Parathyrsoidins A–D, Four New Sesquiterpenoids from the Soft Coral Paralemnalia thyrsoides
Abstract
:1. Introduction
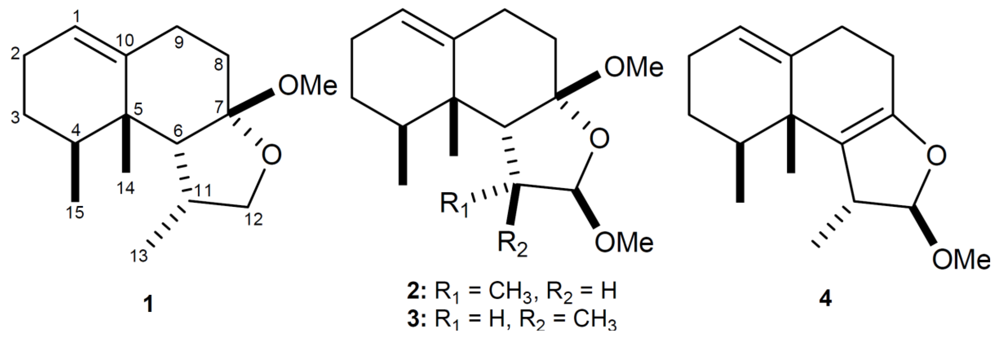
2. Results and Discussion
| Position | 1 | 4 | ||
|---|---|---|---|---|
| δH a | δC b | δH a | δC b | |
| 1 | 5.28 brd (5.2) c | 120.7, CH d | 5.29 brs | 120.9, CH |
| 2 | 1.89 m, 1.94 m | 26.0, CH2 | 1.82 m, 1.88 m | 25.7, CH2 |
| 3α 3β | 1.28 m
1.42 qd (12.0, 6.0) | 27.2, CH2 | 1.28 m
1.37 qd (13.2, 6.4) | 28.9, CH2 |
| 4 | 1.78 dqd (12.0, 6.8, 3.2) | 35.0, CH | 1.71 dqd (13.2, 6.4, 3.6) | 37.8, CH |
| 5 | 38.6, qC | 39.2, qC | ||
| 6 | 2.32 d (6.0) | 52.7, CH | 118.8, qC | |
| 7 | 107.5, qC | 150.1, qC | ||
| 8α 8β | 1.81 td (13.6, 5.2)
2.31 dt (13.6, 5.2,) | 35.3, CH2 | 2.19 m | 26.4, CH2 |
| 9α 9β | 1.98 ddd (13.6, 5.2, 1.2)
2.52 tdq (13.6, 5.2, 2.4) | 29.2, CH2 | 1.95 ddd (12.8, 6.0, 1.2)
2.45 m | 29.9, CH2 |
| 10 | 141.9, qC | 142.7, qC | ||
| 11 | 1.98 qdd (7.2, 6.0, 4.0) | 36.7, CH | 2.99 qd (7.2, 1.2) | 46.4, CH |
| 12α 12β | 3.40 d (8.0)
3.78 dd (8.0, 4.0) | 74.1, CH2 | 4.82 d (1.2) | 112.5, CH |
| 13 | 1.03 d (7.2) | 15.9, CH3 | 1.12 d (7.2) | 19.6, CH3 |
| 14 | 1.19 s | 21.8, CH3 | 1.10 s | 21.5, CH3 |
| 15 | 0.80 d (6.8) | 16.4, CH3 | 0.95 d (6.4) | 18.9, CH3 |
| OMe-7 OMe-12 | 3.26 s | 48.5, CH3 | ||
| 3.28 s | 54.8, CH3 | |||
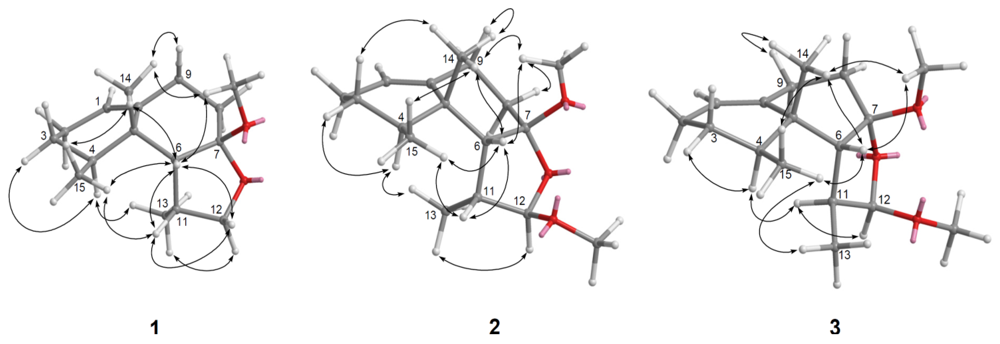
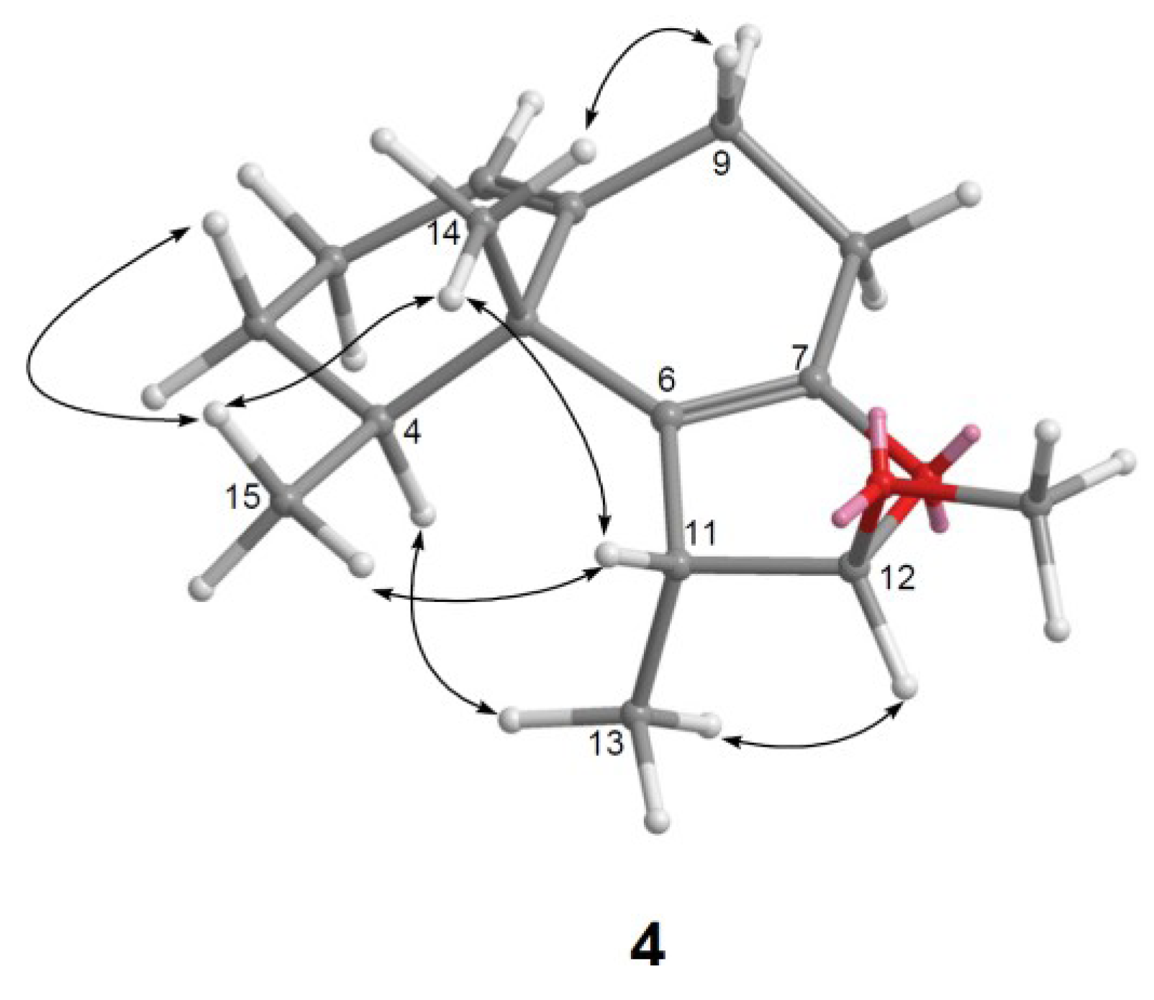
 ) and HMBC (
) and HMBC (  ) correlations of 1–4.
) correlations of 1–4.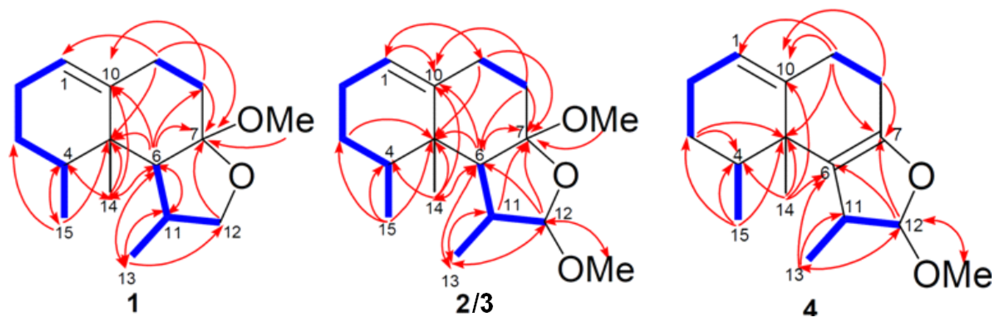
| Position | 2 | 3 | ||
|---|---|---|---|---|
| δH a | δC b | δH a | δC b | |
| 1 | 5.56 t (2.8) c | 120.8, CH | 5.42 brs | 122.7, CH d |
| 2 | 1.84 m, 1.90 m | 26.1, CH2 | 1.90 m, 1.96 m | 25.7, CH2 |
| 3α 3β | 1.23 m
1.35 qd (12.0, 6.0) | 27.1, CH2 | 1.29 m 1.47 qd (13.2, 7.2) | 27.1, CH2 |
| 4 | 1.74 dqd (12.0, 6.8, 3.2) | 35.2, CH | 1.71 dqd (13.2, 6.8, 3.2) | 35.3, CH |
| 5 | 38.4, qC | 40.3, qC | ||
| 6 | 2.86 dd (6.8, 2.4) | 45.8, CH | 2.40 d (10.8) | 53.6, CH |
| 7 | 109.6, qC | 111.9, qC | ||
| 8α 8β | 1.81 td (14.4, 5.2)
2.38 ddt (14.4, 5.6, 2.4) | 38.0, CH2 | 1.95 m | 32.4, CH2 |
| 9α 9β | 1.92 ddd (14.4,5.2, 2.4)
2.62 tdq (14.4, 5.6, 2.4) | 29.7, CH2 | 2.33 m 2.21 tdq (14.0, 5.6, 1.6) | 27.4, CH2 |
| 10 | 142.0, qC | 140.2, qC | ||
| 11 | 2.31 quin (6.8) | 42.0, CH | 1.83 dqd (10.8, 7.2, 5.6) | 42.1, CH |
| 12 | 4.37 s | 109.0, CH | 4.54 d (5.6) | 105.9, CH |
| 13 | 1.00 d (6.8) | 14.2, CH3 | 1.15 d (7.2) | 15.6, CH3 |
| 14 | 1.17 s | 21.0, CH3 | 1.16 s | 21.9, CH3 |
| 15 | 0.83 d (6.8) | 16.2, CH3 | 0.75 d (6.8) | 16.3, CH3 |
| OMe-7 OMe-12 | 3.33 s
3.23 s | 48.7, CH3 54.3, CH3 | 3.32 s 3.27 s | 48.9, CH3 54.5, CH3 |
| Compound | A-549 | HT-29 | P-388 |
|---|---|---|---|
| 1 | >20 | >20 | 7.95 |
| 2 | >20 | >20 | 13.2 |
| 3 | >20 | >20 | 3.63 |
| 4 Mithramycin | >20 0.17 | >20 0.19 | 2.32 0.14 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.4. Cytotoxicity Testing
3.5. Anti-HCMV Assay
4. Conclusions
Acknowledgements
References
- Su, J.-Y.; Zhong, Y.-L.; Zeng, L.-M. Two new sesquiterpenoids from the soft coral Paralemnalia thyrsoides. J. Nat. Prod. 1993, 56, 288–291. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J. Studies of Australian soft corals. XIX. Two new sesquiterpenes with the nardosinane skeleton from a Paralemnalia species. Aust. J. Chem. 1980, 33, 885–890. [Google Scholar] [CrossRef]
- Izac, R.R.; Schneider, P.; Swain, M.; Fenical, W. New nor-sesquiterpenoids of apparent nardosinane origin from the pacific soft-coral Paralemnalia thyrsoides. Tetrahedron Lett. 1982, 23, 817–820. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chao, C.-H.; Su, J.-H.; Hsu, C.-H.; Chen, S.-P.; Kuo, Y.-H.; Sheu, J.-H. Neolemnane-type sesquiterpenoids from a Formosan soft coral Paralemnalia thyrsoides. Chem. Pharm. Bull. 2007, 55, 876–880. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Su, J.-H.; Chen, B.-W.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Sheu, J.-H.; Sung, P.-J. Nardosinane-type sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Mar. Drugs 2011, 9, 1543–1553. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Lin, E.-H.; Huang, J.-S.; Wen, Z.-H.; Duh, C.-Y. Ylangene-type and Nardosinane-type sesquiterpenoids from the soft corals Lemnalia flava and Paralemnalia thyrsoides. Chem. Pharm. Bull. 2010, 58, 381–385. [Google Scholar] [CrossRef]
- Bishara, A.; Yeffet, D.; Sisso, M.; Shmul, G.; Schleyer, M.; Benayahu, Y.; Rudi, A.; Kashman, Y. Nardosinanols A–I and lemnafricanol, sesquiterpenes from several soft corals, Lemnalia sp., Paralemnalia clavata, Lemnalia africana, and Rhytisma fulvum fulvum. J. Nat. Prod. 2008, 71, 375–380. [Google Scholar]
- Zeng, L.-M.; Zhong, Y.-L.; Su, J.-Y.; Zhao, D. Sesquiterpenes from the soft coral Paralemnalia thyrsoides and their biogenetic correlation. Chin. Sci. Bull. 1995, 40, 213–216. [Google Scholar]
- Wang, G.-H.; Huang, H.-C.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Paralemnolins J–P, new sesquiterpenoids from the soft coral Paralemnalia thyrsoides. Chem. Pharm. Bull. 2010, 58, 30–33. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chao, C.-H.; Liao, J.-H.; Chiang, M.Y.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. A novelchlorinated norsesquiterpenoid and two related new metabolites from the soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2005, 46, 7711–7714. [Google Scholar]
- Huang, H.-C.; Wen, Z.-H.; Chao, C.-H.; Ahmed, A.F.; Chiang, M.Y.; Kuo, Y.-H.; Hsu, C.-H.; Chen, S.-P.; Sheu, J.-H. Novel sesquiterpenoids from the Formosan soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2006, 47, 8751–8755. [Google Scholar] [CrossRef]
- Daloze, D.; Braekman, J.C.; Georget, P.; Tursch, B. Chemical studies of marine invertebrates. XXII. Two novel sesquiterpenes from soft corals of the genera Lemnalia and Paralemnalia. Bull. Soc. Chim. Belg. 1977, 86, 47–54. [Google Scholar]
- Wang, S.-K.; Lee, Y.-S.; Duh, C.-Y. Paralemnolide A, an unprecedented bisnorsesquiterpene from the Taiwanese soft coral Paralemnalia thyrsoides. Mar. Drugs 2012, 10, 1528–1535. [Google Scholar] [CrossRef]
- Kapojos, M.M.; Mangindaan, R.E.P.; Nakazawa, T.; Oda, T.; Ukai, K.; Namikoshi, M. Three new nardosinane type sesquiterpenes from an Indonesian soft coral Nephthea sp. Chem. Pharm. Bull. 2008, 56, 332–334. [Google Scholar] [CrossRef]
- Hou, R.-S.; Duh, C.-Y.; Chiang, M.Y.; Lin, C.-N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; MacDonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological syatems. Cancer Chemother. Rep. 1972, 3, 1–91. [Google Scholar]
- Stevens, M.; Balzarini, J.; Tabarrini, O.; Andrei, G.; Snoeck, R.; Cecchetti, V.; Fravolini, A.; de Clercq, E.; Pannecouque, C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother. 2005, 56, 847–855. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tseng, Y.-J.; Lee, Y.-S.; Wang, S.-K.; Sheu, J.-H.; Duh, C.-Y. Parathyrsoidins A–D, Four New Sesquiterpenoids from the Soft Coral Paralemnalia thyrsoides. Mar. Drugs 2013, 11, 2501-2509. https://doi.org/10.3390/md11072501
Tseng Y-J, Lee Y-S, Wang S-K, Sheu J-H, Duh C-Y. Parathyrsoidins A–D, Four New Sesquiterpenoids from the Soft Coral Paralemnalia thyrsoides. Marine Drugs. 2013; 11(7):2501-2509. https://doi.org/10.3390/md11072501
Chicago/Turabian StyleTseng, Yen-Ju, Yu-Sheng Lee, Shang-Kwei Wang, Jyh-Horng Sheu, and Chang-Yih Duh. 2013. "Parathyrsoidins A–D, Four New Sesquiterpenoids from the Soft Coral Paralemnalia thyrsoides" Marine Drugs 11, no. 7: 2501-2509. https://doi.org/10.3390/md11072501




