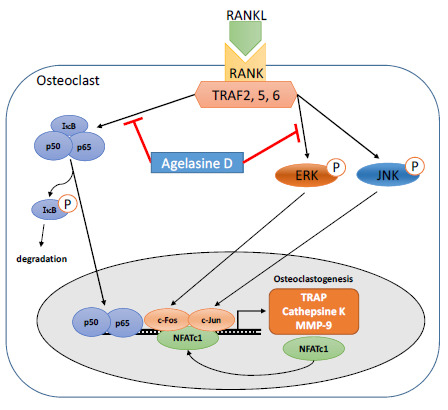
Agelasine D Suppresses RANKL-Induced Osteoclastogenesis via Down-Regulation of c-Fos, NFATc1 and NF-κB
Abstract
:1. Introduction
2. Results and Discussion
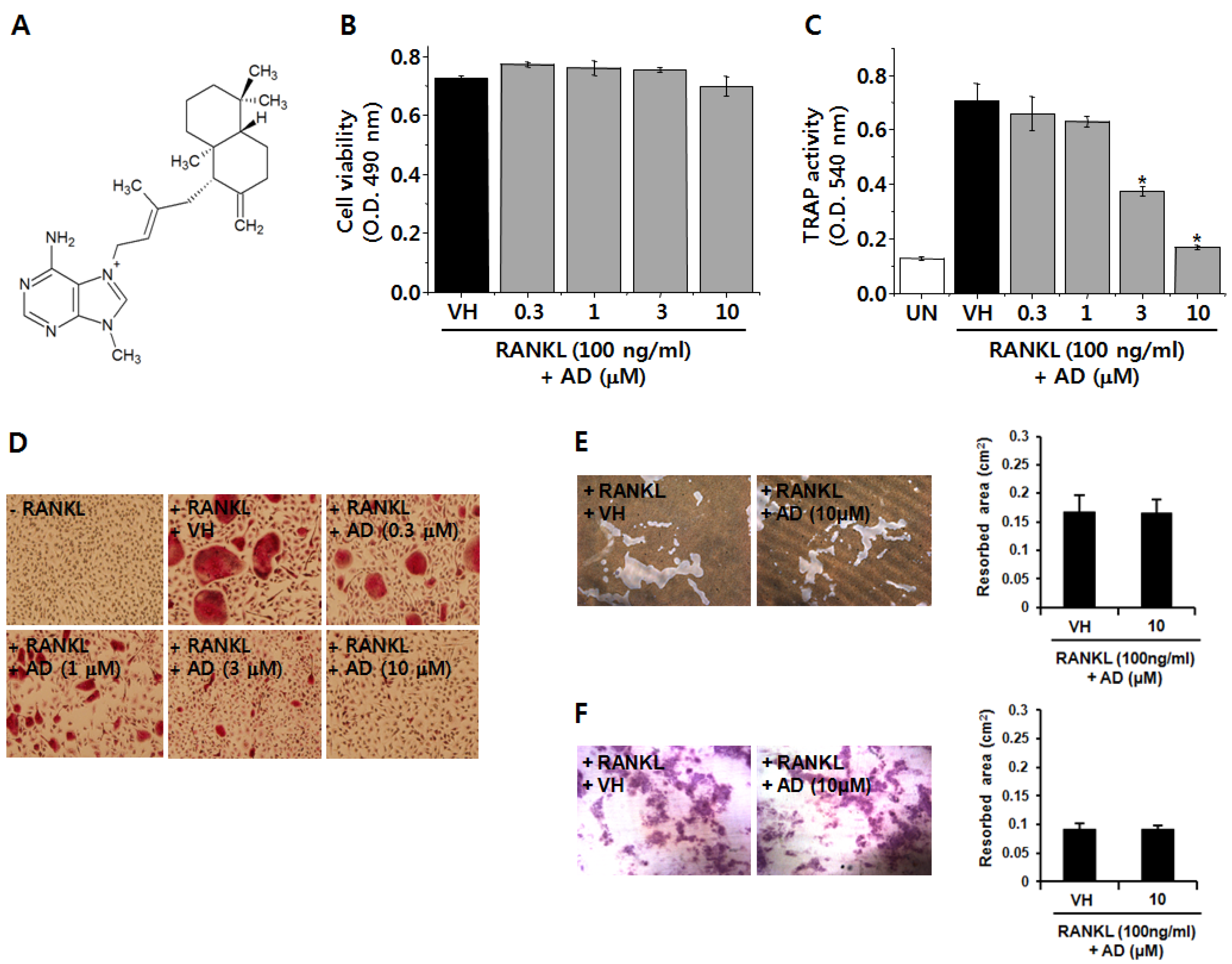
2.1. AD Inhibits RANKL-Induced Osteoclast Differentiation
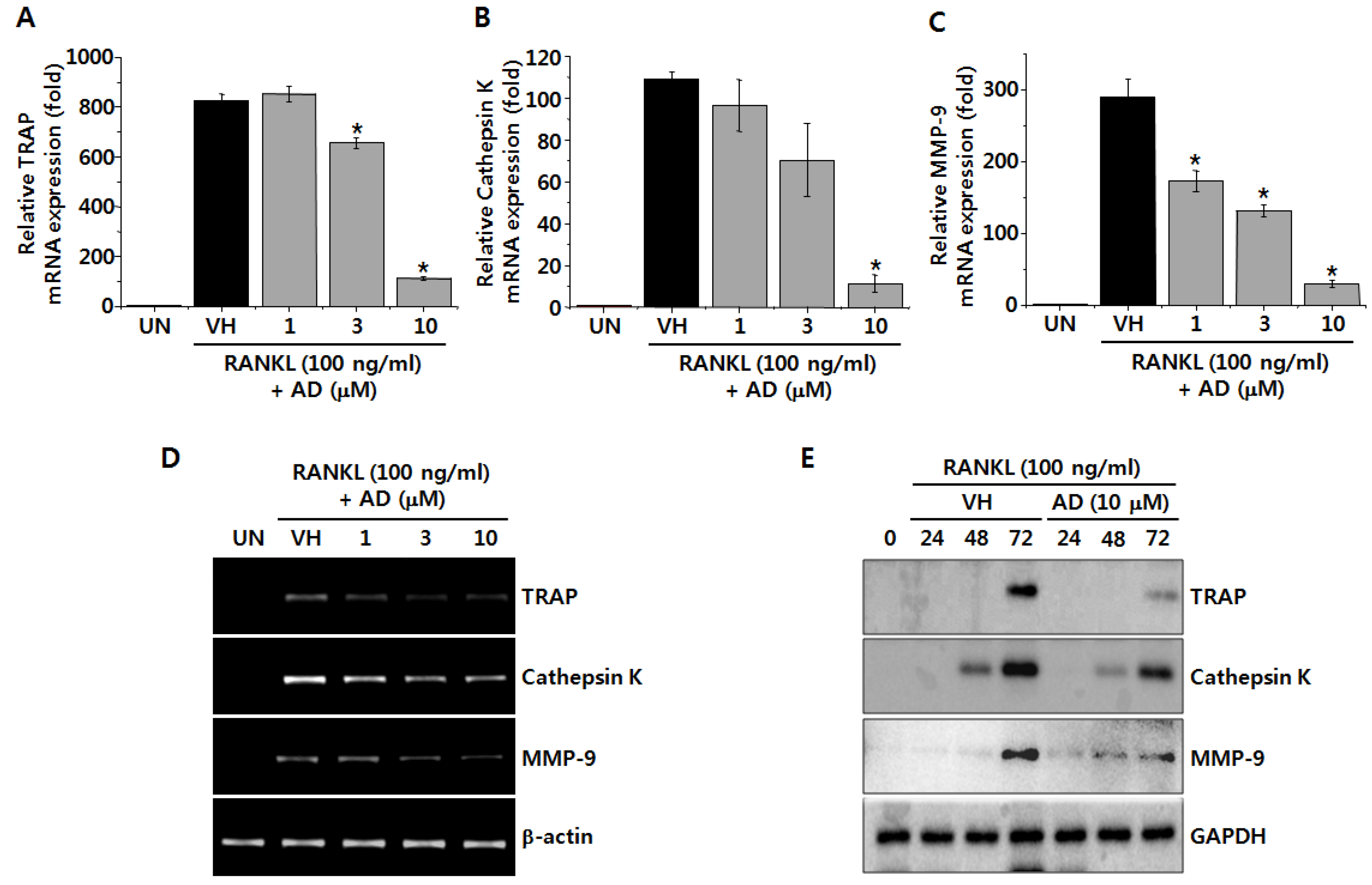
2.2. AD Suppresses RANKL-Induced mRNA and Protein Expression of Osteoclastic Markers
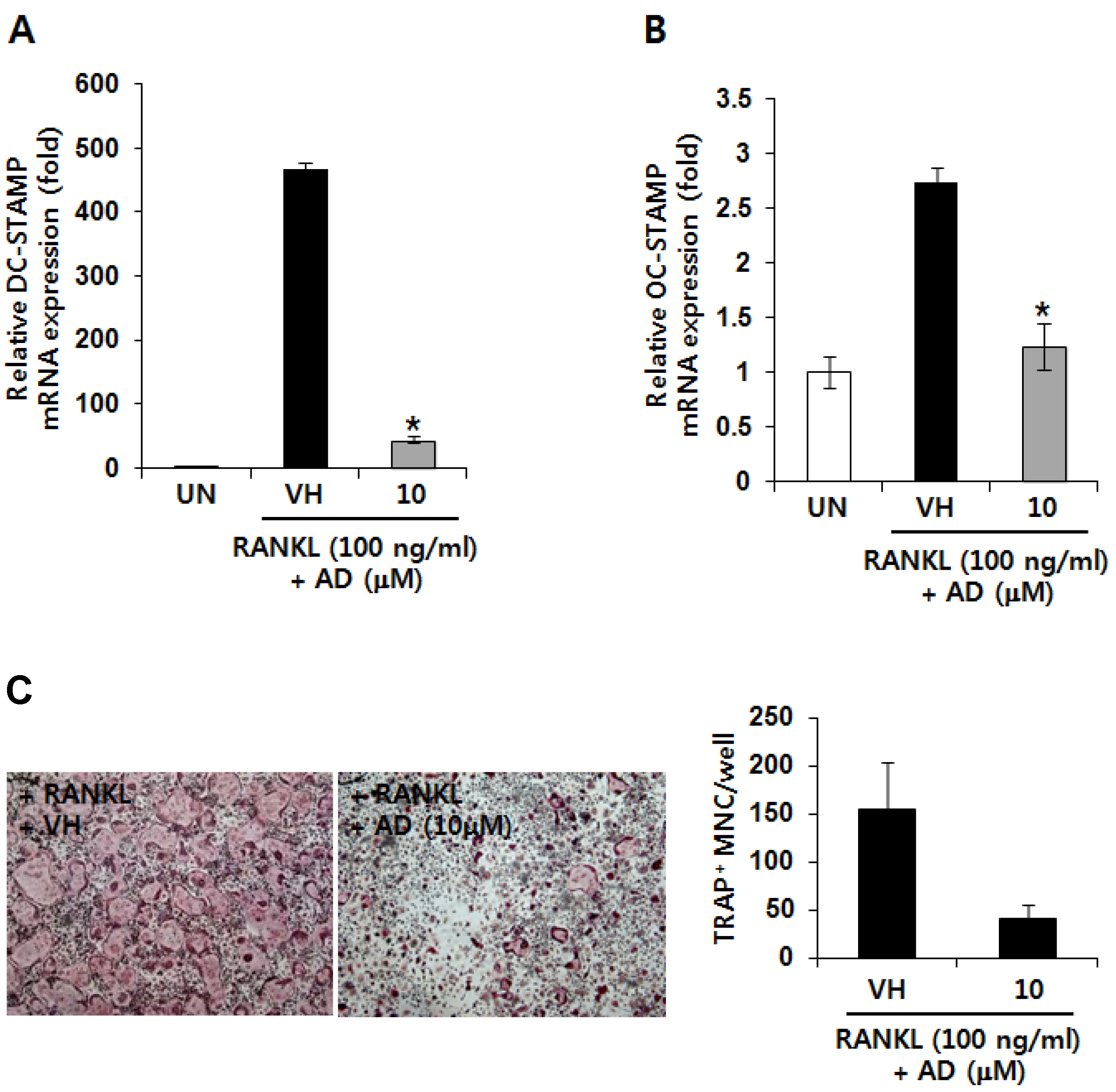
2.3. AD Inhibits RANKL-Induced mRNA Expression of Fusion-Related Molecules
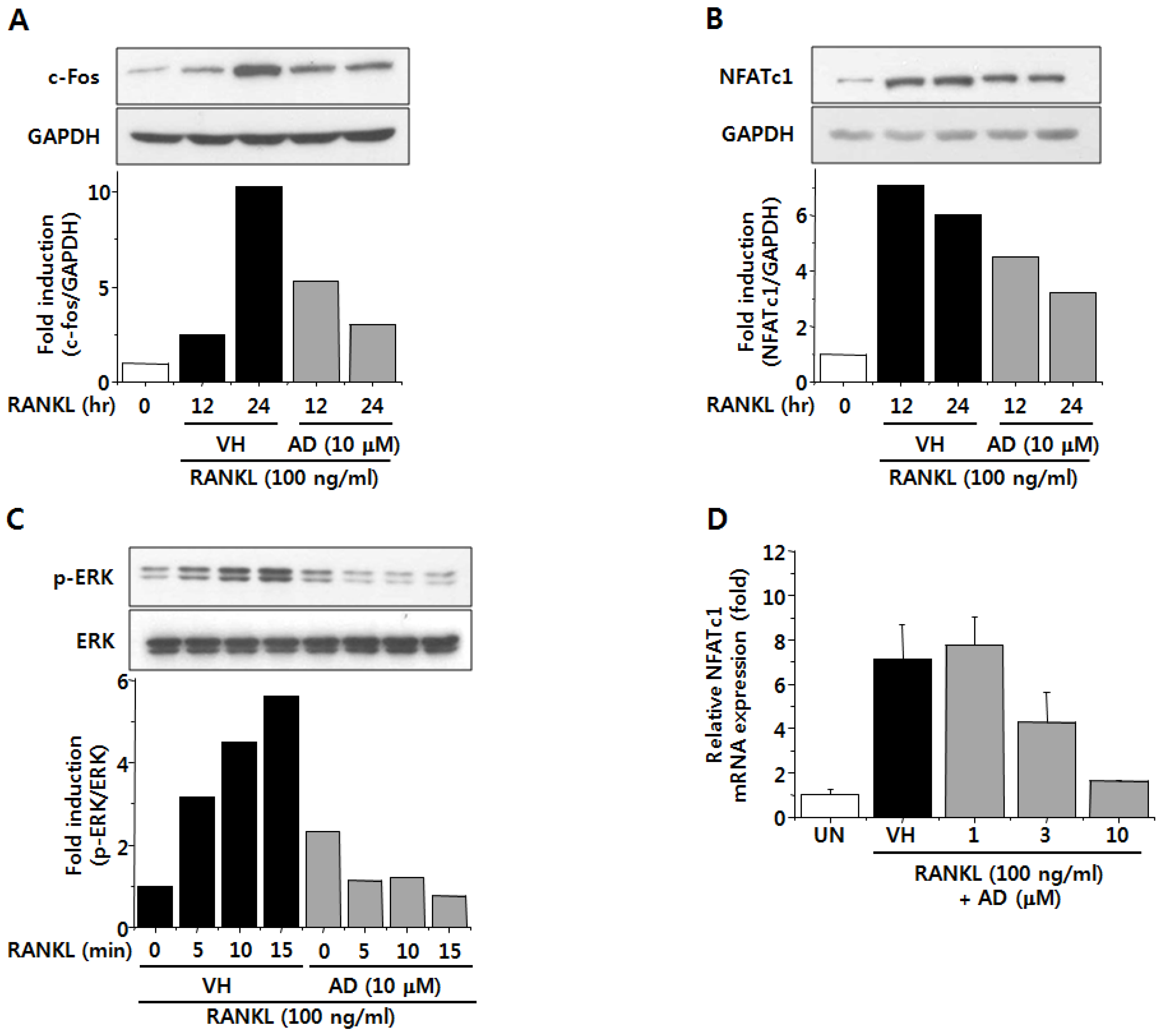
2.4. AD Negatively Regulates RANKL-Induced Expression of c-Fos and NFATc1 and Attenuates RANKL-Induced Phosphorylation of ERK
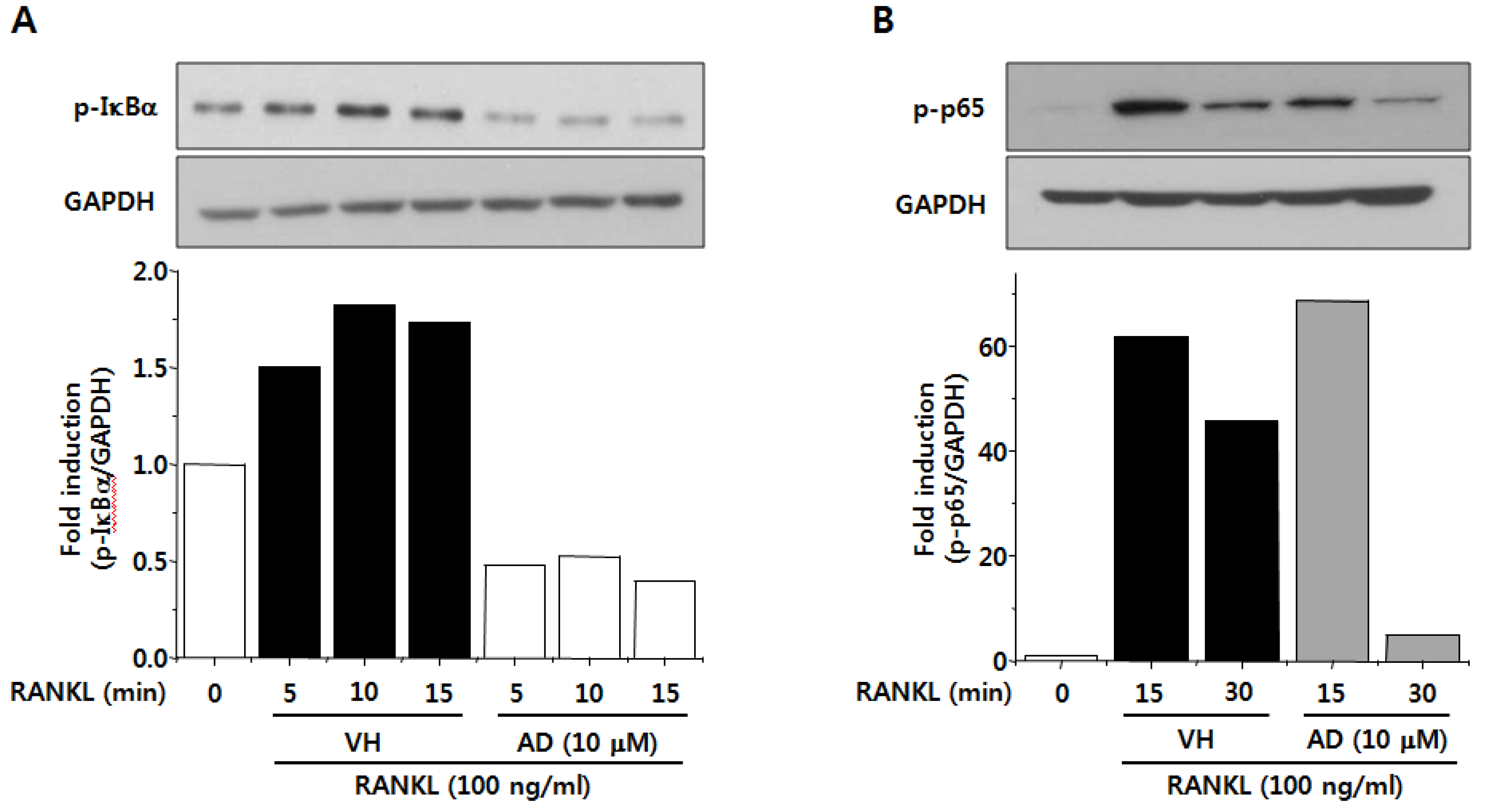
2.5. AD Inhibits RANKL-Induced NF-κB Activation
3. Experimental Section
3.1. Materials
3.2. Bone Marrow Macrophage (BMM) Isolation and Induction of Osteoclast Differentiation
3.3. Cell Viability Assay
3.4. Resorption Assay
3.5. Cell Fusion Assay
3.6. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
| Gene Name | Primer Sequence |
|---|---|
| DC-STAMP | forward: 5′-tggaggttcacttgaaactacgtg-3′ reverse: 5′-ctcggtttcccgtcagcctctctc-3′ |
| OC-STAMP | forward: 5′-cagccacggaacacctct-3′ reverse: 5′-ggacaggctgggagaagg-3′ |
| TRAP | forward: 5′-ctgctgggcctacaaatat-3′ reverse: 5′-ggtagtaagggctgggaag-3′ |
| Cathepsin K | forward: 5′-aggcggctatatgaccactg-3′ reverse: 5′-ccgagccaagagagcatatc-3′ |
| MMP-9 | forward: 5′-cgtcgtgatccccacttact-3′ reverse: 5′-agagtactgcttgcccagga-3′ |
| β-actin | forward: 5′-tggaatcctgtgcgatccatgaaa-3′ reverse: 5′-taaaacgcagctcagtaacagtccg-3′ |
3.7. Western Immunoblot Analysis
3.8. Statistical Analysis
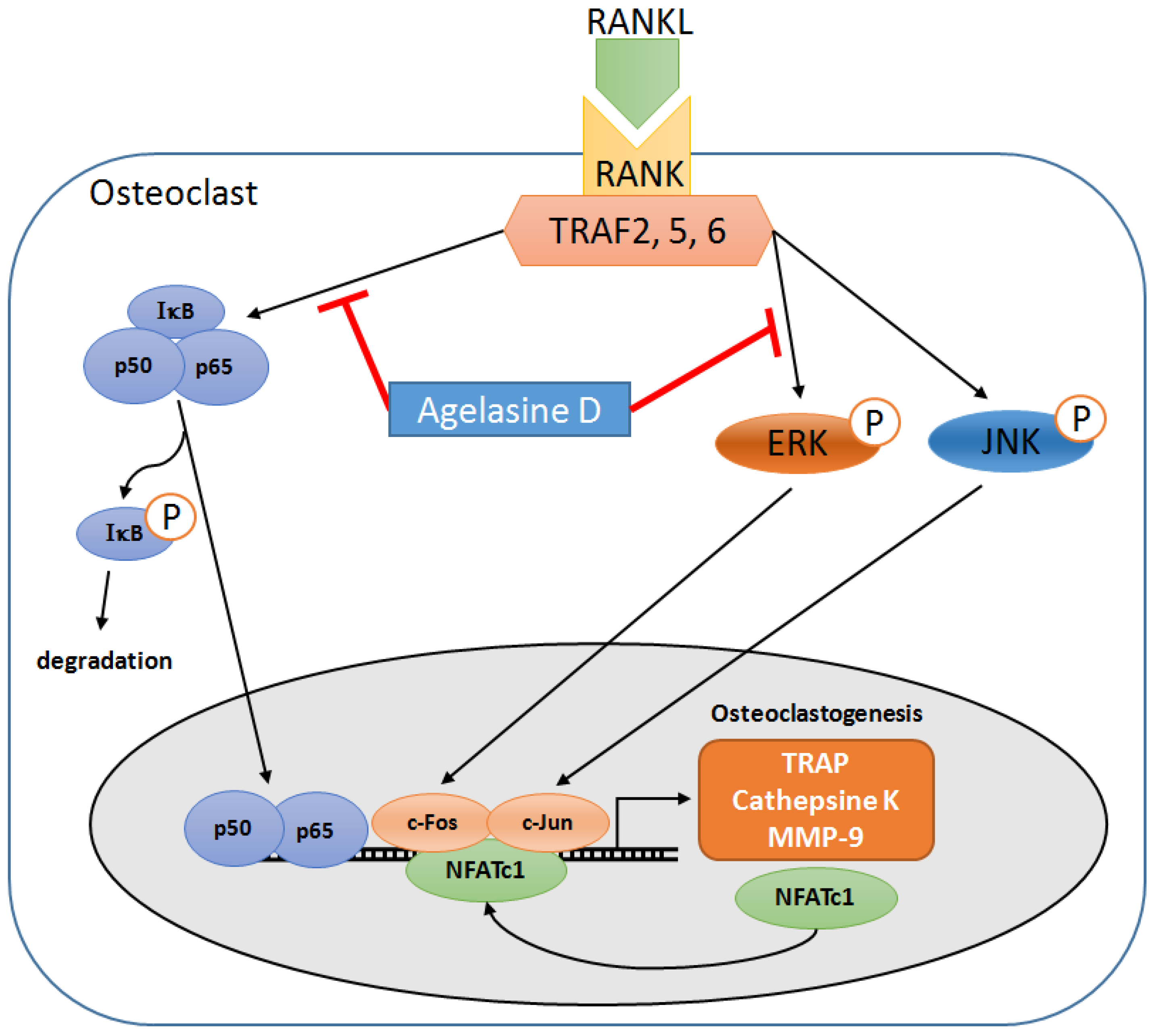
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karsenty, G.; Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2002, 2, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A.; Martin, T.J. Therapeutic approaches to bone diseases. Science 2000, 289, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Martin, T.J. Modulation of osteoclast differentiation. Endocr. Rev. 1992, 13, 66–80. [Google Scholar] [PubMed]
- Roodman, G.D. Cell biology of the osteoclast. Exp. Hematol. 1999, 27, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kobayashi, Y.; Nakamichi, Y.; Udagawa, N.; Takahashi, N.; Im, N.K.; Seo, H.J.; Jeon, W.B.; Yonezawa, T.; Cha, B.Y.; et al. Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice. Biochem. Pharmacol. 2010, 80, 352–361. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Shen, Y.; Chen, X.; Zhou, F.; Peng, D. Schisantherin A suppresses osteoclast formation and wear particle-induced osteolysis via modulating RANKL signaling pathways. Biochem Biophys Res. Commun. 2014, 449, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Inui, M.; Inoue, K.; Kim, S.; Suematsu, A.; Kobayashi, E.; Iwata, T.; Ohnishi, H.; Matozaki, T.; Kodama, T.; et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 2004, 428, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Mocsai, A.; Humphrey, M.B.; van Ziffle, J.A.; Hu, Y.; Burghardt, A.; Spusta, S.C.; Majumdar, S.; Lanier, L.L.; Lowell, C.A.; Nakamura, M.C. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 6158–6163. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. The role of NFAT in osteoclast formation. Ann. N. Y. Acad. Sci. 2007, 1116, 227–237. [Google Scholar] [CrossRef]
- Hertiani, T.; Edrada-Ebel, R.; Ortlepp, S.; van Soest, R.W.; de Voogd, N.J.; Wray, V.; Hentschel, U.; Kozytska, S.; Muller, W.E.; Proksch, P. From anti-fouling to biofilm inhibition: new cytotoxic secondary metabolites from two Indonesian Agelas sponges. Bioorg. Med. Chem. 2010, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Tsuda, M.; Okada, T.; Mitsuhashi, S.; Shima, H.; Kikuchi, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Nagelamides A–H, new dimeric bromopyrrole alkaloids from marine sponge Agelas species. J. Nat. Prod. 2004, 67, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Motoki, K.; Natori, T.; Uchida, T.; Fukushima, H.; Koezuka, Y. Enhancing effects of agelasphin-11 on natural killer cell activities of normal and tumor-bearing mice. Biol. Pharm. Bull. 1996, 19, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Proszenyak, A.; Charnock, C.; Hedner, E.; Larsson, R.; Bohlin, L.; Gundersen, L.L. Synthesis, antimicrobial and antineoplastic activities for agelasine and agelasimine analogs with a beta-cyclocitral derived substituent. Arch. Pharm. 2007, 340, 625–634. [Google Scholar] [CrossRef]
- Sjogren, M.; Dahlstrom, M.; Hedner, E.; Jonsson, P.R.; Vik, A.; Gundersen, L.L.; Bohlin, L. Antifouling activity of the sponge metabolite agelasine D and synthesised analogs on Balanus improvisus. Biofouling 2008, 24, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A.R.; Bune, A.J.; Bradley, J.R.; Rashbass, J.; Cox, T.M. Osteoclastic tartrate-resistant acid phosphatase (Acp 5): Its localization to dendritic cells and diverse murine tissues. J. Histochem. Cytochem. 2000, 48, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.; Lawrence, K.M.; Ross, J.L.; Grabowska, U.B.; Shiroo, M.; Samuelsson, B.; Chambers, T.J. Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone 2008, 42, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Zenger, S.; Hollberg, K.; Ljusberg, J.; Norgard, M.; Ek-Rylander, B.; Kiviranta, R.; Andersson, G. Proteolytic processing and polarized secretion of tartrate-resistant acid phosphatase is altered in a subpopulation of metaphyseal osteoclasts in cathepsin K-deficient mice. Bone 2007, 41, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Lotinun, S.; Kiviranta, R.; Matsubara, T.; Alzate, J.A.; Neff, L.; Luth, A.; Koskivirta, I.; Kleuser, B.; Vacher, J.; Vuorio, E.; et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 2013, 123, 666–681. [Google Scholar] [PubMed]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ninomiya, K.; Fujita, N.; Suzuki, T.; Iwasaki, R.; Morita, K.; Hosogane, N.; Matsuo, K.; Toyama, Y.; Suda, T.; et al. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007, 22, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Birnbaum, M.J.; MacKay, C.A.; Mason-Savas, A.; Thompson, B.; Odgren, P.R. Osteoclast stimulatory transmembrane protein (OC-STAMP), a novel protein induced by RANKL that promotes osteoclast differentiation. J. Cell Physiol. 2008, 215, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, A.E.; Wang, Z.Q.; Cecchini, M.G.; Hofstetter, W.; Felix, R.; Fleisch, H.A.; Wagner, E.F. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 1994, 266, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.; Hernandez-Losa, J.; Lyons, R.J.; Castellone, M.D.; Gutkind, J.S. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J. Biol. Chem. 2005, 280, 35081–35084. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.R.; Ernst, M.K. In vivo control of NF-kappa B activation by I kappa B alpha. EMBO J. 1993, 12, 4685–4695. [Google Scholar] [PubMed]
- Franzoso, G.; Carlson, L.; Xing, L.; Poljak, L.; Shores, E.W.; Brown, K.D.; Leonardi, A.; Tran, T.; Boyce, B.F.; Siebenlist, U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997, 11, 3482–3496. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; De Wilde, G.; Notebaert, S.; Vanden Berghe, W.; Haegeman, G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem. Pharmacol. 2002, 64, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Choi, S.; Park, E.S.; Shin, B.; Lee, S.H.; Takami, M.; Kang, J.S.; Meong, H.; Rho, J. d-chiro-inositol negatively regulates the formation of multinucleated osteoclasts by down-regulating NFATc1. J. Clin. Immunol. 2012, 32, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.R.; Jo, S.A.; Yoon, Y.D.; Park, K.H.; Oh, S.J.; Yun, J.; Lee, C.W.; Nam, K.-H.; Kim, Y.; Han, S.-B.; et al. Agelasine D Suppresses RANKL-Induced Osteoclastogenesis via Down-Regulation of c-Fos, NFATc1 and NF-κB. Mar. Drugs 2014, 12, 5643-5656. https://doi.org/10.3390/md12115643
Kang MR, Jo SA, Yoon YD, Park KH, Oh SJ, Yun J, Lee CW, Nam K-H, Kim Y, Han S-B, et al. Agelasine D Suppresses RANKL-Induced Osteoclastogenesis via Down-Regulation of c-Fos, NFATc1 and NF-κB. Marine Drugs. 2014; 12(11):5643-5656. https://doi.org/10.3390/md12115643
Chicago/Turabian StyleKang, Moo Rim, Sun Ah Jo, Yeo Dae Yoon, Ki Hwan Park, Soo Jin Oh, Jieun Yun, Chang Woo Lee, Ki-Hoan Nam, Youngsoo Kim, Sang-Bae Han, and et al. 2014. "Agelasine D Suppresses RANKL-Induced Osteoclastogenesis via Down-Regulation of c-Fos, NFATc1 and NF-κB" Marine Drugs 12, no. 11: 5643-5656. https://doi.org/10.3390/md12115643