Sinulolides A–H, New Cyclopentenone and Butenolide Derivatives from Soft Coral Sinularia sp.
Abstract
:1. Introduction

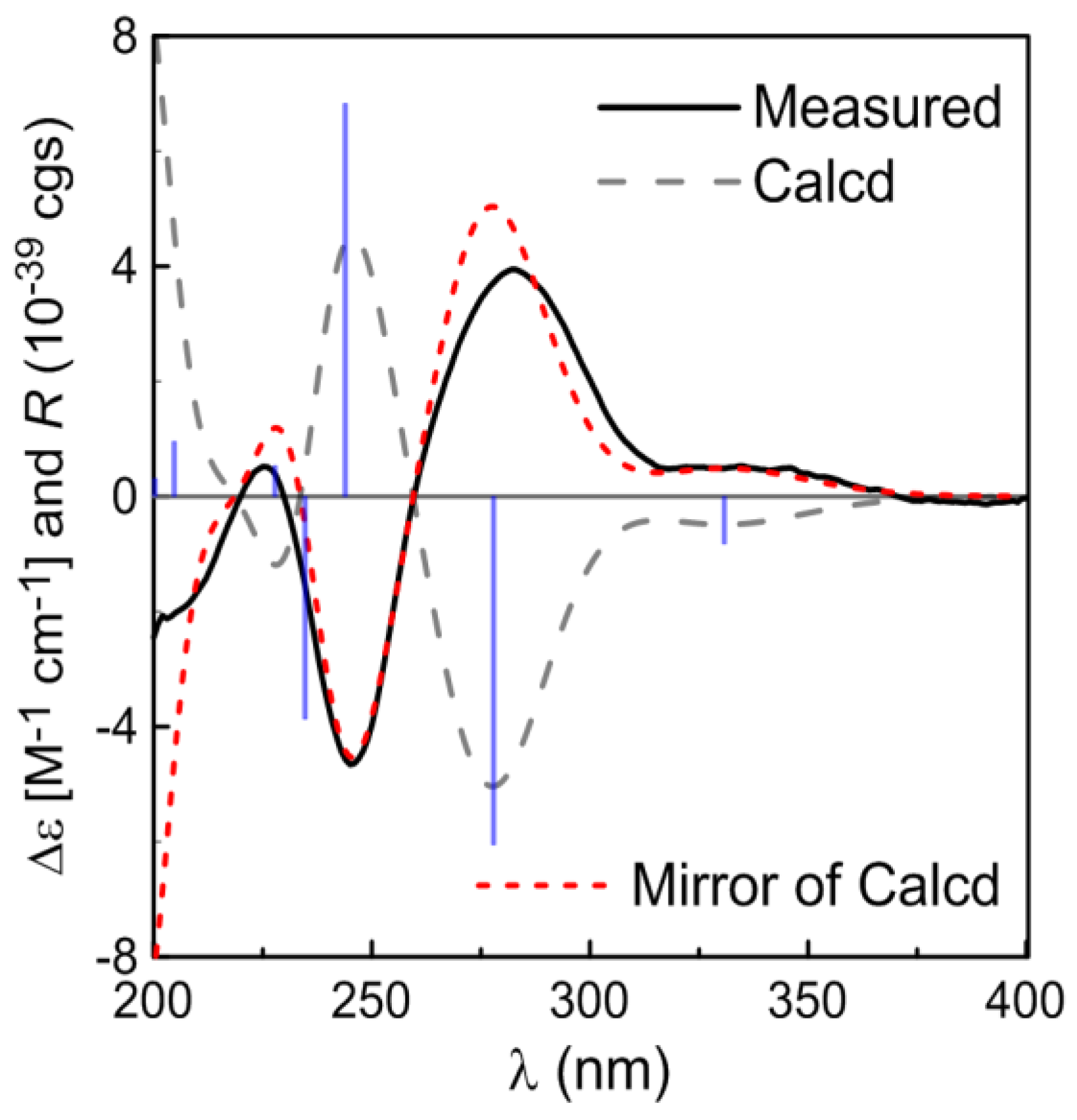
2. Results and Discussion
| Position | 1 | 2 | ||
|---|---|---|---|---|
| 13C | 1H | 13C | 1H | |
| 1 | 25.0 | 2.01 s | 24.8 | 2.01 s |
| 2 | 207.0 | 207.0 | ||
| 3 | 89.1 | 4.72 s | 89.4 | 4.68 s |
| 4 | 163.1 | 163.2 | ||
| 5 | 139.5 | 139.5 | ||
| 6 | 204.7 | 205.0 | ||
| 7 | 92.3 | 92.7 | ||
| 8 | 36.5 | 1.87 m | 36.8 | 1.88 m |
| 1.76 m | 1.76 m | |||
| 9 | 33.0 | 2.41 m | 33.3 | 2.38 m |
| 1.76 m | 1.76 m | |||
| 10 | 86.7 | 86.9 | ||
| 11 | 41.0 | 1.62 m | 41.4 | 1.62 m |
| 12 | 22.5 | 1.41 m | 22.5 | 1.41 m |
| 1.36 m | 1.36 m | |||
| 13 | 34.4 | 1.67 m | 34.2 | 1.64 m |
| 1.41 m | 1.40 m | |||
| 14 | 39.5 | 2.48 m | 39.3 | 2.44 m |
| 15 | 177.4 | 177.2 | ||
| 16 | 51.5 | 3.66 s | 51.5 | 3.67 s |
| 17 | 17.2 | 1.15 d (7.0) | 17.0 | 1.14 d (7.0) |
| 18 | 25.7 | 1.04 s | 25.4 | 1.29 s |
| 19 | 12.0 | 1.83 s | 11.8 | 1.83 s |
| 20 | 8.9 | 1.82 s | 8.7 | 1.81 s |


| Position | 3 | 4 | 5 | 6 | ||||
|---|---|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | |
| 1 | 175.2 | 174.3 | 175.5 | 174.4 | ||||
| 2 | 29.8 | 3.15 dd (4.5, 15.5) | 31.7 | 2.98 m | 29.6 | 3.05 dd (3.0, 18.0) | 28.6 | 3.05 dd (4.5, 8.0) |
| 2.39 dd (7.0, 11.5) | 2.62 d (15.5) | 2.69 dd (6.5, 11.5 ) | 2.61 dd (4.5, 14.5) | |||||
| 3 | 55.0 | 2.85 dd (3.5, 8.5) | 46.6 | 2.93 m | 60.9 | 2.98 dd (3.0, 11.5) | 60.3 | 2.94 dd (7.0,12.5) |
| 4 | 202.8 | 204.1 | 203.1 | 204.4 | ||||
| 5 | 135.5 | 139.0 | 136.9 | 140.2 | ||||
| 6 | 168.7 | 167.1 | 165.9 | 165.7 | ||||
| 7 | 80.4 | 92.3 | 83.7 | 80.8 | ||||
| 8 | 37.0 | 1.76 td (19.0, 5.5) | 34.5 | 1.97 td (14.0, 4.0) | 86.9 | 3.29 dd (4.5, 8.0) | 93.0 | 3.61 dd (2.5, 8.5) |
| 1.52 td (13.0, 3.5) | 1.81 m | |||||||
| 9 | 25.1 | 0.75 m | 23.3 | 0.88 m | 31.3 | 1.64 m | 30.6 | 1.68 m |
| 0.63 m | 1.10 m | 1.22 m | 1.30 m | |||||
| 10 | 31.9 | 1.19 m | 32.5 | 1.32 m | 32.0 | 1.24 m | 32.2 | 1.34 m |
| 11 | 22.4 | 1.19 m | 22.4 | 1.25 m | 22.6 | 1.39 m | 22.8 | 1.34 m |
| 1.07 m | 1.51 m | |||||||
| 12 | 13.9 | 0.83 t (7.0) | 13.9 | 0.89 t (7.0) | 13.9 | 0.83 t (7.0) | 14.0 | 0.92 t (7.0 ) |
| 13 | 7.8 | 1.72 s | 8.2 | 1.75 s | 8.0 | 1.72 s | 8.3 | 1.75 s |
| 14 | 11.5 | 2.00 s | 12.2 | 2.06 s | 11.8 | 1.99 s | 13.0 | 2.06 s |
| 15 | 52.4 | 3.75 s | 52.1 | 3.74 s | 44.0 | 3.38 s | ||
| 16 | 54.7 | 3.42 s | ||||||
| Concentration | IR (%) | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 10 | |
| 10 μg/mL | 27.85 | 28.75 | 38.12 | 28.24 | 27.08 | 25.28 | 43.00 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Assays for Bioactivities
3.5. Computational Calculation
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Hsieh, C.H.; Chen, S.P.; Lu, C.K.; Dai, C.F.; Sheu, J.H. Sinularianins A and B, novel sesquiterpenoids from the Formosan soft coral Sinularia sp. Tetrahedron Lett. 2006, 47, 5889–5891. [Google Scholar] [CrossRef]
- Huang, C.Y.; Su, J.H.; Liu, Y.C.; Wen, Z.H.; Hsu, C.H.; Chiang, M.Y.; Sheu, J.H. Oppositane-type sesquiterpenoids from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2010, 83, 678–682. [Google Scholar] [CrossRef]
- Yang, B.; Liao, S.R.; Lin, X.P.; Wang, J.F.; Liu, J.; Zhou, X.F.; Yang, X.W.; Liu, Y.H. New sinularianin sesquiterpenes from soft coral Sinularia sp. Mar. Drugs 2013, 11, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Wen, Z.H.; Su, J.H.; Hsieh, Y.T.; Wu, Y.C.; Hu, W.P.; Sheu, J.H. Oxygenated cembranoids from a Formosan soft coral Sinulatia gibberosa. J. Nat. Prod. 2008, 71, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.W.; Chao, C.H.; Su, J.H.; Huang, C.Y.; Dai, C.F.; Wen, Z.H.; Sheu, J.H. A novel symmetric sulfur-containing biscembranoid from the Formosan soft coral Sinularia flexibilis. Tetrahedron Lett. 2010, 51, 5764–5766. [Google Scholar] [CrossRef]
- Haidy, N.K.; Marc, S. Terpenoids of sinularia: Chemistry and biomedical applications. Pharm. Biol. 2005, 43, 253–269. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.F.; Lin, X.P.; Liu, J.; Peng, Y.; Yang, X.W.; Liu, Y.H. Cembrane diterpenes chemistry and biological properties. Curr. Org. Chem. 2012, 16, 1512–1539. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.F.; Huang, H.; Yang, X.W.; Liu, J.; Lin, X.P.; Li, X.B.; Peng, Y.; Liu, Y.H. New cembrane diterpenoids from a Hainan soft coral Sinularia sp. Mar. Drugs 2012, 10, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.F.; Deng, Z.W.; Pei, Y.H.; Fu, H.Z.; Li, J.; Van Ofwegen, L.; Proksch, P.; Lin, W.H. Polyhydroxylated steroids from the soft coral Sinularia dissecta. Steroids 2005, 70, 487–493. [Google Scholar] [CrossRef]
- Chen, B.W.; Su, J.H.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Polyoxygenated steroids from a Formosan soft coral Sinularia facile. Bull. Chem. Soc. Jpn. 2008, 81, 1304–1307. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Hsieh, Y.T.; Wen, Z.H.; Wu, Y.C.; Sheu, J.H. Polyoxygenated sterols from the formosan soft coral Sinularia gibberosa. J. Nat. Prod. 2006, 69, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Lo, C.L.; Lu, Y.; Wen, Z.H.; Huang, C.Y.; Dai, C.F.; Sheu, J.H. Anti-Inflammatory polyoxygenated steroids from the soft coral Sinularia sp. B. Chem. Soc. Jap. 2008, 81, 1616–1620. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, C.Y.; Lin, Y.F.; Wen, Z.H.; Su, J.H.; Kuo, Y.H.; Chiang, M.Y.; Sheu, J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; Hou, R.S. Cytotoxic cembranoids from the soft corals Sinularia gibberosa and Sarcophyton trocheliophorum. J. Nat. Prod. 1996, 59, 595–598. [Google Scholar] [CrossRef]
- Li, G.Q.; Zhang, Y.L.; Deng, Z.W.; van Ofwegen, L.P.; Proksch, P.; Lin, W.H. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Umeyama, A.; Takei, M.; Arihara, S. Potent inhibitors of histamine release polyhydroxylated sterols from the Okinawan soft coral Sinularia abrupta. J. Pharm. Sci. 1994, 83, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Kuo, Y.H. Two novel alpha-tocopheroids from the aerial roots of Ficus microcarpa. Tetrahedron Lett. 2003, 44, 5125–5128. [Google Scholar] [CrossRef]
- Matsuo, M.; Matsumoto, S.; Iitaka, Y. Oxygenations of vitamin-E (alpha-tocopherol) and its model-compound 2,2,5,7,8-pentamethylchroman-6-ol in the presence of the superoxide radical solubilized in aprotic-solvents-unique epoxidations and recyclizatons. J. Org. Chem. 1987, 52, 3514–3520. [Google Scholar] [CrossRef]
- Yuan, Z.Z.; Duan, H.M.; Xu, Y.Y.; Wang, A.L.; Gan, L.S.; Li, J.J.; Liu, M.T.; Shang, X.Y. α-Tocospiro C, a novel cytotoxic α-tocopheroid from Cirsium setosum. Phytochem. Lett. 2014, 8, 116–120. [Google Scholar] [CrossRef]
- Shi, H.Y.; Yu, S.J.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Sinularones A-I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs 2012, 10, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wang, W.; Hong, J.; Lee, C.O.; Shin, S.; Im, K.S.; Jung, J.H. A new 2,3-dimethyl butenolide from the brittle star Ophiomastix mixta. Chem. Pharm. Bull. 2007, 55, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Kusumi, T.; Ishitsuka, M.; Kakisawa, H. 2,3-dimethyl-4-methoxybuenolides from red algae, Coeloseira pacifica and Ahnfeltia paradoxa. Chem. Lett. 1980, 955–956. [Google Scholar]
- Koshino, H.; Yoshihara, T.; Sakamura, S.; Shimanuki, T.; Sato, T.; Tajimi, A. Novel C-11 expoxy fatty-acid from stromata of Epichloe typhina on Phleum pratense. Agric. Biol. Chem. 1989, 53, 2527–2528. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhang, Z.Y.; Zhang, M.; Mais, D.E.; Wang, M.W. High throughput screening for bioactive components from traditional Chinese medicine. Comb. Chem. High Throughput. Screen. 2010, 13, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Zanella, F.; Rosado, A.; Garcia, B.; Carnero, A.; Link, W. Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. Chembiochem 2008, 9, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.M.P.; Reisen, F.; Zgraggen, S.; Fischer, S.; Yuen, D.; Kang, G.J.; Chen, L.; Schneider, G.; Detmar, M. Phenotype-based high-content chemical library screening identifies statins as inhibitors of in vivo lymphangiogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, E2665–E2674. [Google Scholar] [CrossRef]
- Kang, M.I.; Henrich, C.J.; Bokesch, H.R.; Gustafson, K.R.; McMahon, J.B.; Baker, A.R.; Young, M.R.; Colburn, N.H. A selective small-molecule nuclear factor-kappa B inhibitor from a high-throughput cell-based assay for “activator protein-1 hits”. Mol. Cancer Ther. 2009, 8, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Wagner, B.K.; Ma, Y.; Chinsomboon, J.; Laznik, D.; Spiegelman, B.M. Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1 alpha and oxidative phosphorylation. Proc. Natl. Acad. Sci. USA 2008, 105, 4721–4726. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, H.P.; Zhou, Y.Y.; Du, J.Q.; Shen, Q.; Li, J.Y.; Li, J. Discovery of a novel competitive inhibitor of PTP1B by high-throughput screening. Acta Pharmacol. Sin. 2008, 29, 278–284. [Google Scholar] [CrossRef]
- Farrelly, E.; Amaral, M.C.; Marshall, L.; Huang, S.G. A high-throughput assay for mitochondrial membrane potential in permeabilized yeast cells. Anal. Biochem. 2001, 293, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.N.; Cool, B.L.; Kifle, L.; Chiou, W.; Egan, D.A.; Barrett, L.W.; Richardson, P.L.; Frevert, E.U.; Warrior, U.; Kofron, J.L.; et al. Microarrayed compound screening (mu ARCS) to identify activators and inhibitors of AMP-activated protein kinase. J. Biomol. Screen. 2004, 9, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumloffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental clectronic circular dichroism dpectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Wei, X.; Huang, J.; Lin, X.; Liu, J.; Liao, S.; Wang, J.; Zhou, X.; Wang, L.; Liu, Y. Sinulolides A–H, New Cyclopentenone and Butenolide Derivatives from Soft Coral Sinularia sp. Mar. Drugs 2014, 12, 5316-5327. https://doi.org/10.3390/md12105316
Yang B, Wei X, Huang J, Lin X, Liu J, Liao S, Wang J, Zhou X, Wang L, Liu Y. Sinulolides A–H, New Cyclopentenone and Butenolide Derivatives from Soft Coral Sinularia sp. Marine Drugs. 2014; 12(10):5316-5327. https://doi.org/10.3390/md12105316
Chicago/Turabian StyleYang, Bin, Xiaoyi Wei, Jingxia Huang, Xiuping Lin, Juan Liu, Shengrong Liao, Junfeng Wang, Xuefeng Zhou, Lishu Wang, and Yonghong Liu. 2014. "Sinulolides A–H, New Cyclopentenone and Butenolide Derivatives from Soft Coral Sinularia sp." Marine Drugs 12, no. 10: 5316-5327. https://doi.org/10.3390/md12105316





