Effect of Grazing-Mediated Dimethyl Sulfide (DMS) Production on the Swimming Behavior of the Copepod Calanus helgolandicus
Abstract
:1. Introduction
2. Experimental Section
2.1. Cultures
2.2. Tethering
2.3. Filming Set-Up
2.4. Treatments
2.5. DMS Quantification
2.6. Behavioral Analysis

2.7. Statistical Analyses
3. Results and Discussion
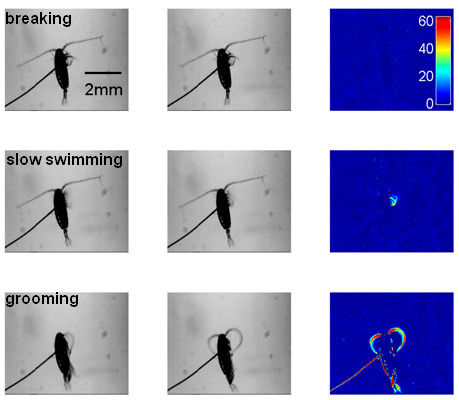
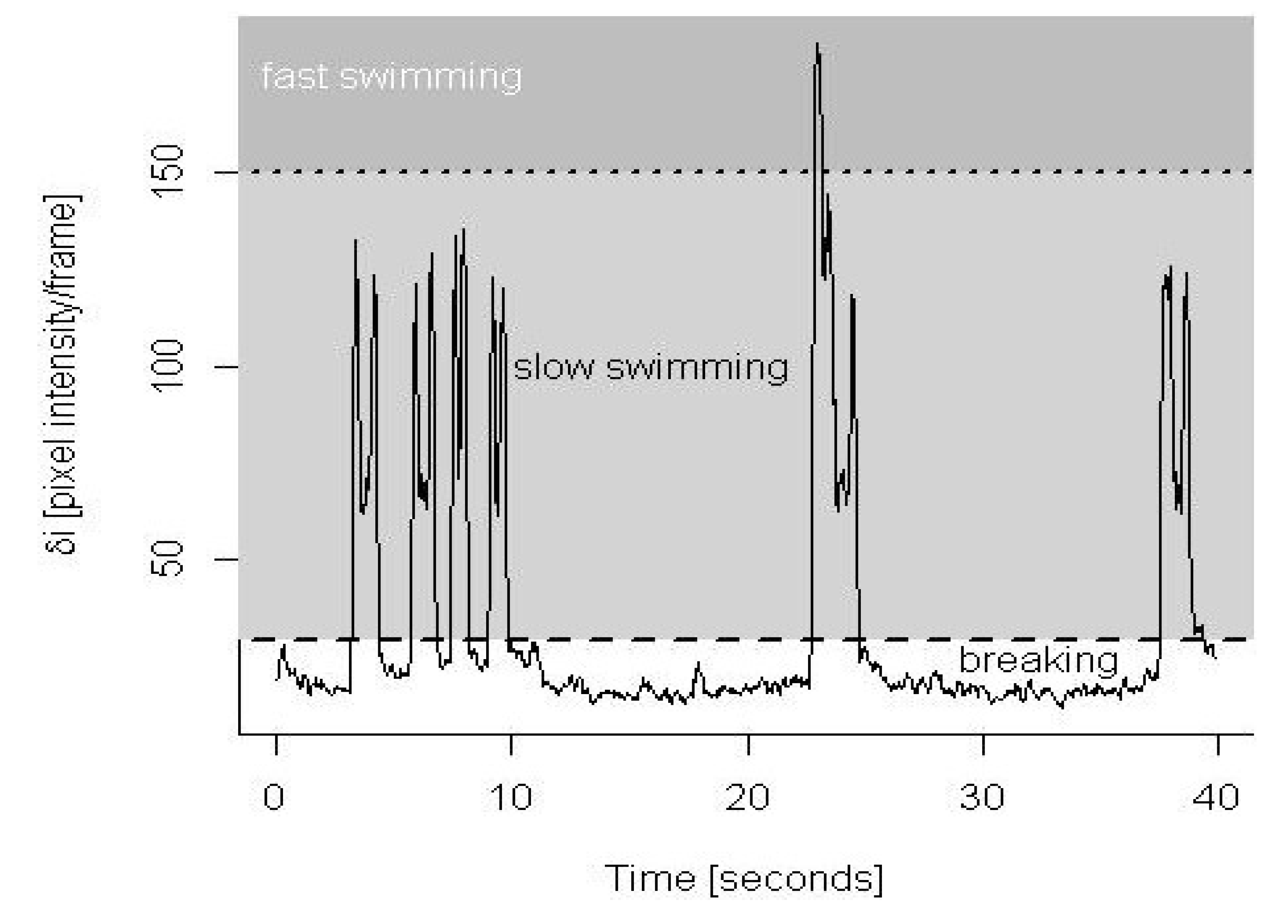
3.1. Validation of Behavioral Analysis
3.2. DMS Quantification
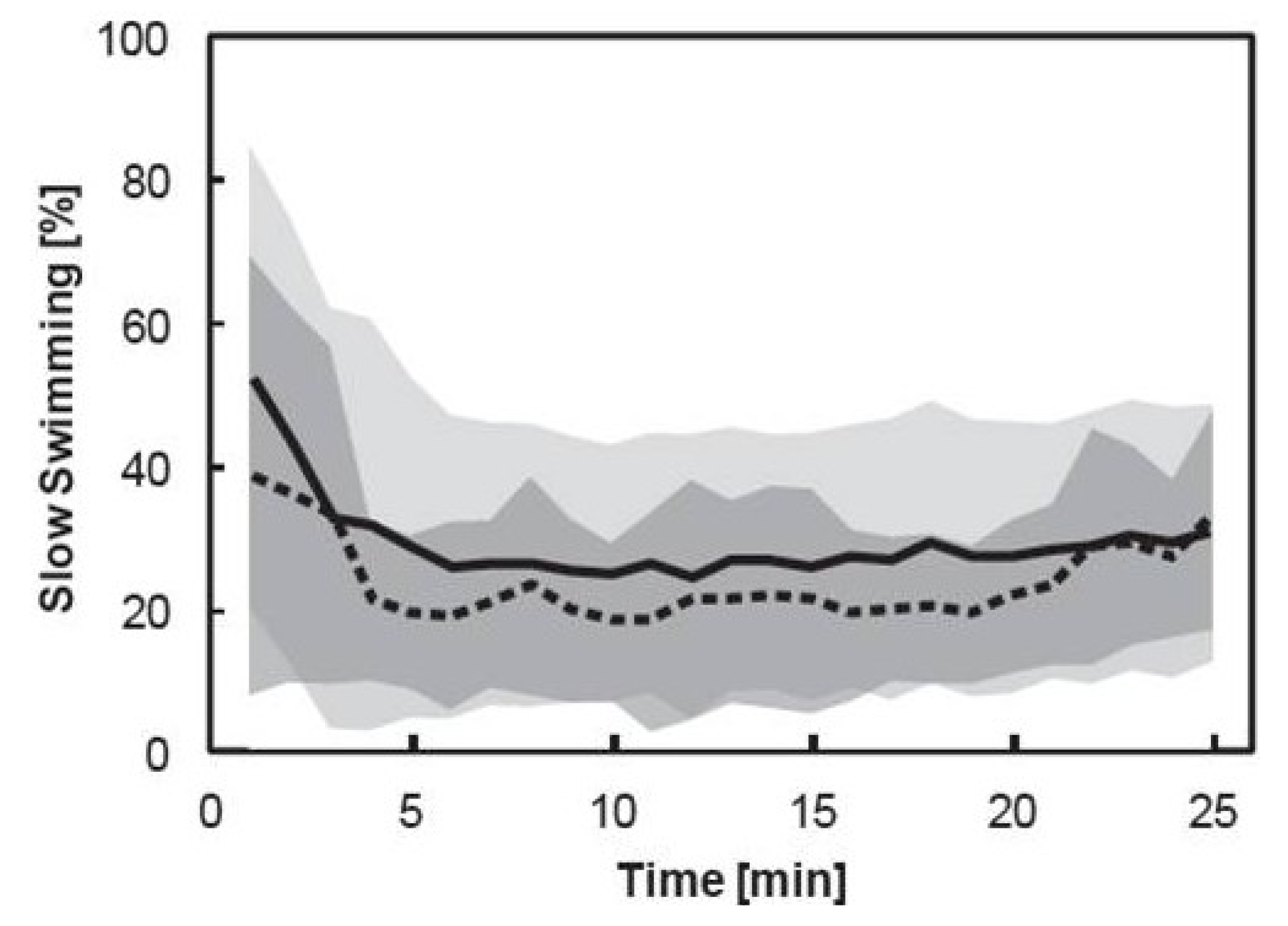
3.3. Pooled Copepod Behavior
| d.f. | MS | F | p | |
|---|---|---|---|---|
| DMS | 1 | 0.727 | 2.11 | 0.177 |
| Error (DMS) | 10 | 0.354 | ||
| Time | 24 | 0.112 | 3.88 | <0.001 |
| Error (Time) | 240 | 0.029 | ||
| DMS × Time | 24 | 0.015 | 1.45 | 0.087 |
| Error (DMS × Time) | 240 | 0.100 |
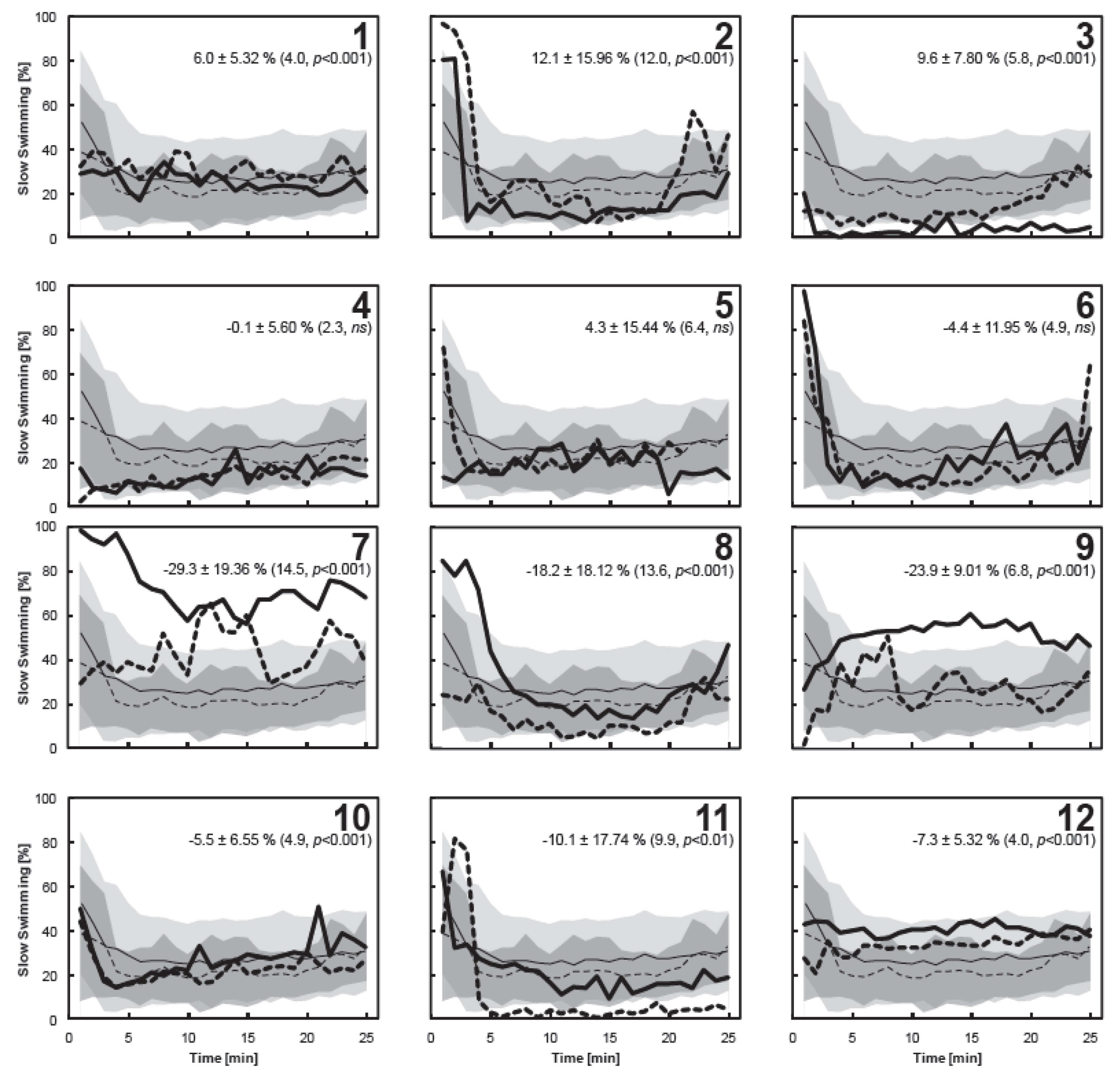
3.4. Individual Copepod Behavior
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Hay, M.E. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193. [Google Scholar] [CrossRef]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engström-Öst, J.; Halsband, C.; Sonnenschein, E.; Legrand, C.; Llewellyn, C.A. The relevance of marine chemical ecology to plankton and ecosystem function: An emerging field. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef] [Green Version]
- Amo, L.; Rodríguez-Gironés, M.Á.; Barbosa, A. Olfactory detection of dimethyl sulphide in a krill-eating Antarctic penguin. Mar. Ecol. Prog. Ser. 2013, 474, 277–285. [Google Scholar] [CrossRef]
- Cunningham, G.B.; Strauss, V.; Ryan, P.G. African penguins (Spheniscus demersus) can detect dimethyl sulphide, a prey-related odour. J. Exp. Biol. 2008, 211, 3123–3127. [Google Scholar] [CrossRef]
- Kowalewsky, S.; Dambach, M.; Mauck, B.; Dehnhardt, G. High olfactory sensitivity for dimethyl sulphide in harbour seals. Biol. Lett. 2006, 2, 106–109. [Google Scholar] [CrossRef]
- Nevitt, G.A.; Veit, R.R.; Kareiva, P. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature 1995, 376, 680–682. [Google Scholar] [CrossRef]
- Steinke, M.; Stefels, J.; Stamhuis, E. Dimethyl sulfide triggers search behavior in copepods. Limnol. Oceanogr. 2006, 51, 1925–1930. [Google Scholar] [CrossRef]
- Yang, E.J.; Kang, H.-K.; Yoo, S.; Hyun, J.-H. Contribution of auto-and heterotrophic protozoa to the diet of copepods in the Ulleung Basin, East Sea/Japan Sea. J. Plankton Res. 2009, 31, 647–659. [Google Scholar] [CrossRef]
- Fileman, E.; Petropavlovsky, A.; Harris, R. Grazing by the copepods Calanus helgolandicus and Acartia clausi on the protozooplankton community at station L4 in the Western English Channel. J. Plankton Res. 2010, 32, 709–724. [Google Scholar] [CrossRef]
- Lewis, N.D.; Breckels, M.N.; Archer, S.D.; Morozov, A.; Pitchford, J.W.; Steinke, M.; Codling, E.A. Grazing-induced production of DMS can stabilize food-web dynamics and promote the formation of phytoplankton blooms in a multitrophic plankton model. Biogeochemistry 2012, 110, 303–313. [Google Scholar] [CrossRef]
- Ferrer, R.P.; Zimmer, R.K. Molecules of keystone significance: Crucial agents in ecology and resource management. BioScience 2013, 63, 428–438. [Google Scholar] [CrossRef]
- Steinke, M.; Malin, G.; Liss, P.S. Trophic interactions in the sea: An ecological role for climate relevant volatiles? J. Phycol. 2002, 38, 630–638. [Google Scholar] [CrossRef]
- Widdicombe, C.E.; Eloire, D.; Harbour, D.; Harris, R.P.; Somerfield, P.J. Long-term phytoplankton community dynamics in the Western English Channel. J. Plankton Res. 2010, 32, 643–655. [Google Scholar] [CrossRef]
- Huskin, I.; Anadon, R.; Alvarez-Marques, F.; Harris, R.P. Ingestion, faecal pellet and egg production rates of Calanus helgolandicus feeding coccolithophorid vs. non-coccolithophorid diets. J. Exp. Mar. Biol. Ecol. 2000, 248, 239–254. [Google Scholar] [CrossRef]
- Hansen, F.C.; Reckermann, M.; Klein Breteler, W.C.; Riegman, R. Phaeocystis blooming enhanced by copepod predation on protozoa: Evidence from incubation experiments. Mar. Ecol. Prog. Ser. 1993, 102, 51–57. [Google Scholar] [CrossRef]
- Wolfe, G.V.; Steinke, M. Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnol. Oceanogr. 1996, 41, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Archer, S.D.; Stelfox-Widdicombe, C.E.; Burkill, P.H.; Malin, G. A dilution approach to quantify the production of dissolved dimethylsulphoniopropionate and dimethyl sulphide due to microzooplankton herbivory. Aquat. Microb. Ecol. 2001, 23, 131–145. [Google Scholar] [CrossRef]
- Costello, J.H.; Strickler, J.R.; Marrasé, C.; Trager, G.; Zeller, R.; Freise, A.J. Grazing in a turbulent environment: Behavioral response of a calanoid copepod, Centropages hamatus. Proc. Natl. Acad. Sci. USA 1990, 87, 1648–1652. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Costello, J.H.; Strickler, J.R. Copepod grazing in turbulent flow: Elevated foraging behavior and habituation of escape responses. J. Plankton Res. 1994, 16, 421–431. [Google Scholar] [CrossRef]
- Cowles, T.J.; Strickler, J.R. Characterization of feeding activity patterns in the planktonic copepod Centropages typicus Kroyer under various food conditions. Limnol. Oceanogr. 1983, 28, 106–115. [Google Scholar] [CrossRef]
- Vlymen, W.J. Energy expenditure of swimming copepods. Limnol. Oceanogr. 1970, 15, 348–356. [Google Scholar] [CrossRef]
- Marrasé, C.; Costello, J.H.; Granata, T.; Strickler, J.R. Grazing in a turbulent environment: Energy dissipation, encounter rates, and efficacy of feeding currents in Centropages hamatus. Proc. Natl. Acad. Sci. USA 1990, 87, 1653–1657. [Google Scholar]
- Fields, D.; Yen, J. Outer limits and inner structure: The 3-dimensional flow field of Pleuromamma xiphias (Calanoida: Metridinidae). Bull. Mar. Sci. 1993, 53, 84–95. [Google Scholar]
- Van Duren, L.A.; Stamhuis, E.J.; Videler, J.J. Copepod feeding currents: Flow patterns, filtration rates and energetics. J. Exp. Biol. 2003, 206, 255–267. [Google Scholar] [CrossRef]
- Van Duren, L.A.; Videler, J.J. The trade-off between feeding, mate seeking and predator avoidance in copepods: Behavioural responses to chemical cues. J. Plankton Res. 1996, 18, 805–818. [Google Scholar] [CrossRef]
- Kiørboe, T. How zooplankton feed: Mechanisms, traits and trade-offs. Biol. Rev. 2011, 86, 311–339. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Strickler, R. Can copepods differentiate prey from predator hydromechanically? Zool. Stud. 2001, 40, 1–6. [Google Scholar]
- Gill, C.W.; Harris, R.P. Behavioural responses of the copepods Calanus helgolandicus and Temora longicornis to dinoflagellate diets. J. Mar. Biol. Assoc. UK 1987, 67, 785–801. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Archer, S.D.; Cummings, D.G.; Llewellyn, C.A.; Fishwick, J.R. Phytoplankton taxa, irradiance and nutrient availability determine the seasonal cycle of DMSP in temperate shelf seas. Mar. Ecol. Prog. Ser. 2009, 394, 111–124. [Google Scholar] [CrossRef]
- R Development Core Team, R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2012.
- Mendenhall, W.; Sincich, T. Statistics for Engineering and the Sciences, 5th ed.; Pearson Prentice-Hall: New Jersey, NJ, USA, 2007. [Google Scholar]
- Breckels, M.N. The Infochemical Role of Dimethyl Sulphide (DMS) and Related Compounds in Trophic Interactions. Ph.D. Thesis, The University of Essex, Colchester, UK, July 2010. [Google Scholar]
- Mather, J.A.; Logue, D.M. The Bold and the Spineless: Invertebrate Personalities. In Animal Personalities Behavior, Physiology, and Evolution; Carere, C., Maestripieri, D., Eds.; The University of Chicago Press: Chicago, IL, USA, 2013; pp. 13–35. [Google Scholar]
- Gherardi, F.; Aquiloni, L.; Tricarico, E. Behavioral plasticity, behavioral syndromes and animal personality in crustacean decapods: An imperfect map is better than no map. Curr. Zool. 2012, 58, 567–579. [Google Scholar]
- Dingemanse, N.J.; Wolf, M. Between-individual differences in behavioural plasticity within populations: Causes and consequences. Anim. Behav. 2012, 85, 1031–1039. [Google Scholar] [CrossRef]
- Woodson, C.B.; Webster, D.R.; Weissburg, M.J.; Yen, J. Cue hierarchy and foraging in calanoid copepods: Ecological implications of oceanographic structure. Mar. Ecol. Prog. Ser. 2007, 330, 163–177. [Google Scholar] [CrossRef]
- Svensen, C.; Kiørboe, T. Remote prey detection in Oithona similis: Hydromechanical versus chemical cues. J. Plankton Res. 2000, 22, 1155–1166. [Google Scholar] [CrossRef]
- Strickler, J.R. Calanoid copepods, feeding currents, and the role of gravity. Science 1982, 218, 158–160. [Google Scholar]
- Andrews, J.C. Deformation of the active space in the low Reynolds number feeding current of calanoid copepods. Can. J. Fish. Aquat. Sci. 1983, 40, 1293–1302. [Google Scholar] [CrossRef]
- Yen, J. Life in transition: Balancing inertial and viscous forces by planktonic copepods. Biol. Bull. 2000, 198, 213–224. [Google Scholar] [CrossRef]
- Jiang, H.; Osborn, T.R.; Meneveau, C. Chemoreception and the deformationof the active space in freely swimming copepods: A numerical study. J. Plankton Res. 2002, 24, 495–510. [Google Scholar] [CrossRef]
- Bruno, E.; Andersen Borg, C.M.; Kiørboe, T. Prey detection and prey capture in copepod nauplii. PLoS One 2012, 7, e47906. [Google Scholar]
- Tiselius, P.; Saiz, E.; Kiørboe, T. Sensory capabilities and food capture of two small copepods Paracalanus parvus and Pseudocalanus sp. Limnol. Oceanogr. 2013. [Google Scholar] [CrossRef]
- Poulet, S.A.; Gill, C.W. Spectral analyses of movements made by the cephalic appendages of copepods. Mar. Ecol. Prog. Ser. 1988, 43, 259–267. [Google Scholar] [CrossRef]
- Buskey, E.J. Swimming pattern as an indicator of the roles of copepod sensory systems in the recognition of food. Mar. Biol. 1984, 79, 165–175. [Google Scholar] [CrossRef]
- Visser, A.W.; Kiørboe, T. Plankton motility patterns and encounter rates. Oecologia 2006, 148, 538–546. [Google Scholar] [CrossRef]
- Poulet, S.A.; Ouellet, G. The role of amino acids in the chemosensory swarming and feeding of marine copepods. J. Plankton Res. 1982, 4, 341–361. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Breckels, M.N.; Bode, N.W.F.; Codling, E.A.; Steinke, M. Effect of Grazing-Mediated Dimethyl Sulfide (DMS) Production on the Swimming Behavior of the Copepod Calanus helgolandicus. Mar. Drugs 2013, 11, 2486-2500. https://doi.org/10.3390/md11072486
Breckels MN, Bode NWF, Codling EA, Steinke M. Effect of Grazing-Mediated Dimethyl Sulfide (DMS) Production on the Swimming Behavior of the Copepod Calanus helgolandicus. Marine Drugs. 2013; 11(7):2486-2500. https://doi.org/10.3390/md11072486
Chicago/Turabian StyleBreckels, Mark N., Nikolai W. F. Bode, Edward A. Codling, and Michael Steinke. 2013. "Effect of Grazing-Mediated Dimethyl Sulfide (DMS) Production on the Swimming Behavior of the Copepod Calanus helgolandicus" Marine Drugs 11, no. 7: 2486-2500. https://doi.org/10.3390/md11072486