Exploring Bioactive Properties of Marine Cyanobacteria Isolated from the Portuguese Coast: High Potential as a Source of Anticancer Compounds
Abstract
:1. Introduction
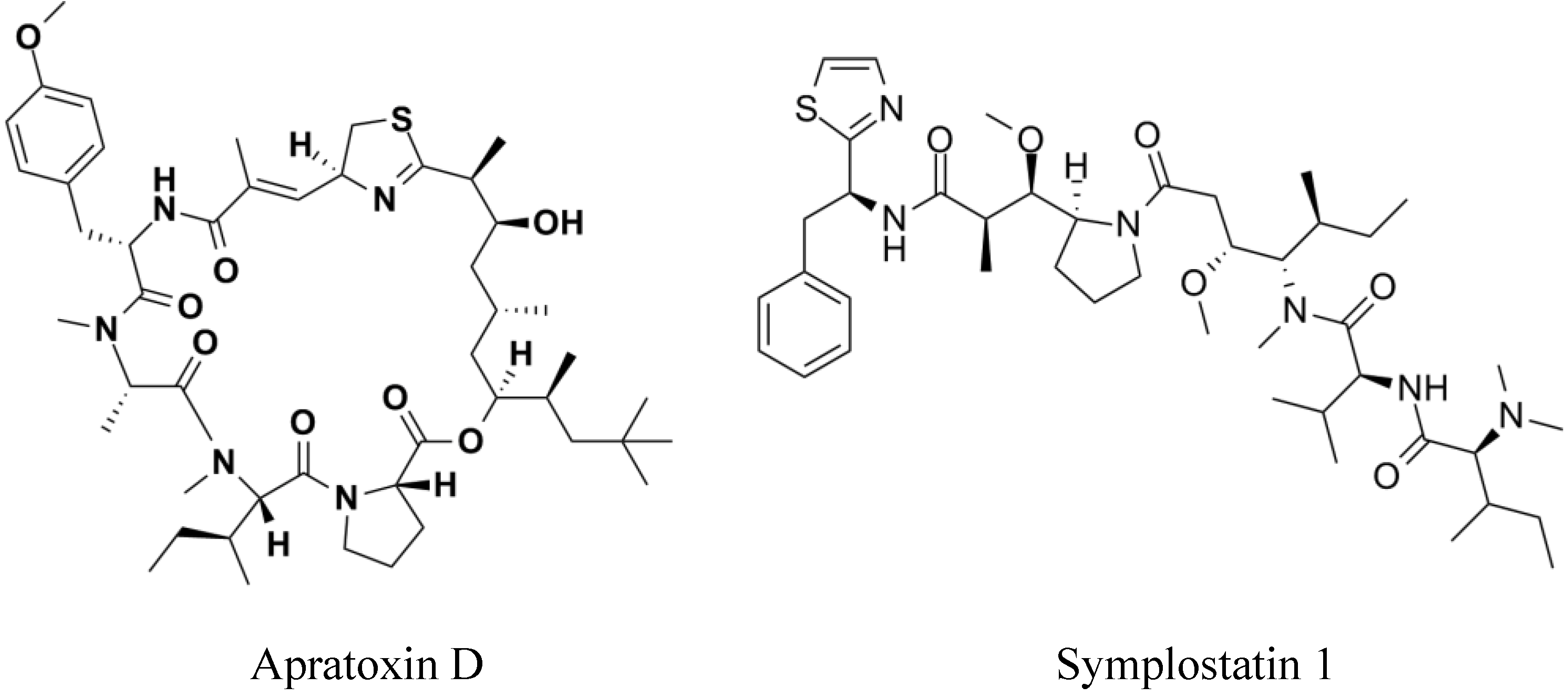
2. Results
| Strain | Cancer Cell Lines | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HepG2 | RKO | MG-63 | SK-BR-3 | T47D | HT-29 | SH-SY5Y | PC-3 | ||
| Nodosilinea nodulosa | LEGE 06152 | − | − | − | − | − | +++ | + | +++ |
| Leptolyngbya cf. halophila | LEGE 06102 | +++ | + | ++ | − | − | ++ | + | ++ |
| Leptolyngbya mycoidea | LEGE 06108 | − | − | − | − | ++ | − | − | − |
| Leptolyngbya mycoidea | LEGE 06118 | + | + | ++ | − | ++ | + | ++ | − |
| Leptolyngbya mycoidea | LEGE 06009 | − | − | − | − | ++ | +++ | + | ++ |
| Leptolyngbya fragilis | LEGE 07167 | ++ | ++ | +++ | ++ | +++ | + | +++ | +++ |
| Pseudanabaena aff. curta | LEGE 07160 | − | + | + | − | − | ++ | − | ++ |
| Pseudanabaena aff. curta | LEGE 07169 | ++ | − | +++ | + | ++ | +++ | − | +++ |
| Pseudanabaena aff. persicina | LEGE 07163 | − | − | − | − | − | − | − | − |
| Pseudanabaena sp. | LEGE 06144 | +++ | − | − | +++ | + | − | − | − |
| Pseudanabaena sp. | LEGE 06194 | − | − | − | − | − | − | − | − |
| Cyanobium sp. | LEGE 06098 | − | − | ++ | + | − | − | ++ | − |
| Cyanobium sp. | LEGE 06134 | − | − | + | − | − | + | − | − |
| Cyanobium sp. | LEGE 07175 | + | − | ++ | − | ++ | − | − | − |
| Cyanobium sp. | LEGE 07186 | − | − | ++ | − | ++ | ++ | − | ++ |
| Cyanobium sp. | LEGE 06113 | +++ | − | + | ++ | − | − | ++ | − |
| Cyanobium sp. | LEGE 06137 | − | ++ | − | − | − | − | + | − |
| Cyanobium sp. | LEGE 06097 | + | − | − | − | ++ | + | − | − |
| Cyanobium sp. | LEGE 06139 | − | ++ | − | − | + | − | + | − |
| Synechococcus nidulans | LEGE 07171 | + | − | − | +++ | − | − | ++ | − |
| Synechococcus sp. | LEGE 07172 | − | ++ | − | − | − | − | ++ | − |
| Synechococcus sp. | LEGE 06005 | − | − | ++ | − | − | − | − | − |
| Synechococcus sp. | LEGE 06026 | − | − | − | + | − | + | − | − |
| Synechocystis salina | LEGE 06099 | +++ | − | ++ | +++ | − | − | + | − |
| Synechocystis salina | LEGE 06155 | +++ | +++ | + | ++ | + | + | ++ | − |
| Synechocystis salina | LEGE 07173 | + | − | − | − | + | − | ++ | − |
| Romeria sp. | LEGE 06013 | − | ++ | − | − | − | − | + | − |
| Romeria aff. gracilis | LEGE 07310 | + | − | + | +++ | ++ | − | ++ | − |
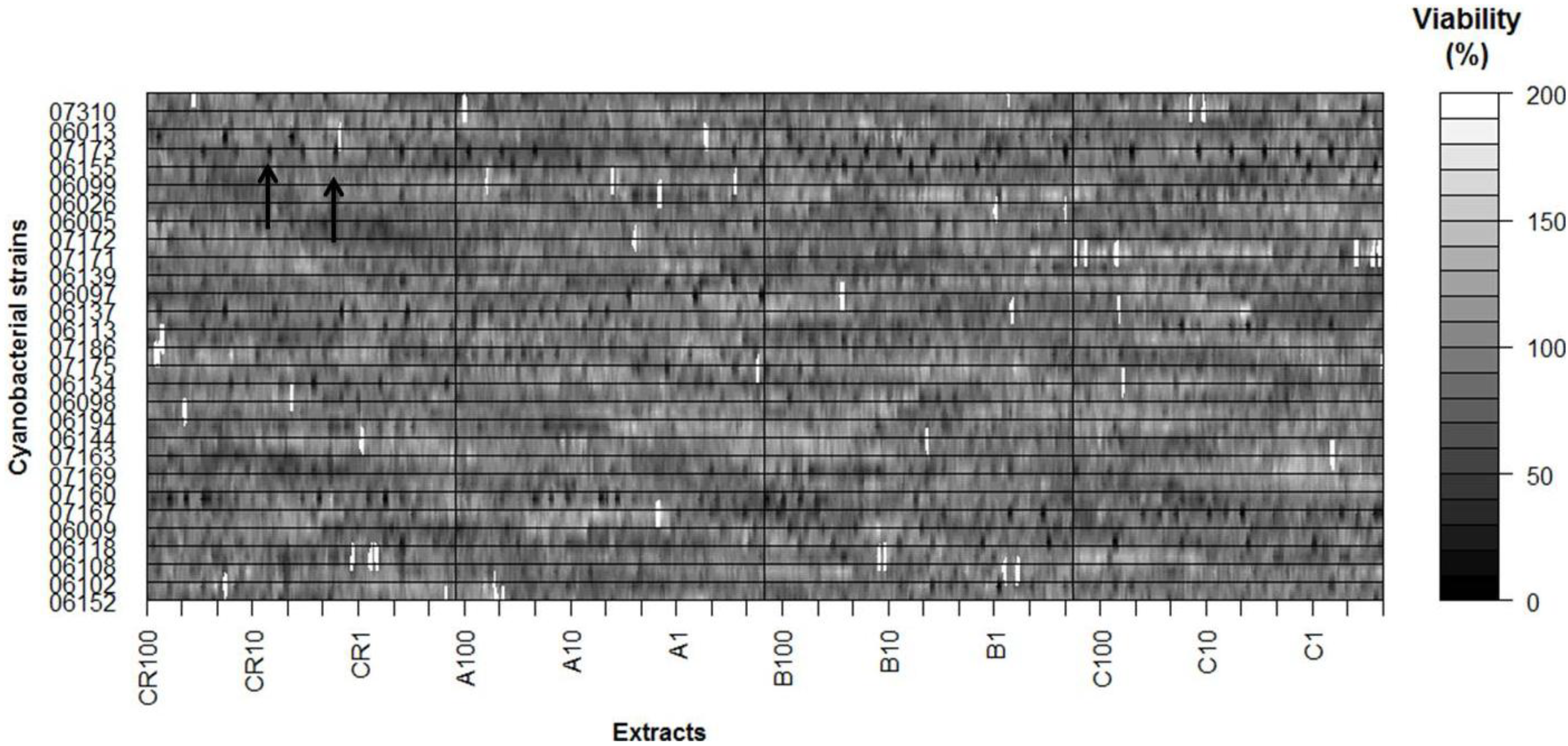

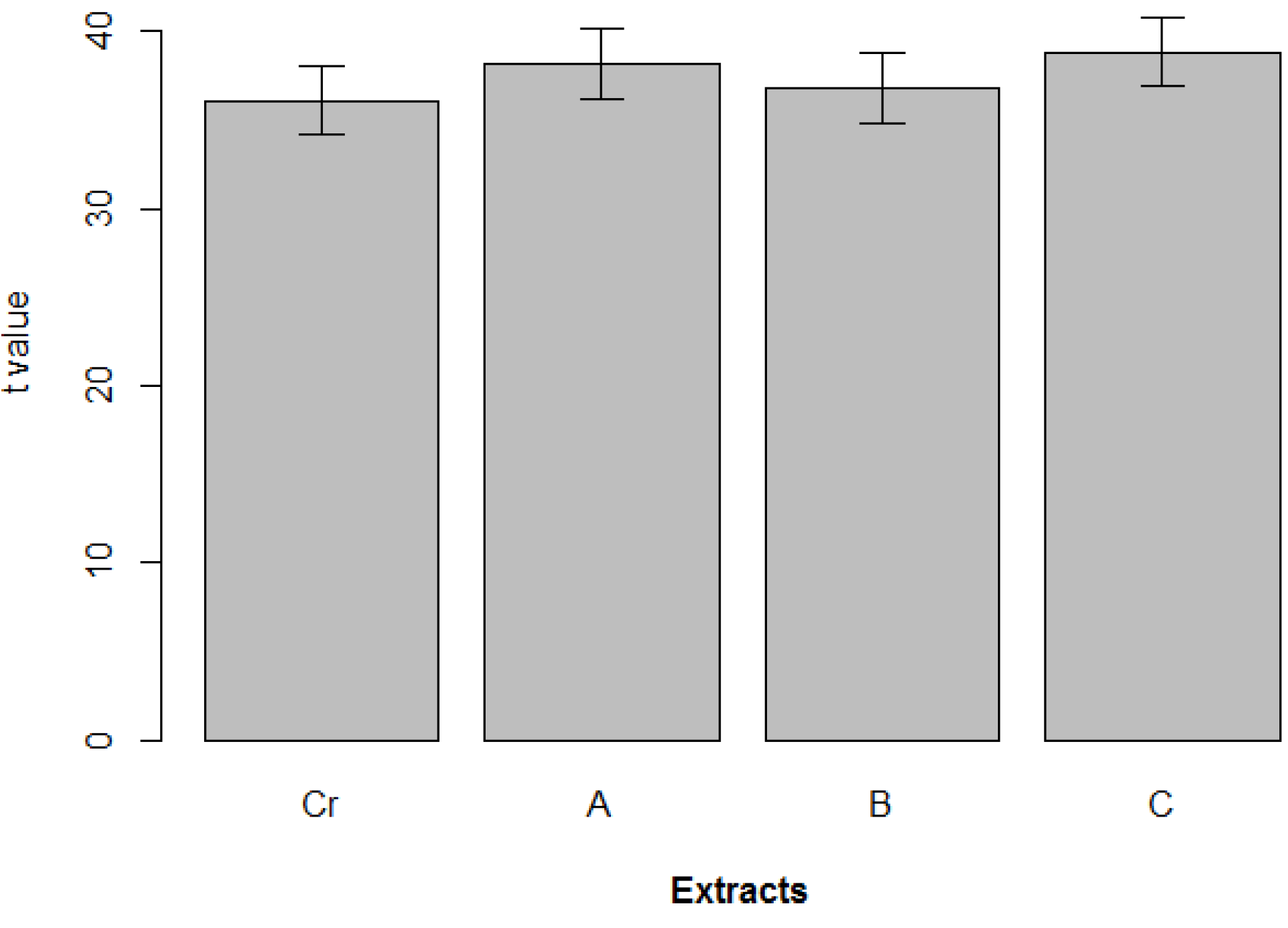
| Cell line | Strain | MTT | LDH |
|---|---|---|---|
| HepG2 | LEGE 06155 | B | B |
| LEGE 06099 | A | A | |
| LEGE 06102 | A | A | |
| LEGE 06113 | B | A | |
| LEGE 06144 | Crude | - | |
| HT-29 | LEGE 07169 | C | A |
| LEGE 06009 | Crude | - | |
| LEGE 06152 | B | B | |
| MG-63 | LEGE 07169 | B | - |
| LEGE 07167 | Crude, A, B, C | - | |
| PC-3 | LEGE 07169 | A | - |
| LEGE 06009 | A | A | |
| LEGE 06152 | Crude, A, B, C | - | |
| RKO | LEGE 06155 | B | B |
| SH-SY5Y | LEGE 07167 | B | - |
| T47D | LEGE 07167 | B | Crude |
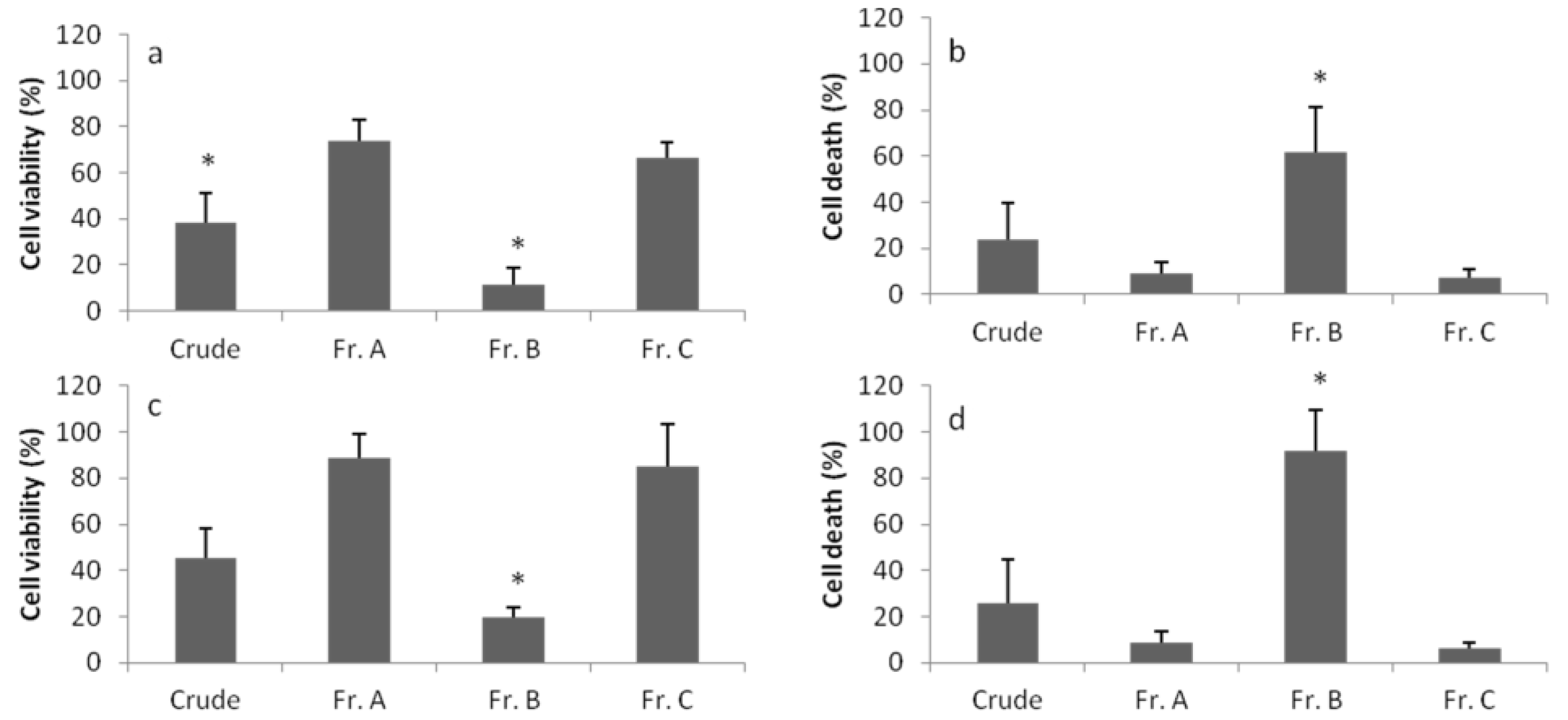
3. Discussion
4. Experimental Section
4.1. Cyanobacteria Strains and Culture
| Taxon | Code | Sampling Location | Accession Number | Reference | Medium |
|---|---|---|---|---|---|
| Nodosilinea nodulosa | LEGE 06152 | Lavadores (4) | HQ832915 | [31] | Z8 |
| Leptolyngbya cf. halophila | LEGE 06102 | S. Bartolomeu do Mar (2) | HQ832906 | [43] | Z8 |
| Leptolyngbya mycoidea | LEGE 06108 | Luz (11) | HQ832942 | [43] | Z8 |
| Leptolyngbya mycoidea | LEGE 06118 | Luz (11) | HQ832943 | [43] | Z8 |
| Leptolyngbya mycoidea | LEGE 06009 | Foz do Arelho (7) | JF708121 | [43] | Z8 |
| Leptolyngbya fragilis | LEGE 07167 | Lavadores (4) | HQ832917 | [43] | MN |
| Pseudanabaena aff. curta | LEGE 07160 | Olhos d’Água (12) | HQ832948 | [43] | MN |
| Pseudanabaena aff. curta | LEGE 07169 | Aguda (5) | HQ832923 | [43] | MN |
| Pseudanabaena aff. persicina | LEGE 07163 | Moledo (1) | HQ832900 | [43] | MN |
| Pseudanabaena sp. | LEGE 06144 | Burgau (10) | HQ832937 | [43] | MN |
| Pseudanabaena sp. | LEGE 06194 | Luz (11) | - | - | MN |
| Cyanobium sp. | LEGE 06098 | Martinhal (9) | KC469572 | [44] | Z8 |
| Cyanobium sp. | LEGE 06134 | Moledo (1) | KC469573 | [44] | Z8 |
| Cyanobium sp. | LEGE 07175 | Martinhal (9) | KC469575 | [44] | Z8 |
| Cyanobium sp. | LEGE 07186 | Martinhal (9) | KC469576 | [44] | Z8 |
| Cyanobium sp. | LEGE 06113 | Aguda (5) | KC469577 | [45] | Z8 |
| Cyanobium sp. | LEGE 06137 | Lavadores (4) | HQ832914 | [43] | Z8 |
| Cyanobium sp. | LEGE 06097 | Martinhal (9) | HQ832928 | [43] | Z8 |
| Cyanobium sp. | LEGE 06139 | Aguda (5) | KC469574 | [44] | Z8 |
| Synechococcus nidulans | LEGE 07171 | Burgau (10) | HQ832939 | [43] | Z8 |
| Synechococcus sp. | LEGE 07172 | Olhos d’Água (12) | HQ832950 | [43] | Z8 |
| Synechococcus sp. | LEGE 06005 | São Pedro de Moel (6) | HM124558 | [37] | Z8 |
| Synechococcus sp. | LEGE 06026 | Empa (8) | - | - | Z8 |
| Synechocystis salina | LEGE 06099 | Moledo (1) | HQ832895 | [43] | Z8 |
| Synechocystis salina | LEGE 06155 | S. Bartolomeu do Mar (2) | HQ832911 | [43] | Z8 |
| Synechocystis salina | LEGE 07173 | Olhos d’Água (12) | HQ832951 | [43] | Z8 |
| Romeria sp. | LEGE 06013 | Foz do Arelho (7) | HQ832927 | [43] | Z8 |
| Romeria aff. gracilis | LEGE 07310 | Minho estuary (3) | HM217057 | [46] | Z8 |

4.2. Extract Preparation
4.3. Cell Lines
4.4. Cytotoxicity Assays
4.4.1. MTT Assay
4.4.2. LDH Release Assay
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Nagarajan, M.; Maruthanayagam, V.; Sundararaman, M. A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J. Appl. Toxicol. 2012, 32, 153–185. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Vansach, T.; Yoshida, W.Y.; Sakamoto, B.; Porzgen, P.; Horgen, F.D. Halogenated fatty acid amides and cyclic depsipeptides from an eastern Caribbean collection of the cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2009, 72, 1573–1578. [Google Scholar] [CrossRef]
- Gutierrez, M.; Tidgewell, K.; Capson, T.L.; Engene, N.; Almanza, A.; Schemies, J.; Jung, M.; Gerwick, W.H. Malyngolide dimer, a bioactive symmetric cyclodepside from the panamanian marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 709–711. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.Y.; Tan, L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2011, 72, 2369–2375. [Google Scholar] [CrossRef]
- Malloy, K.L.; Villa, F.A.; Engene, N.; Matainaho, T.; Gerwick, L.; Gerwick, W.H. Malyngamide 2, an oxidized lipopeptide with nitric oxide inhibiting activity from a Papua New Guinea marine cyanobacterium. J. Nat. Prod. 2011, 74, 95–98. [Google Scholar] [CrossRef]
- Mevers, E.; Liu, W.T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef]
- Gutierrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a potent cytotoxic cyclodepsipeptide from papua new guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, cancer cell toxins from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Leal, R.M.; Tinley, T.L.; Luesch, H.; Moore, R.E.; Corbett, T.H. The molecular pharmacology of symplostatin 1: A new antimitotic dolastatin 10 analog. Int. J. Cancer 2003, 104, 512–521. [Google Scholar] [CrossRef]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barria, E.; Gerwick, W.H.; McPhail, K.L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef]
- Rubio, B.K.; Parrish, S.M.; Yoshida, W.; Schupp, P.J.; Schils, T.; Williams, P.G. Depsipeptides from a Guamanian marine cyanobacterium, Lyngbya bouillonii, with Selective Inhibition of Serine Proteases. Tetrahedron Lett. 2010, 51, 6718–6721. [Google Scholar]
- Chen, X.X.; Smith, G.D.; Waring, P. Human cancer cell (Jurkat) killing by the cyanobacterial metabolite calothrixin A. J. Appl. Phycol. 2003, 15, 269–277. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L. Isolation, structure determination, and biological activity of Lyngbyabellin A from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 611–615. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Ou, X.L.; Adil, M.R.; Rosati, R.; Khoulani, M.M.; Madan, S.K.; Pettit, G.R. Activity of dolastatin 10 against small-cell lung cancer in vitro and in vivo: Induction of apoptosis and bcl-2 modification. Cancer Chemother. Pharmacol. 1999, 43, 507–515. [Google Scholar] [CrossRef]
- LePage, K.T.; Goeger, D.; Yokokawa, F.; Asano, T.; Shioiri, T.; Gerwick, W.H.; Murray, T.F. The neurotoxic lipopeptide kalkitoxin interacts with voltage-sensitive sodium channels in cerebellar granule neurons. Toxicol. Lett. 2005, 158, 133–139. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Coates, R.; Engene, N.; Gerwick, L.; Grindberg, R.; Jones, A.C.; Sorrels, C.M. Giant marine cyanobacteria produce exciting potential pharmaceuticals. Microbe 2008, 3, 8. [Google Scholar]
- Engene, N.; Choi, H.; Esquenazi, E.; Rottacker, E.C.; Ellisman, M.H.; Dorrestein, P.C.; Gerwick, W.H. Underestimated biodiversity as a major explanation for the perceived rich secondary metabolite capacity of the cyanobacterial genus Lyngbya. Environ. Microbiol. 2011, 13, 1601–1610. [Google Scholar] [CrossRef]
- Engene, N.; Rottacker, E.C.; Kastovsky, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komarek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171–1178. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a cyclic depsipeptide from a Palmyra Atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef]
- McPhail, K.L.; Correa, J.; Linington, R.G.; Gonzalez, J.; Ortega-Barria, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef]
- Matthew, S.; Paul, V.J.; Luesch, H. Largamides A–C, tiglic acid-containing cyclodepsipeptides with elastase-inhibitory activity from the marine cyanobacterium Lyngbya confervoides. Planta Med. 2009, 75, 528–533. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Thimmaiah, M.; Shaala, L.A.; Hau, A.M.; Malmo, J.M.; Ishmael, J.E.; Youssef, D.T.; McPhail, K.L. Cyclic depsipeptides, grassypeptolides D and E and Ibu-epidemethoxylyngbyastatin 3, from a Red Sea Leptolyngbya cyanobacterium. J. Nat. Prod. 2011, 74, 1677–1685. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Kitamura, K.; Nakayama, T.; Suenaga, K. Biselyngbyaside, a macrolide glycoside from the marine cyanobacterium Lyngbya sp. Org. Lett. 2009, 11, 2421–2424. [Google Scholar] [CrossRef]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8, 8. [Google Scholar] [CrossRef]
- Vaucher, R.A.; da Motta Ade, S.; Brandelli, A. Evaluation of the in vitro cytotoxicity of the antimicrobial peptide P34. Cell Biol. Int. 2010, 34, 317–323. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, S.C.; Lee, T.Y.; Jeong, D. Discriminative cytotoxicity assessment based on various cellular damages. Toxicol. Lett. 2009, 184, 13–17. [Google Scholar] [CrossRef]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Garcia-Blairsy Reina, G.; Herrero, M.; Senorans, F.J.; Ibanez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Leao, P.N.; Ramos, V.; Goncalves, P.B.; Viana, F.; Lage, O.M.; Gerwick, W.H.; Vasconcelos, V.M. Chemoecological screening reveals high bioactivity in diverse culturable portuguese marine cyanobacteria. Mar. Drugs 2013, 11, 1316–1335. [Google Scholar] [CrossRef]
- Hrouzek, P.; Tomek, P.; Lukesova, A.; Urban, J.; Voloshko, L.; Pushparaj, B.; Ventura, S.; Lukavsky, J.; Stys, D.; Kopecky, J. Cytotoxicity and secondary metabolites production in terrestrial Nostoc strains, originating from different climatic/geographic regions and habitats: Is their cytotoxicity environmentally dependent? Environ. Toxicol. 2011, 26, 345–358. [Google Scholar] [CrossRef]
- Mian, P.; Heilmann, J.; Burgi, H.R.; Sticher, O. Biological screening of terrestrial and freshwater cyanobacteria for antimicrobial activity, brine shrimp lethality, and cytotoxicity. Pharm. Biol. 2003, 41, 243–247. [Google Scholar] [CrossRef]
- Surakka, A.; Sihvonen, L.M.; Lehtimaki, J.M.; Wahlsten, M.; Vuorela, P.; Sivonen, K. Benthic cyanobacteria from the Baltic Sea contain cytotoxic Anabaena, Nodularia, and Nostoc strains and an apoptosis-inducing Phormidium strain. Environ. Toxicol. 2005, 20, 285–292. [Google Scholar] [CrossRef]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a cytotoxic peptolide from a guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116. [Google Scholar] [CrossRef]
- Martins, R.; Pereira, P.; Welker, M.; Fastner, J.; Vasconcelos, V.M. Toxicity of culturable cyanobacteria strains isolated from the Portuguese coast. Toxicon 2005, 46, 454–464. [Google Scholar] [CrossRef]
- Frazao, B.; Martins, R.; Vasconcelos, V. Are known cyanotoxins involved in the toxicity of picoplanktonic and filamentous North Atlantic marine cyanobacteria? Mar. Drugs 2010, 8, 1908–1919. [Google Scholar] [CrossRef]
- Martins, R.; Fernandez, N.; Beiras, R.; Vasconcelos, V. Toxicity assessment of crude and partially purified extracts of marine Synechocystis and Synechococcus cyanobacterial strains in marine invertebrates. Toxicon 2007, 50, 791–799. [Google Scholar] [CrossRef]
- Martins, R.F.; Ramos, M.F.; Herfindal, L.; Sousa, J.A.; Skaerven, K.; Vasconcelos, V.M. Antimicrobial and cytotoxic assessment of marine cyanobacteria—Synechocystis and Synechococcus. Mar. Drugs 2008, 6, 1–11. [Google Scholar] [CrossRef]
- Lopes, V.R.; Schmidtke, M.; Fernandes, M.H.; Martins, R.; Vasconcelos, V. Cytotoxicity in L929 fibroblasts and inhibition of herpes simplex virus type 1 Kupka by estuarine cyanobacteria extracts. Toxicol. In Vitro 2011, 25, 944–950. [Google Scholar] [CrossRef]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae. NIVA B-11/69. NIVA 1976. Estimation of Algal Growth Potential; Norwegian Institute for Water Research: Oslo, Norway, 1972. [Google Scholar]
- Rippka, R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar]
- Brito, A.; Ramos, V.; Seabra, R.; Santos, A.; Santos, C.L.; Lopo, M.; Ferreira, S.; Martins, A.; Mota, R.; Frazao, B. Culture-dependent characterization of cyanobacterial diversity in the intertidal zones of the Portuguese coast: A polyphasic study. Syst. Appl. Microbiol. 2012, 35, 110–119. [Google Scholar] [CrossRef]
- Costa, M.S.; Costa, M.; Ramos, V.; Leão, P.N.; Barreiro, A.; Vasconcelos, V.; Martins, R.; Interdisciplinary Center of Marine and Environmental Research (CIIMAR/CIMAR), University of Porto, Porto, Portugal. Toxicity of Picocyanobacteria Isolates from a Clade of Marine. Cyanobium, 2013; unpublished work. [Google Scholar]
- Leao, P.N.; Costa, M.; Ramos, V.; Pereira, A.R.; Fernandes, V.C.; Domingues, V.F.; Gerwick, W.H.; Vasconcelos, V.M.; Martins, R. Antitumor activity of hierridin B, a cyanobacterial secondary metabolite found in both filamentous and unicellular marine strains. PLoS One 2013, 8, e69562. [Google Scholar] [CrossRef]
- Lopes, V.R.; Ramos, V.; Martins, A.; Sousa, M.; Welker, M.; Antunes, A.; Vasconcelos, V.M. Phylogenetic, chemical and morphological diversity of cyanobacteria from Portuguese temperate estuaries. Mar. Environ. Res. 2012, 73, 7–16. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Costa, M.; Garcia, M.; Costa-Rodrigues, J.; Costa, M.S.; Ribeiro, M.J.; Fernandes, M.H.; Barros, P.; Barreiro, A.; Vasconcelos, V.; Martins, R. Exploring Bioactive Properties of Marine Cyanobacteria Isolated from the Portuguese Coast: High Potential as a Source of Anticancer Compounds. Mar. Drugs 2014, 12, 98-114. https://doi.org/10.3390/md12010098
Costa M, Garcia M, Costa-Rodrigues J, Costa MS, Ribeiro MJ, Fernandes MH, Barros P, Barreiro A, Vasconcelos V, Martins R. Exploring Bioactive Properties of Marine Cyanobacteria Isolated from the Portuguese Coast: High Potential as a Source of Anticancer Compounds. Marine Drugs. 2014; 12(1):98-114. https://doi.org/10.3390/md12010098
Chicago/Turabian StyleCosta, Margarida, Mónica Garcia, João Costa-Rodrigues, Maria Sofia Costa, Maria João Ribeiro, Maria Helena Fernandes, Piedade Barros, Aldo Barreiro, Vitor Vasconcelos, and Rosário Martins. 2014. "Exploring Bioactive Properties of Marine Cyanobacteria Isolated from the Portuguese Coast: High Potential as a Source of Anticancer Compounds" Marine Drugs 12, no. 1: 98-114. https://doi.org/10.3390/md12010098





