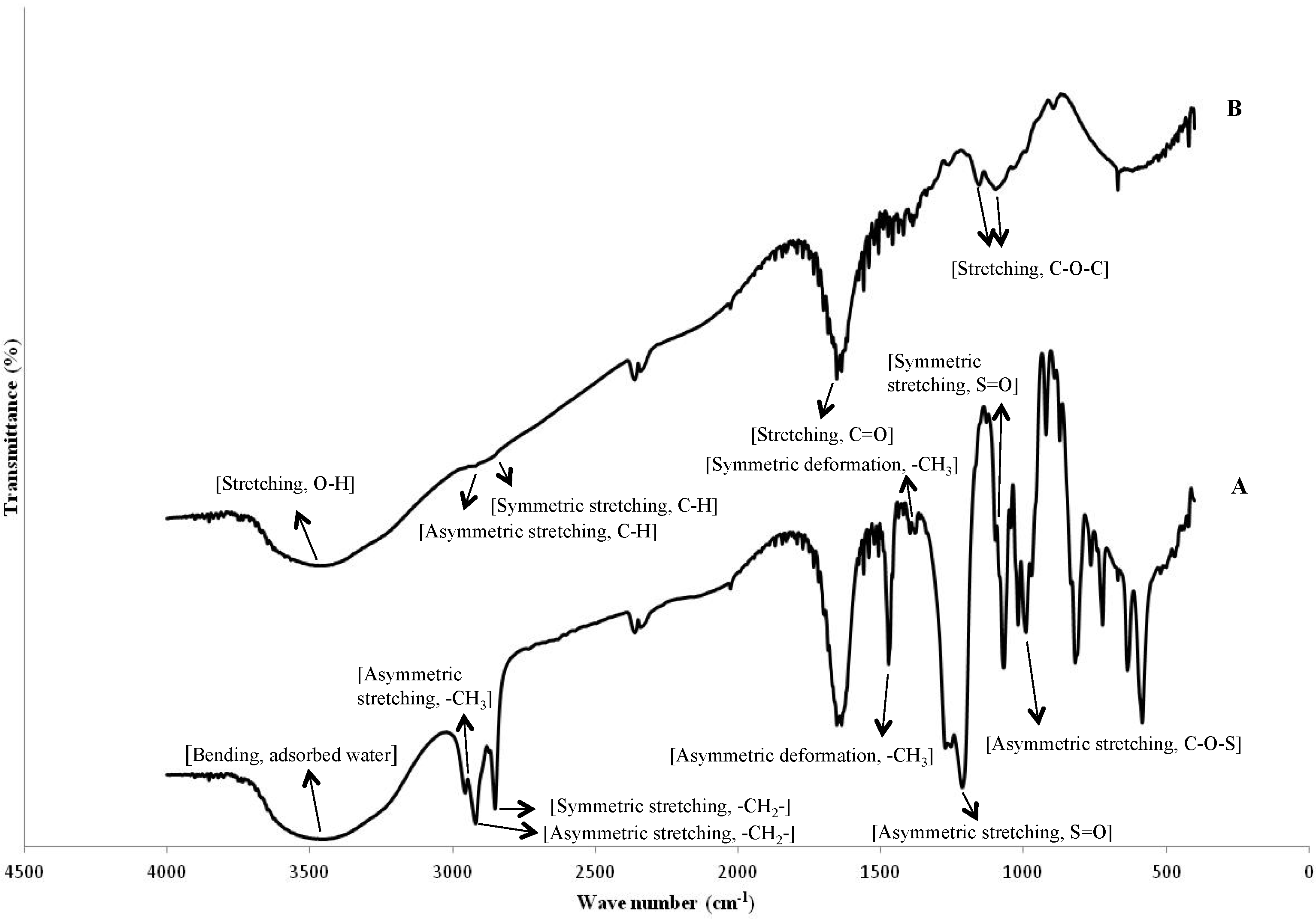
2.2. Influence of Butanol Stabilization on Alkali Treatment of Microcapsules
| Microcapsule Samples | Alkali Treated Samples a | Alkali Treated Samples b | ||||
|---|---|---|---|---|---|---|
| Mean Diameter (μm) | Zeta Potential (mV) | pH | Mean Diameter (μm) | Zeta Potential (mV) | pH | |
| 1 layer | 2.3 ± 0.8 | −12.3 | 5.4 | 3.3 ± 1.1 | −1.8 | 7.0 |
| 4 layers | 4.5 ± 2.5 | +7.7 | 5.4 | 5.4 ± 4.2 | −1.7 | 7.0 |
| 10 layers | 61.5 ± 5.2 | +19.5 | 5.4 | 69.7 ± 18.8 | −2.3 | 7.0 |

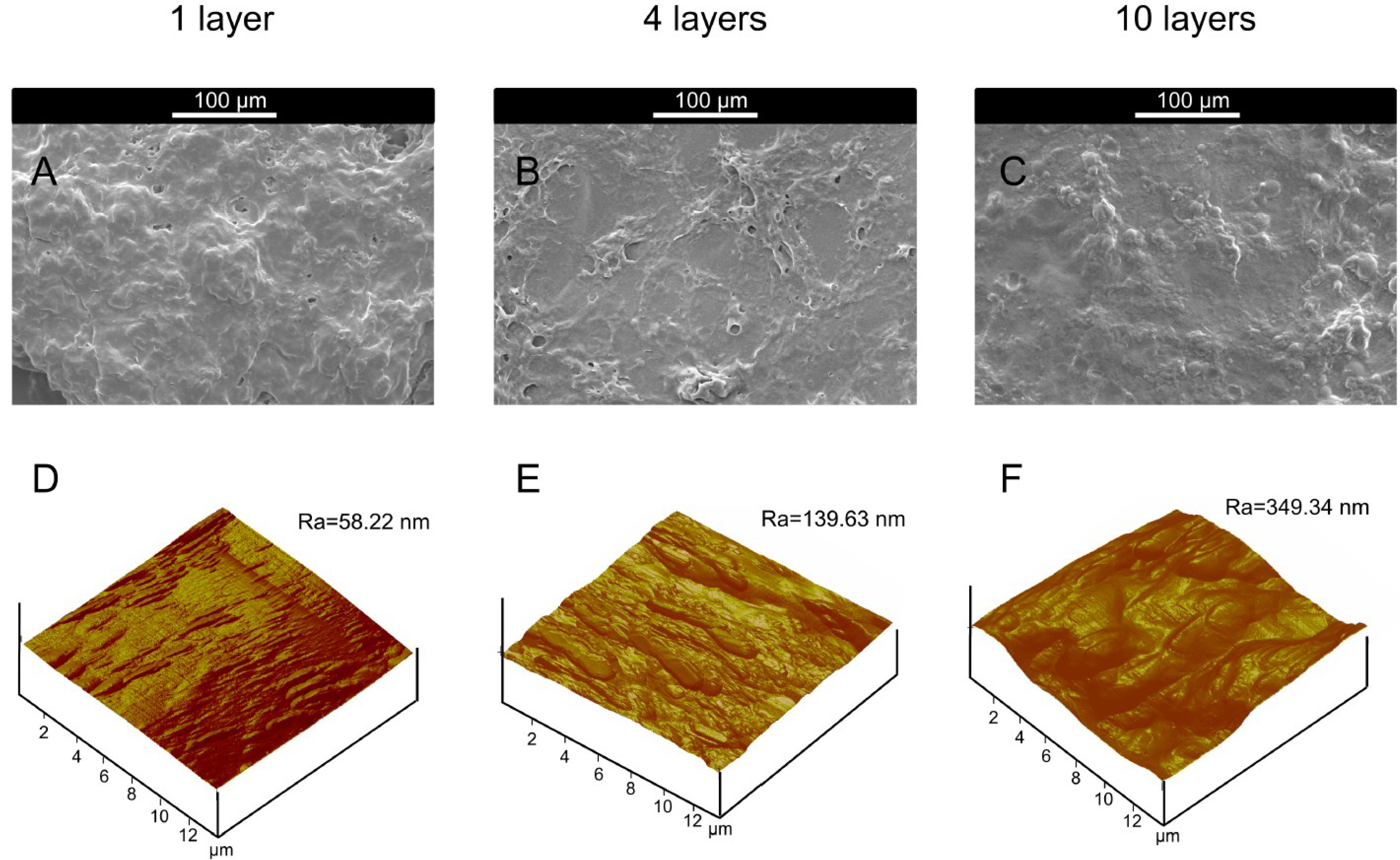
| Alkali Treated Microcapsules Samples (Butanol Stabilization) | θwater a (°) | θdiiodomethane a (°) | γp (mN/m) | γd (mN/m) | γ (mN/m) | Ra b (nm) |
|---|---|---|---|---|---|---|
| 1 layers | 55.8 | 46.6 | 21.0 | 26.6 | 47, 6 | 58.22 |
| 4 layers | 23.6 | 60.5 | 52.2 | 15.0 | 67,2 | 139.63 |
| 10 layers | 29.4 | 25.5 | 33.9 | 32.2 | 66,1 | 349.34 |
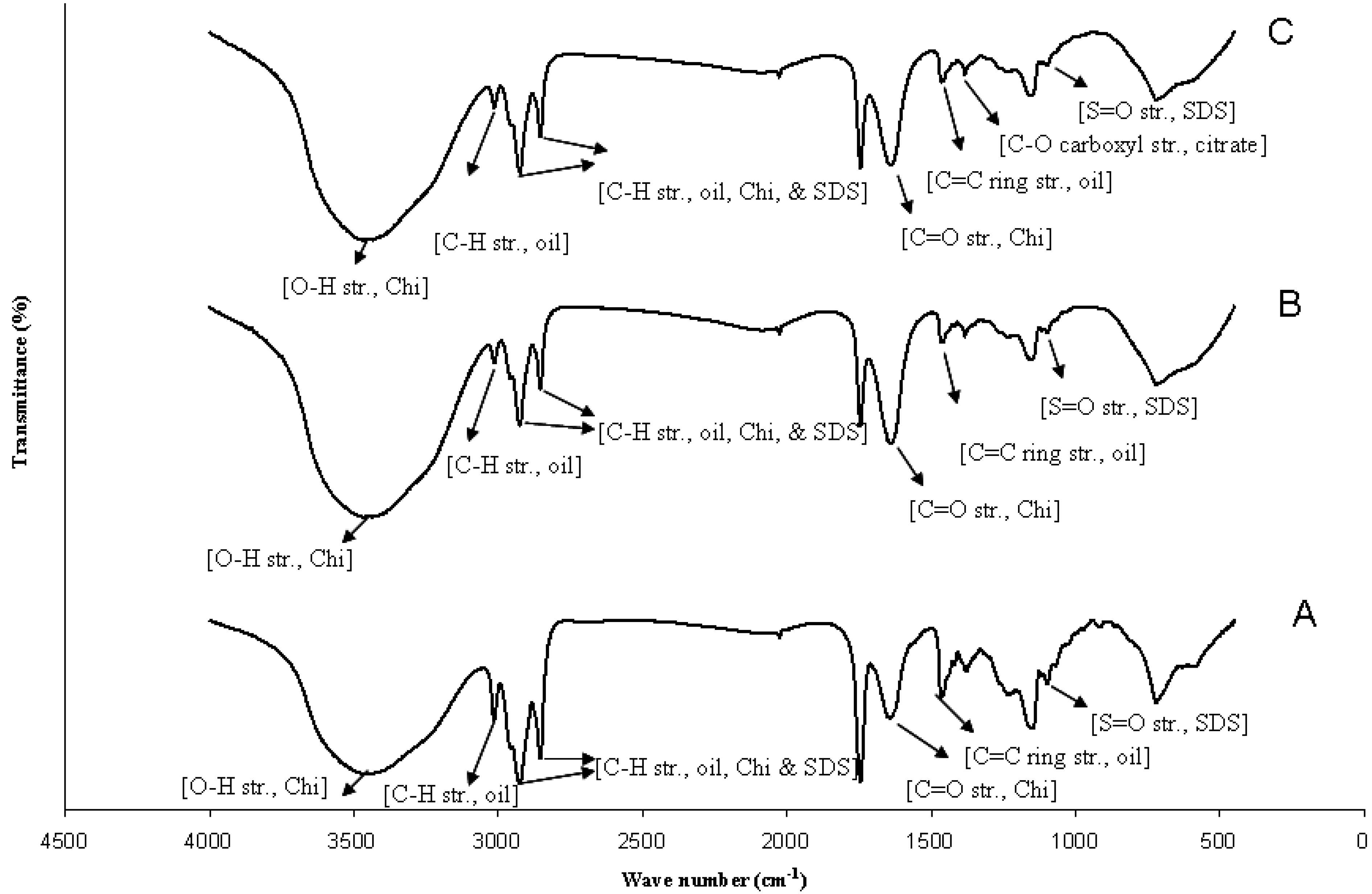
2.3. Influence of Citrate Treatment on Butanol Stabilized Alkali-Treated Microcapsules
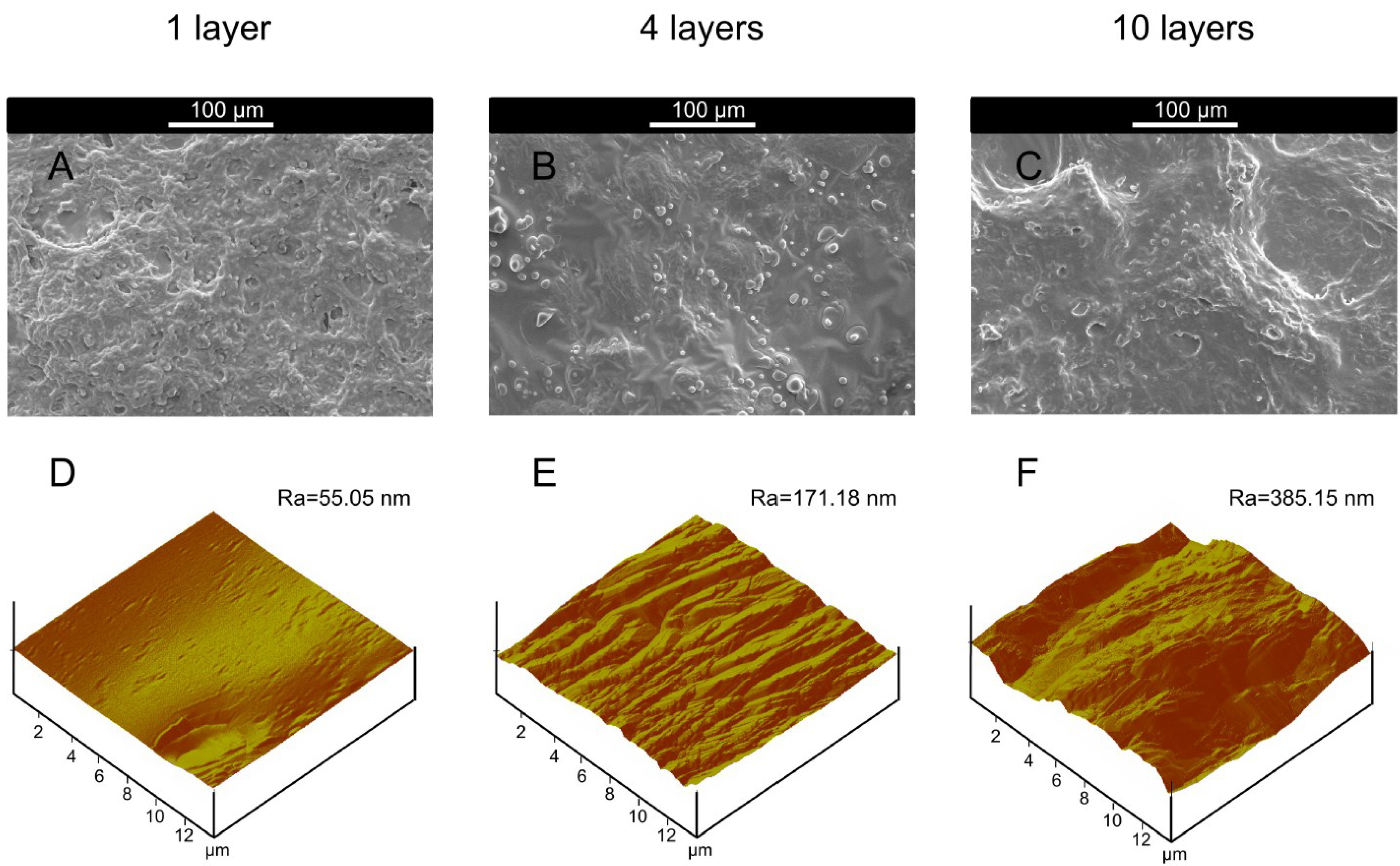
| Alkali Treated Microcapsules Samples (Butanol Stabilization + Citrate Treatment) | θwater a (°) | θdiiodomethane a (°) | γp (mN/m) | γd (mN/m) | γ (mN/m) | Ra b (nm) |
|---|---|---|---|---|---|---|
| 1 layers | 55.4 | 43.1 | 20.3 | 28.4 | 48.7 | 55.05 |
| 4 layers | 38.9 | 51.3 | 36.4 | 21.3 | 57.7 | 171.78 |
| 10 layers | 37.1 | 33.3 | 31.2 | 30.0 | 61.2 | 385.15 |
3. Experimental Section
3.1. Materials
3.2. Preparation of Microcapsules
3.3. Modification of Alkali Treatment Step of Microcapsule Suspension
3.4. Characterization
3.4.1. Zeta Potential Measurement
3.4.2. Size Distribution Analysis by Granulometry
3.4.3. Chemical Characterization
3.4.4. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
3.4.5. Wetting Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.C. Challenges in polymer science: Controlling vesicle-substrate interactions. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3357–3360. [Google Scholar] [CrossRef]
- Fournier, E.; Passirani, C.; Montero-Menei, C.N.; Benoit, J.P. Biocompatibility of implantable synthetic polymeric drug carriers: Focus on brain biocompatibility. Biomaterials 2003, 24, 3311–3331. [Google Scholar] [CrossRef] [PubMed]
- Lensen, D.; Vriezema, D.M.; Hest, J.C.M.V. Polymeric microcapsules for synthetic applications. Macromol. Biosci. 2008, 8, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Badulescu, R.; Vivod, V.; Jausovec, D.; Voncina, B. Grafting of ethylcellulose microcapsules onto cotton fibers. Carbohydr. Polym. 2008, 71, 85–91. [Google Scholar] [CrossRef]
- Salaün, F.; Creach, G.; Rault, F.; Giraud, S. Microencapsulation of bisphenol-A bis (diphenyl phosphate) and influence of particle loading on thermal and fire properties of polypropylene and polyethylene terephtalate. Polym. Degrad. Stab. 2013, 98, 2663–2671. [Google Scholar] [CrossRef]
- Wang, C.; Ye, S.; Dai, L.; Liu, X.; Tong, Z. Enzymatic desorption of layer-by-layer assembled multilayer films and effects on the release of encapsulated indomethacin microcrystals. Carbohydr. Res. 2007, 342, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nagarwal, R.C.; Dhanawat, M.; Pandit, J.K. In-vitro and in vivo study of indomethacin loaded gelatin nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 1–9. [Google Scholar]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahi, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 88, 1962–1987. [Google Scholar] [CrossRef]
- Sacco, L.D.; Masotti, A. Chitin and chitosan as multipurpose natural polymers for groundwater arsenic removal and As2O3 delivery in tumor therapy. Mar. Drugs 2010, 8, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan based micro- and nanoparticles in drug delivery. J. Controlled Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Shi, X.Y.; Tan, T.W. Preparation of chitosan/ethylcellulose complex microcapsule and its application in controlled release of Vitamin D2. Biomaterials 2002, 23, 4469–4473. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.K. Chitosan composites for bone tissue engineering—An overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.; Gimeno, M.; Sepúlveda-Sánchez, J.D.; Shirai, K. Chitosan-based microcapsules containing grapefruit seed extract grafted onto cellulose fibers by a non-toxic procedure. Carbohydr. Res. 2010, 345, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Salaün, F.; Campagne, C. Development of multilayer microcapsules by a phase coacervation method based on ionic interactions for textile applications. Pharmaceutics 2014, 6, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.W.M.; Yip, J.; Liu, L.; Cheuk, K.; Kan, C.W.; Cheung, H.C.; Cheng, S.Y. Chitosan microcapsules loaded with either miconazole nitrate or clotrimazole, prepared via emulsion technique. Carbohydr. Polym. 2012, 89, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Jin, L.J.; Mcallister, T.; Stanford, K.; Xu, J.Y.; Lu, Y.N.; Zhen, Y.H.; Sun, Y.X.; Xu, Y.P. Chitosan−Alginate Microcapsules for Oral Delivery of Egg Yolk Immunoglobulin (IgY). J. Agric. Food Chem. 2007, 55, 2911–2917. [Google Scholar] [PubMed]
- Wang, X.; Zhu, K.X.; Zhou, H.M. Immobilization of glucose oxidase in alginate-chitosan microcapsules. Int. J. Mol. Sci. 2011, 12, 3042–3054. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Salaün, F.; Campagne, C.; Vaupre, S.; Beirão, A. Preparation of microcapsules with multi-layers structure stabilized by chitosan and sodium dodecyl sulfate. Carbohydr. Polym. 2012, 90, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Ashby, R.D.; Foglia, T.A.; Solaiman, D.K.Y.; Liu, C.K.; Nuñez, A.; Eggink, G. Viscoelastic properties of linseed oil-based medium chain length poly(hydroxyalkanoate) films: Effects of epoxidation and curing. Int. J. Biol. Macromol. 2000, 27, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Decker, E.A.; McClements, D.J. Effect of molecular weight and degree of deacetylation of chitosan on the formation of oil-in-water emulsions stabilized by surfactant–chitosan membranes. J. Colloid Interface Sci. 2006, 296, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Decker, E.A.; McClements, D. Influence of environmental stresses on stability of O/W emulsions containing droplets stabilized by multilayered membranes produced by a layer-by-layer electrostatic deposition technique. J. Food Hydrocoll. 2005, 19, 209–220. [Google Scholar] [CrossRef]
- Liu, Y.C.; Ny, A.L.M.L.; Schmidt, J.; Talmon, Y.; Chmelka, B.F.; Lee, C.T., Jr. Photo-assisted gene delivery using light-responsive catanionic vesicles. Langmuir 2009, 25, 5713–5724. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.S.; Kwon, Y.J. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv. Drug Deliv. Rev. 2012, 64, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Bamgbose, J.T.; Bamigbade, A.A.; Adewuyi, S.; Dare, E.O.; Lasisi, A.A.; Njah, A.N. Equilibrium swelling and kinetic studies of highly swollen chitosan film. J. Chem. Chem. Eng. 2012, 6, 272–283. [Google Scholar]
- Rana, R.K.; Murthy, V.S.; Yu, J.; Wong, M.S. Nanoparticle self-assembly of hierarchically ordered microcapsule structures. Adv. Mater. 2005, 17, 1145–1150. [Google Scholar]
- Shu, X.Z.; Zhu, K.J.; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm. 2001, 212, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Babita, K.; Goyal, D.; Tiwary, A. Sodium citrate cross-linked chitosan films: Optimization as substitute for human/rat/rabbit epidermal sheets. J. Pharm. Pharm. Sci. 2005, 8, 10–17. [Google Scholar]
- Oomah, D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Chatterjee, S.; Salaün, F.; Campagne, C.; Vaupre, S.; Beirão, A.; El-Achari, A. Synthesis and characterization of chitosan droplet particles by ionic gelation and phase coacervation. Polym. Bull. 2014, 71, 1001–1013. [Google Scholar]
- Grehk, T.M.; Berger, R.; Bexell, U. Investigation of the drying process of linseed oil using FTIR and ToF-SIMS. J. Phys. Conf. Ser. 2008, 100, 012019. [Google Scholar] [CrossRef]
- Rao, C.N.R. Chemical Application of Infrared Spectroscopy; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Salaün, F.; Campagne, C. The Influence of 1-Butanol and Trisodium Citrate Ion on Morphology and Chemical Properties of Chitosan-Based Microcapsules during Rigidification by Alkali Treatment. Mar. Drugs 2014, 12, 5801-5816. https://doi.org/10.3390/md12125801
Chatterjee S, Salaün F, Campagne C. The Influence of 1-Butanol and Trisodium Citrate Ion on Morphology and Chemical Properties of Chitosan-Based Microcapsules during Rigidification by Alkali Treatment. Marine Drugs. 2014; 12(12):5801-5816. https://doi.org/10.3390/md12125801
Chicago/Turabian StyleChatterjee, Sudipta, Fabien Salaün, and Christine Campagne. 2014. "The Influence of 1-Butanol and Trisodium Citrate Ion on Morphology and Chemical Properties of Chitosan-Based Microcapsules during Rigidification by Alkali Treatment" Marine Drugs 12, no. 12: 5801-5816. https://doi.org/10.3390/md12125801





