Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications
Abstract
:1. Introduction
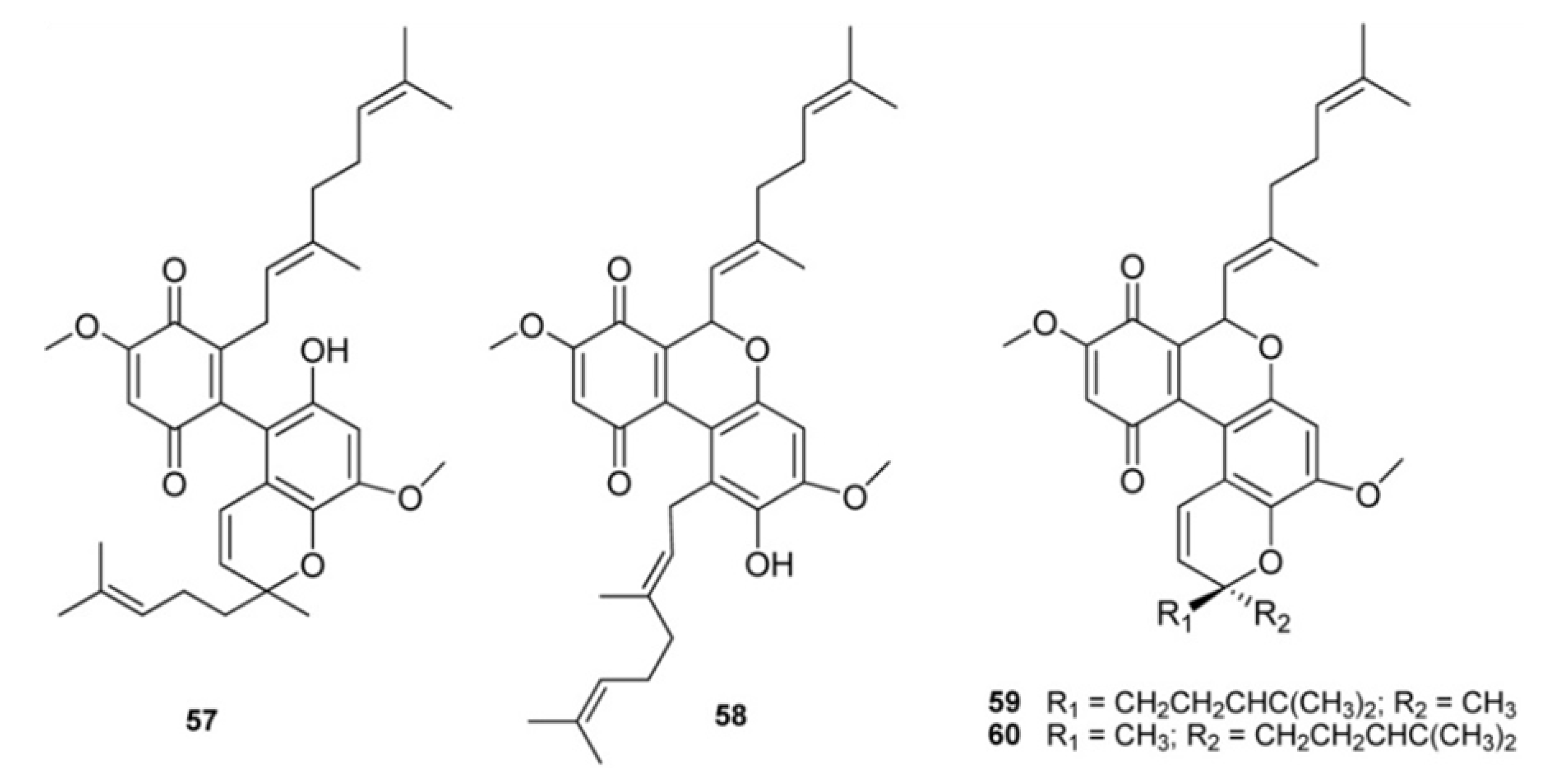
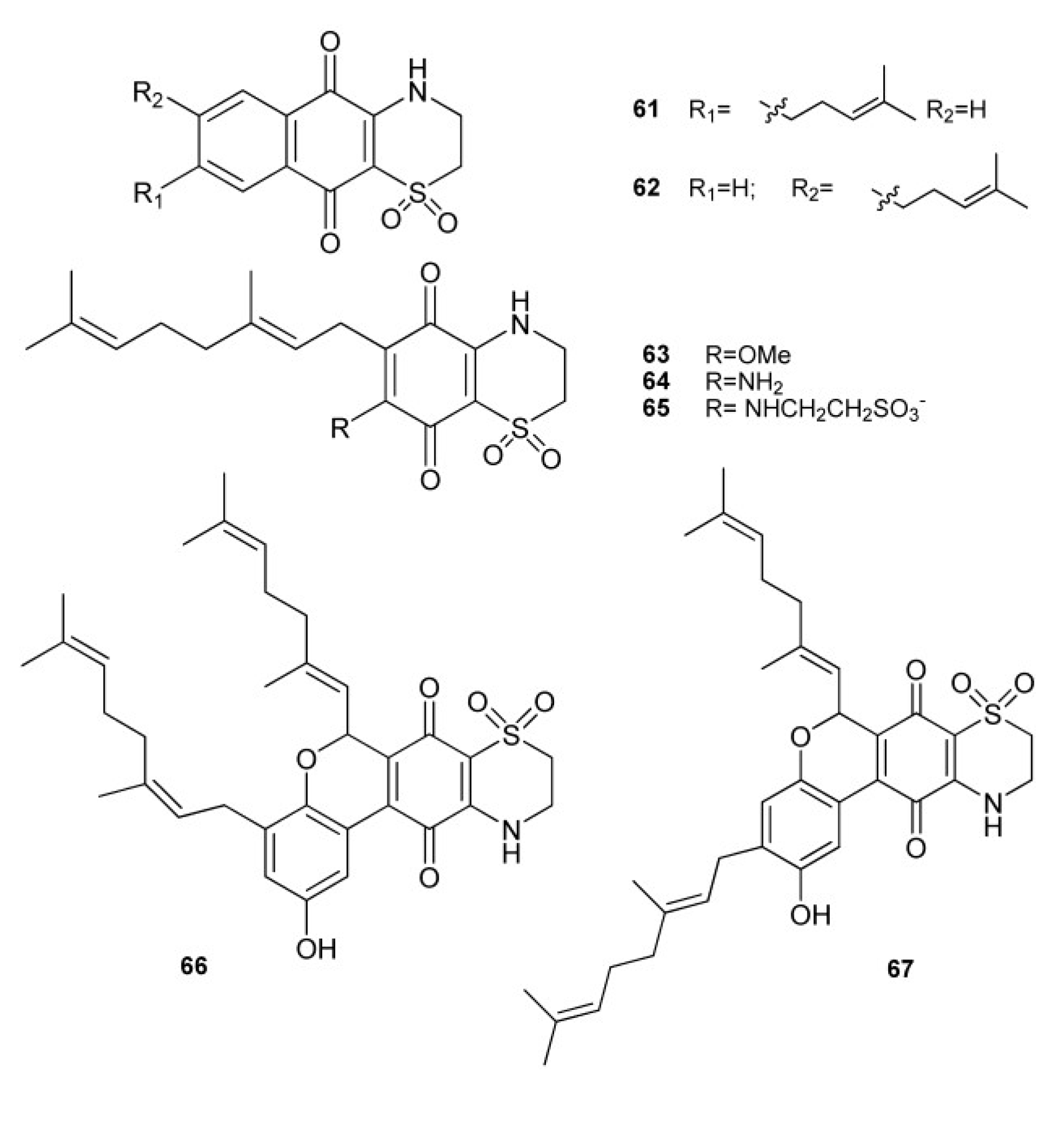
2. Meroterpenes from Ascidians

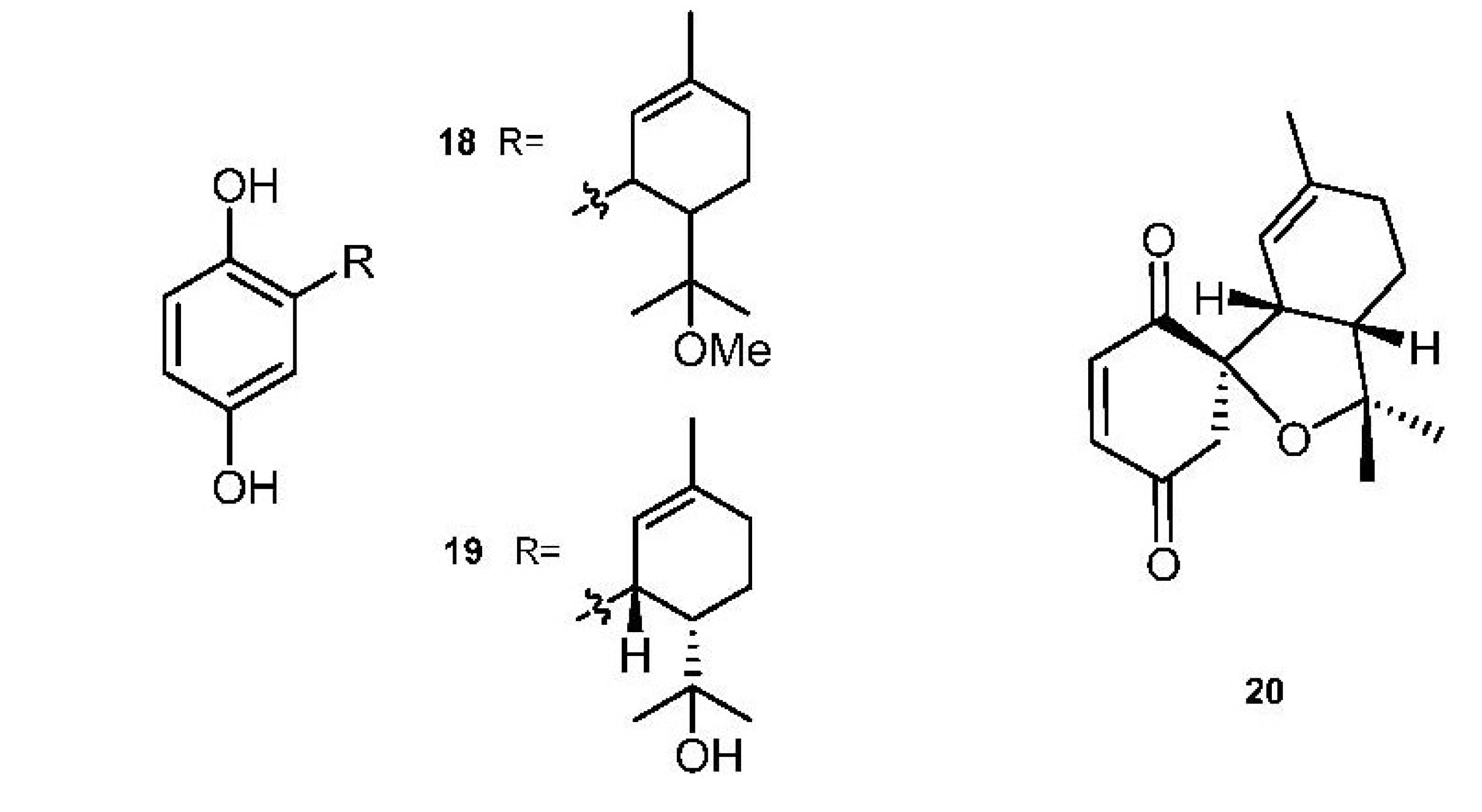

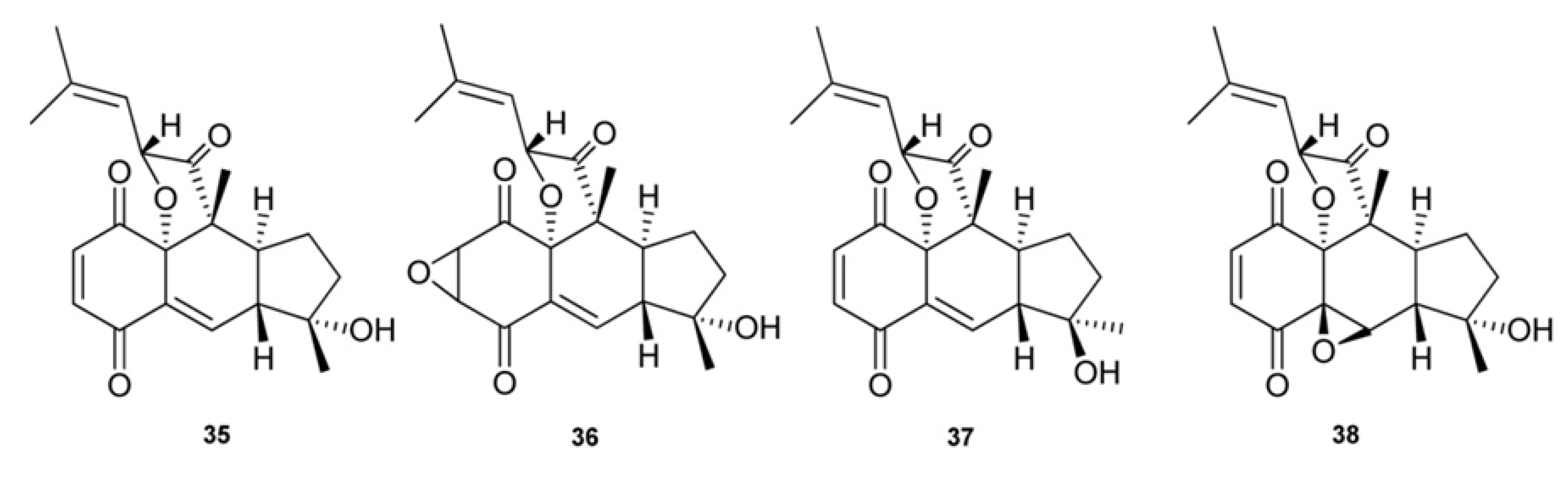
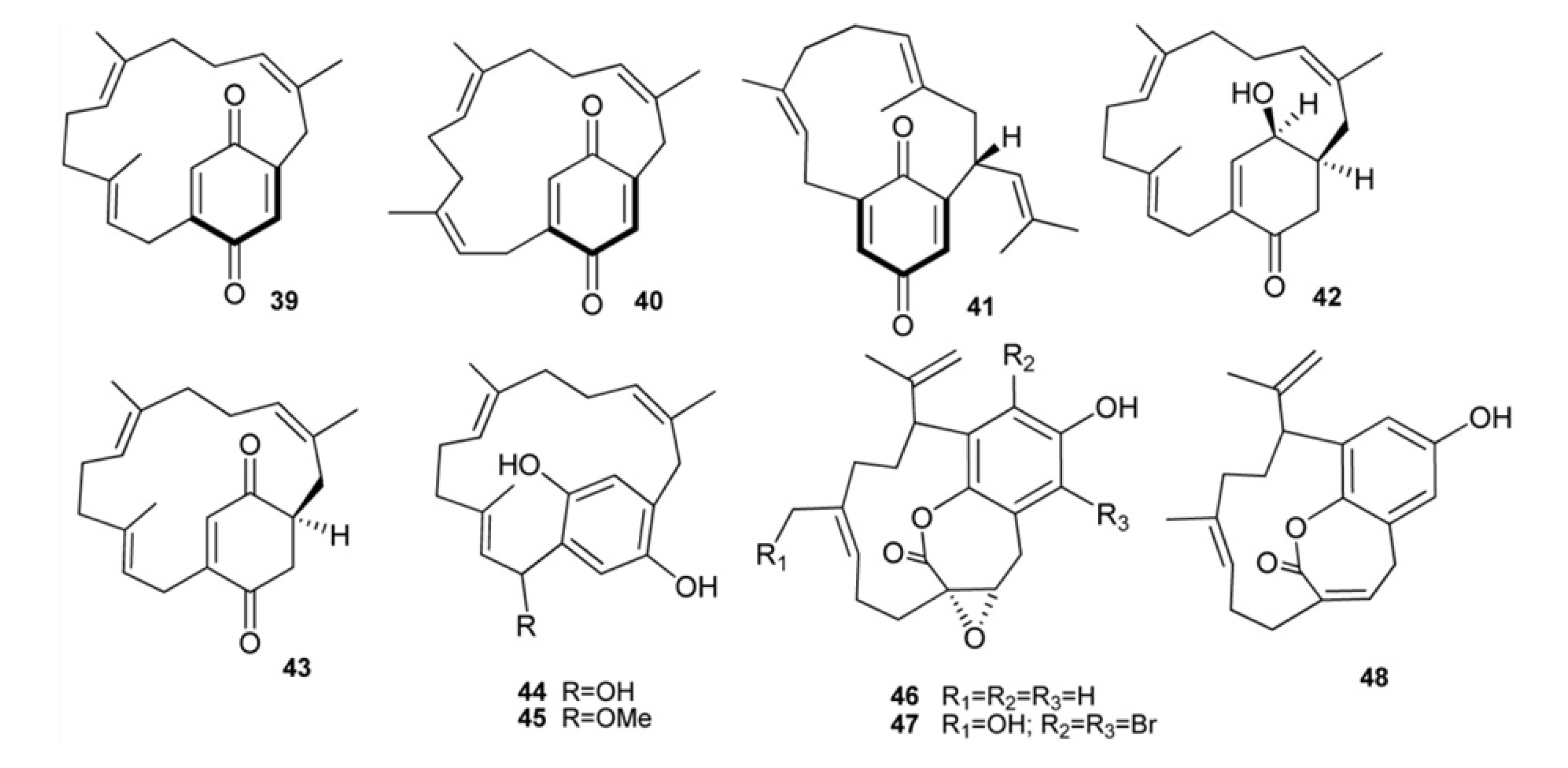
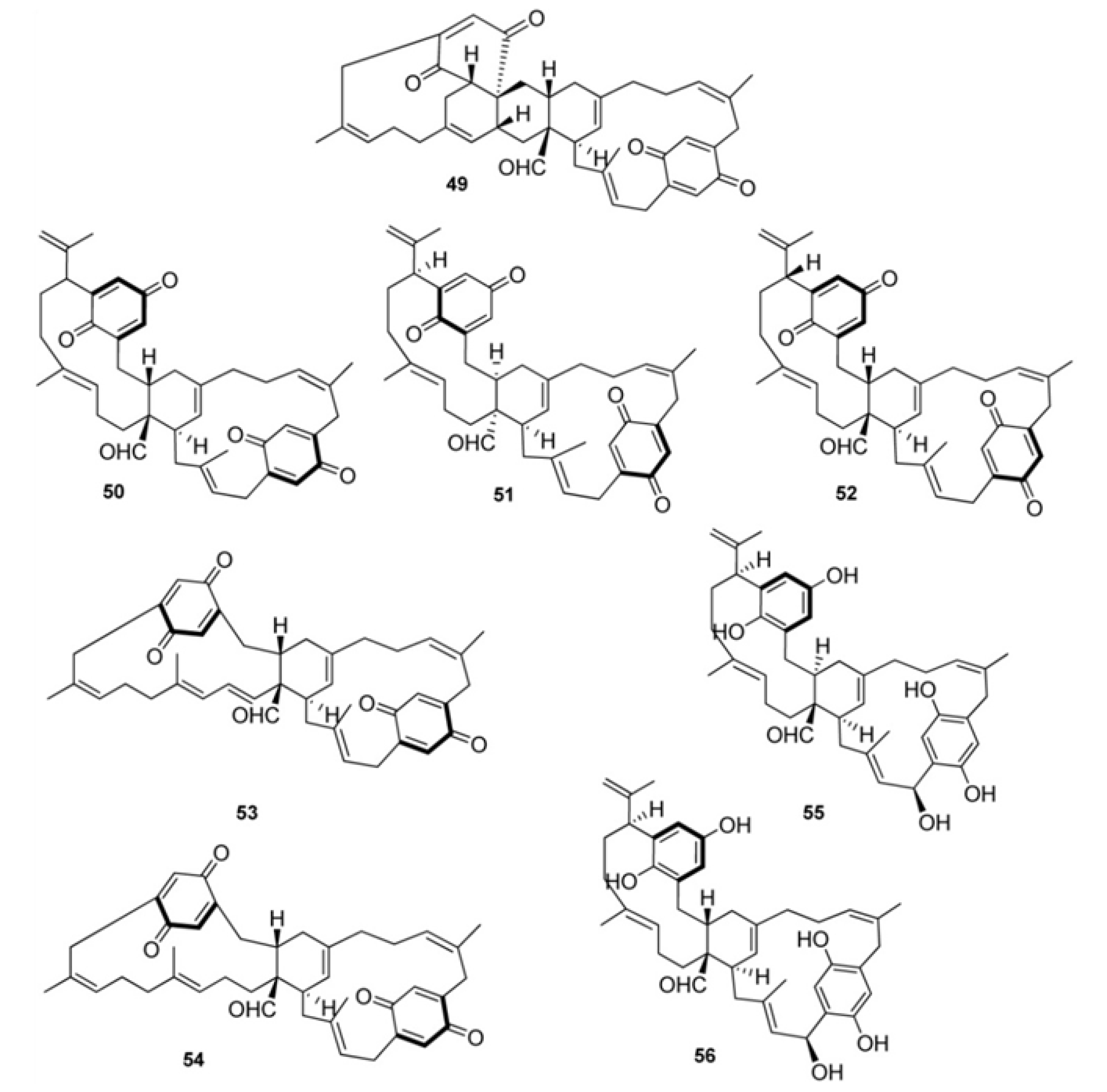
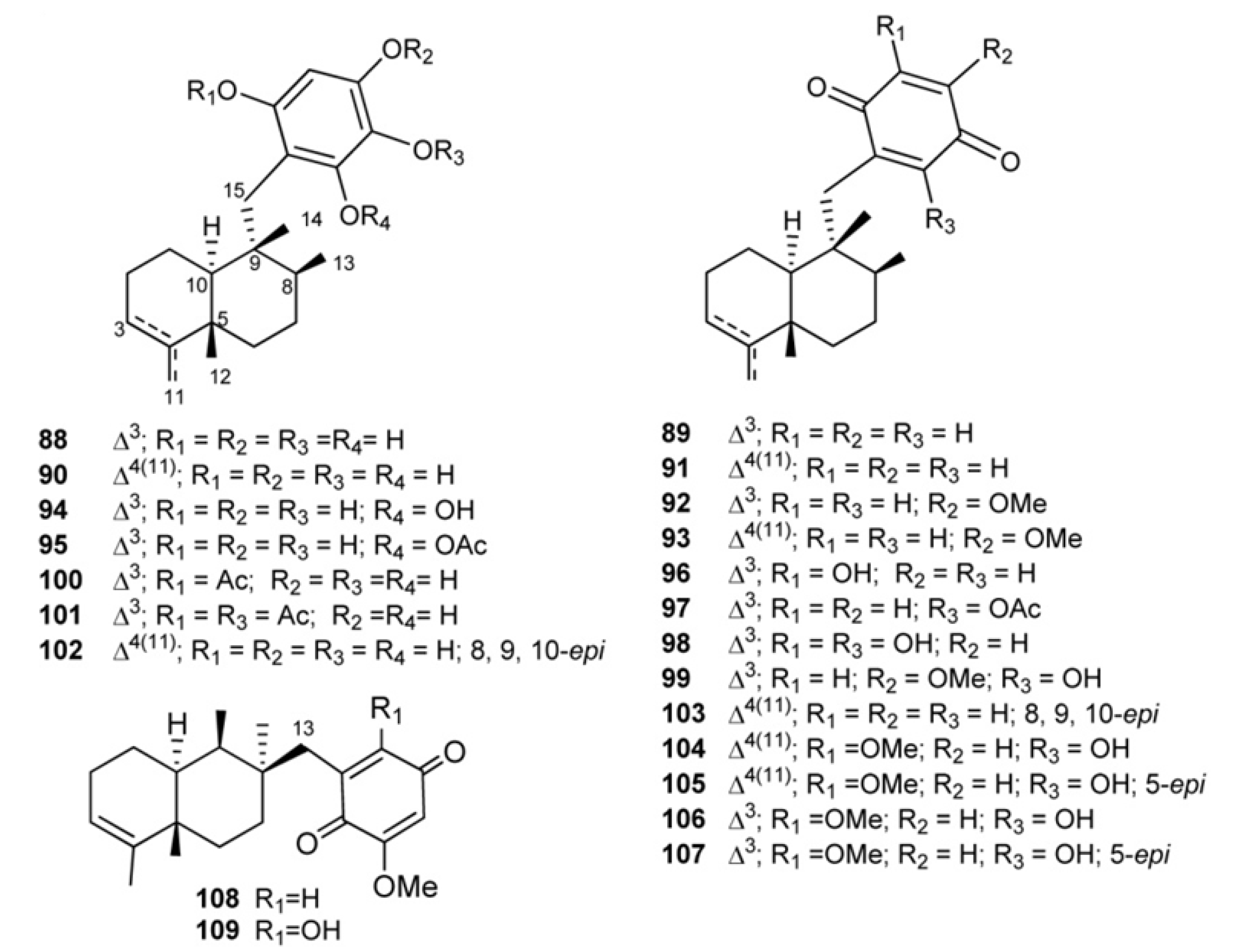
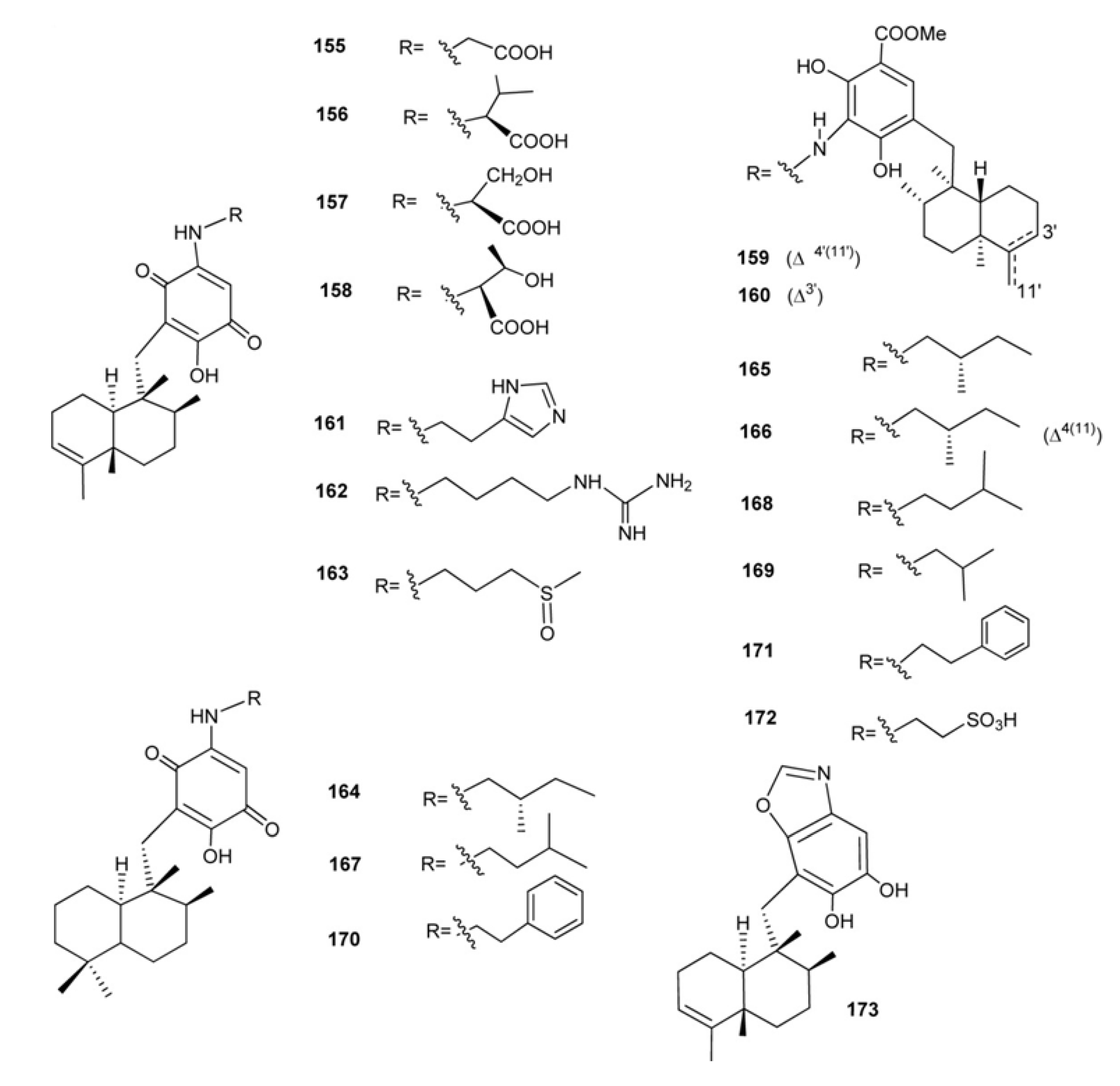
3. Meroterpenes from Sponges



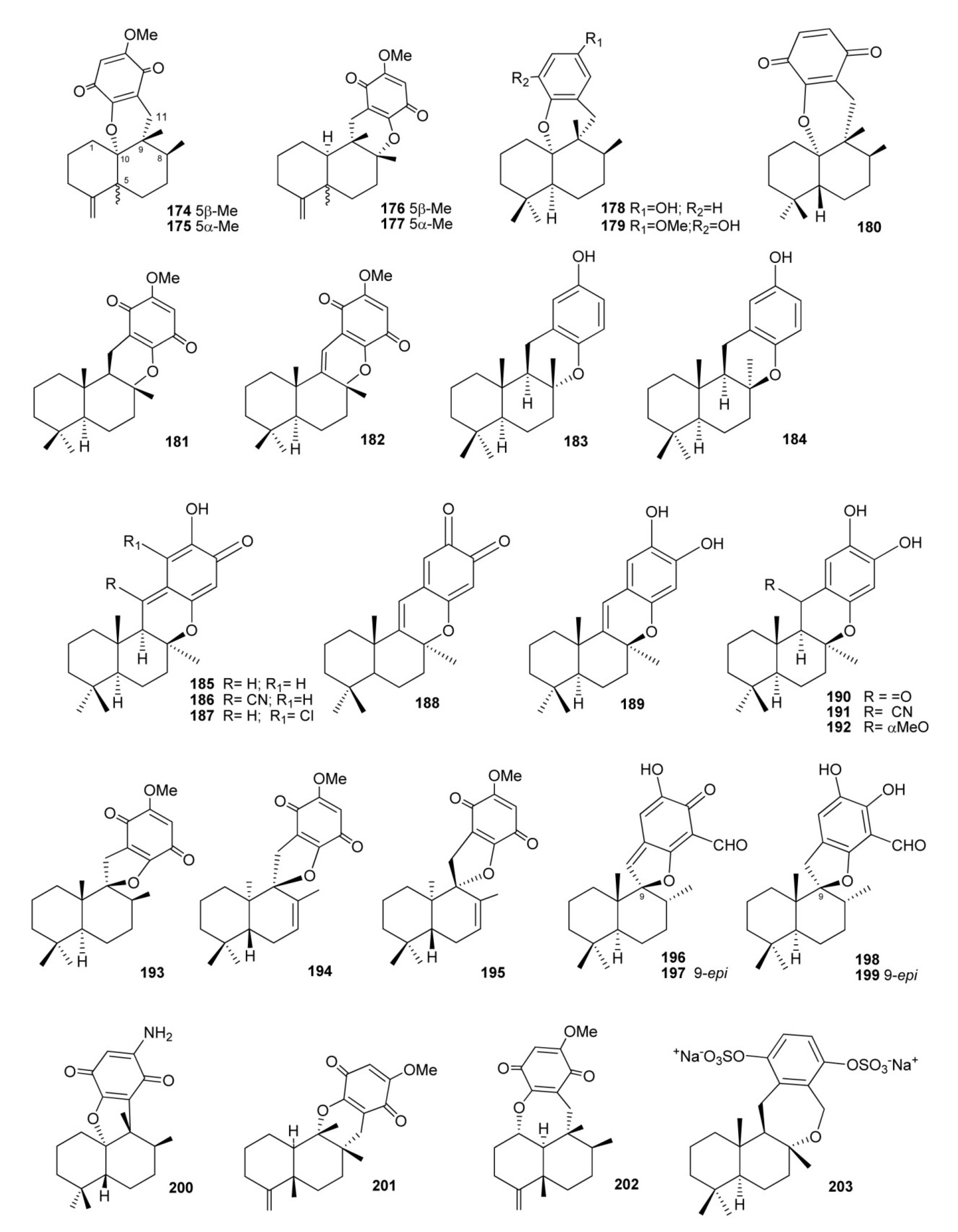
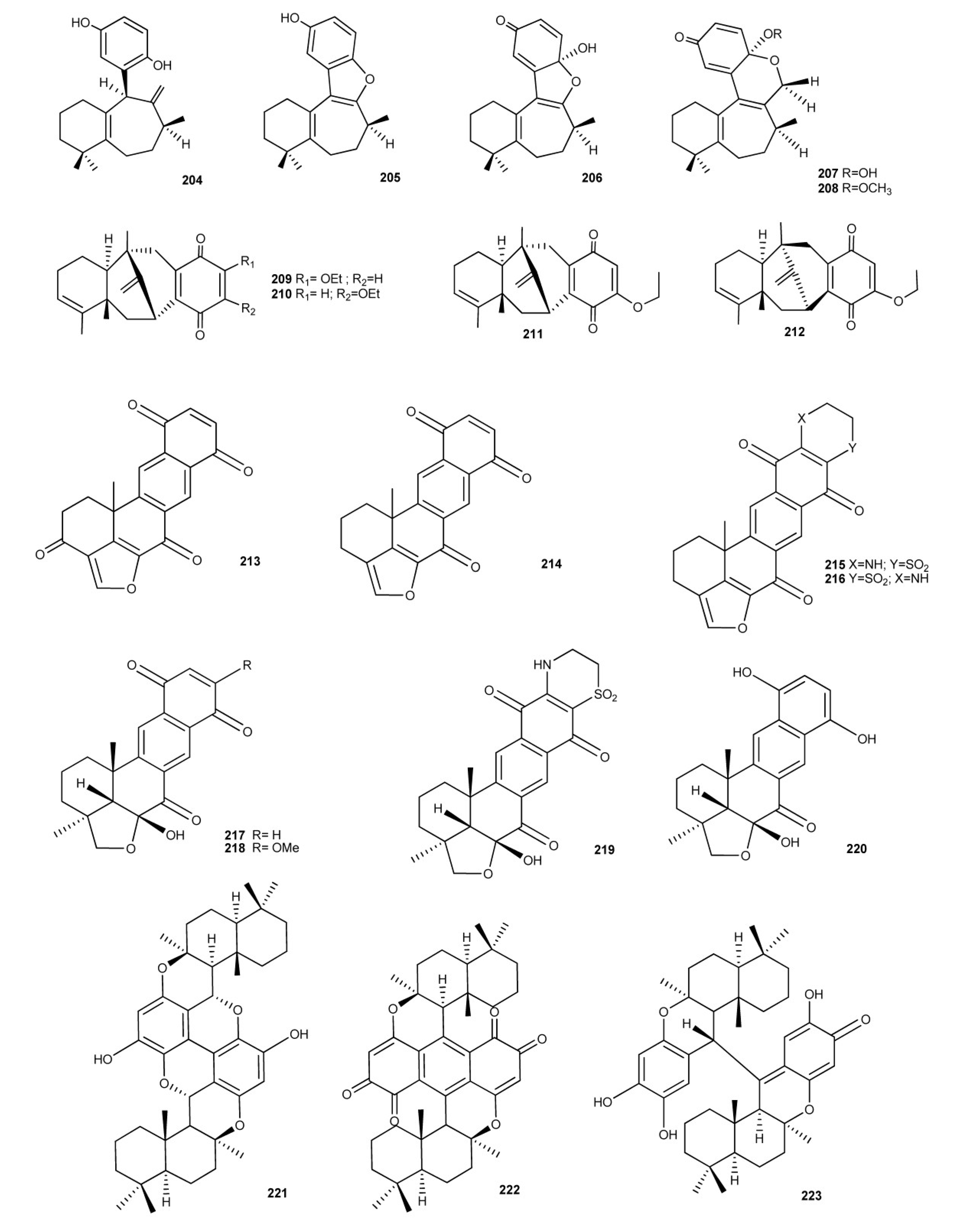
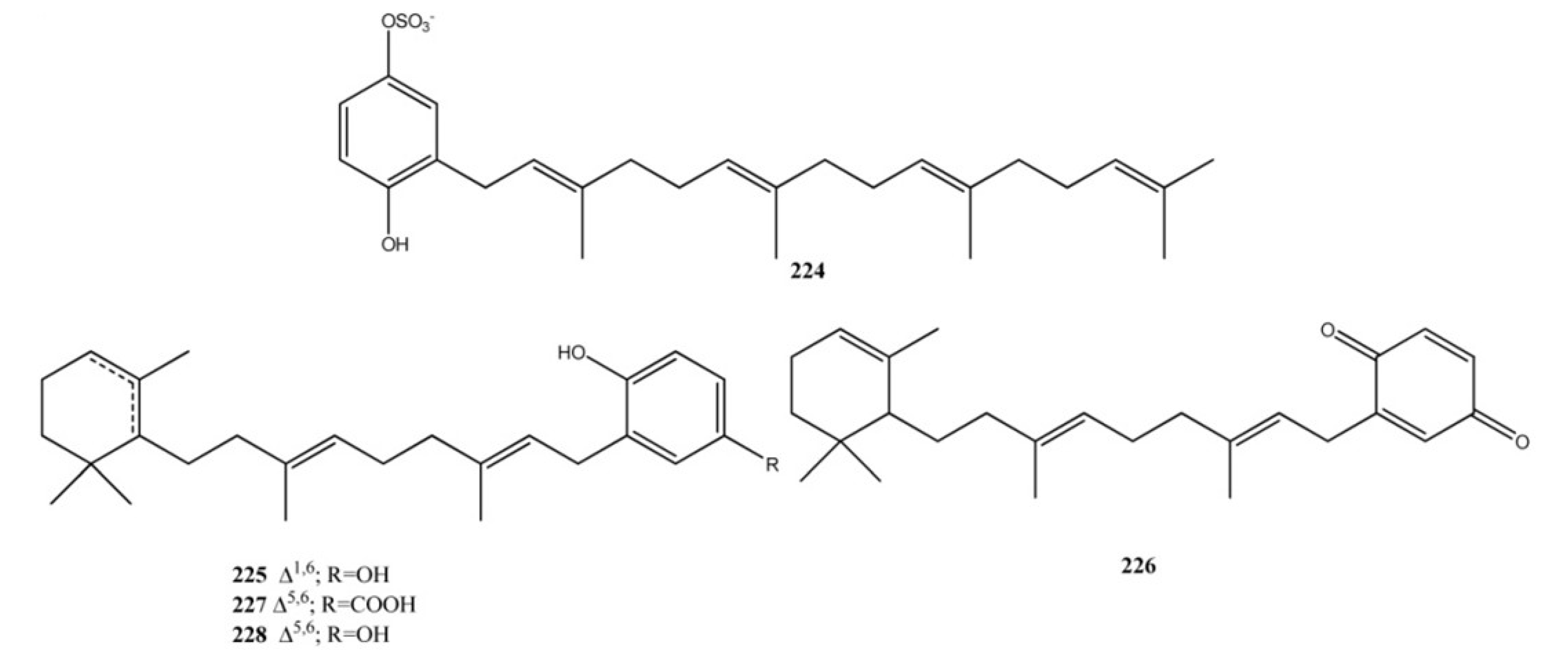
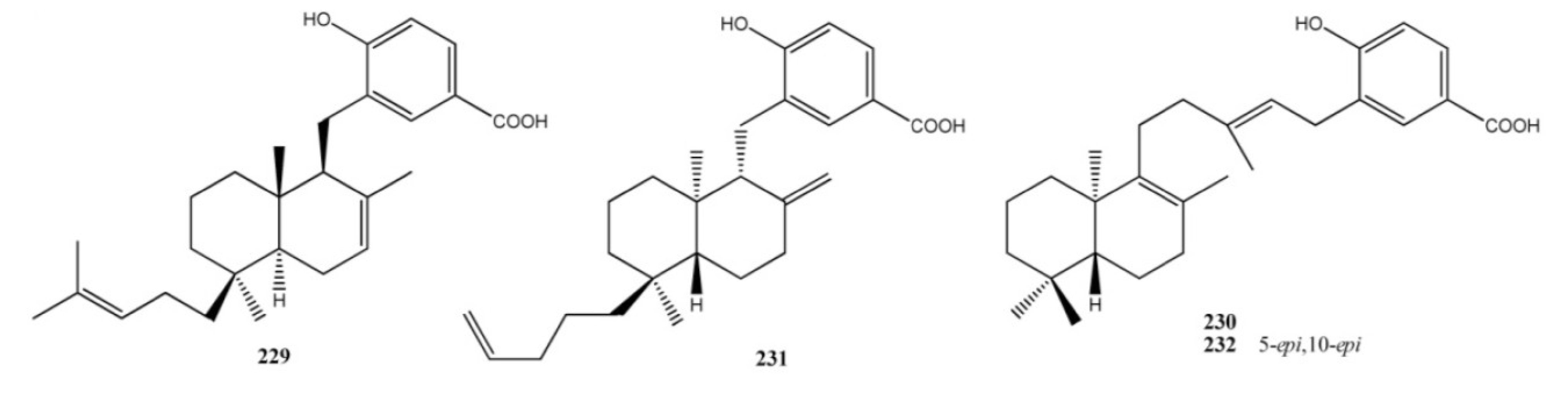
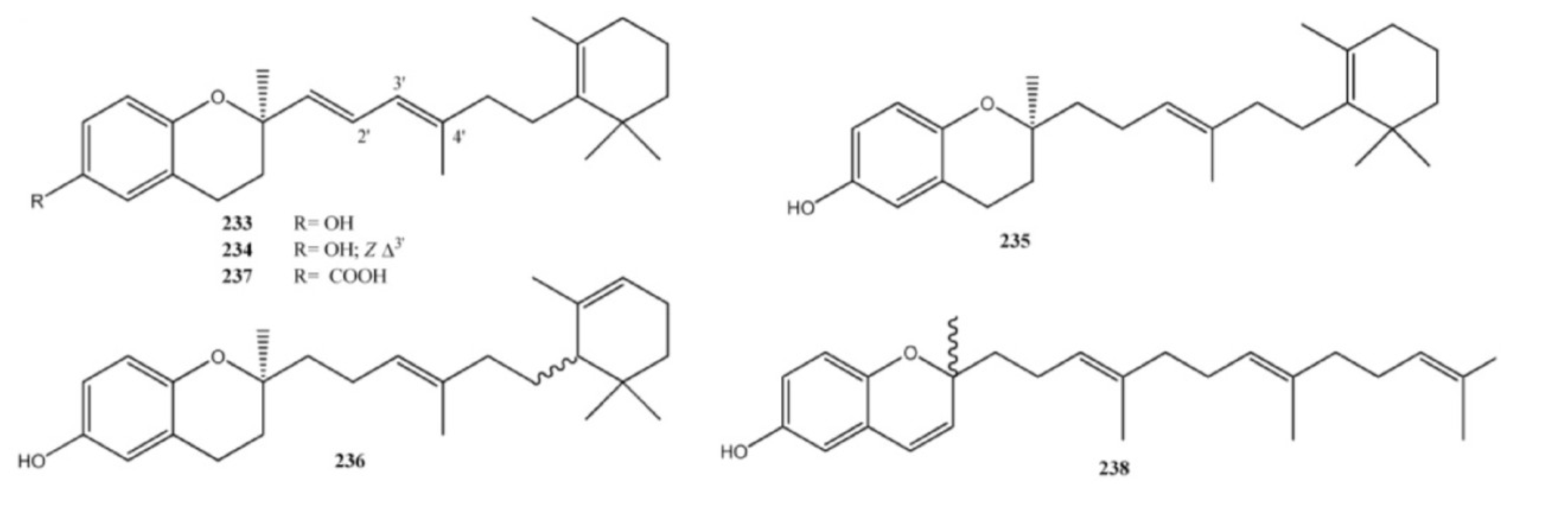
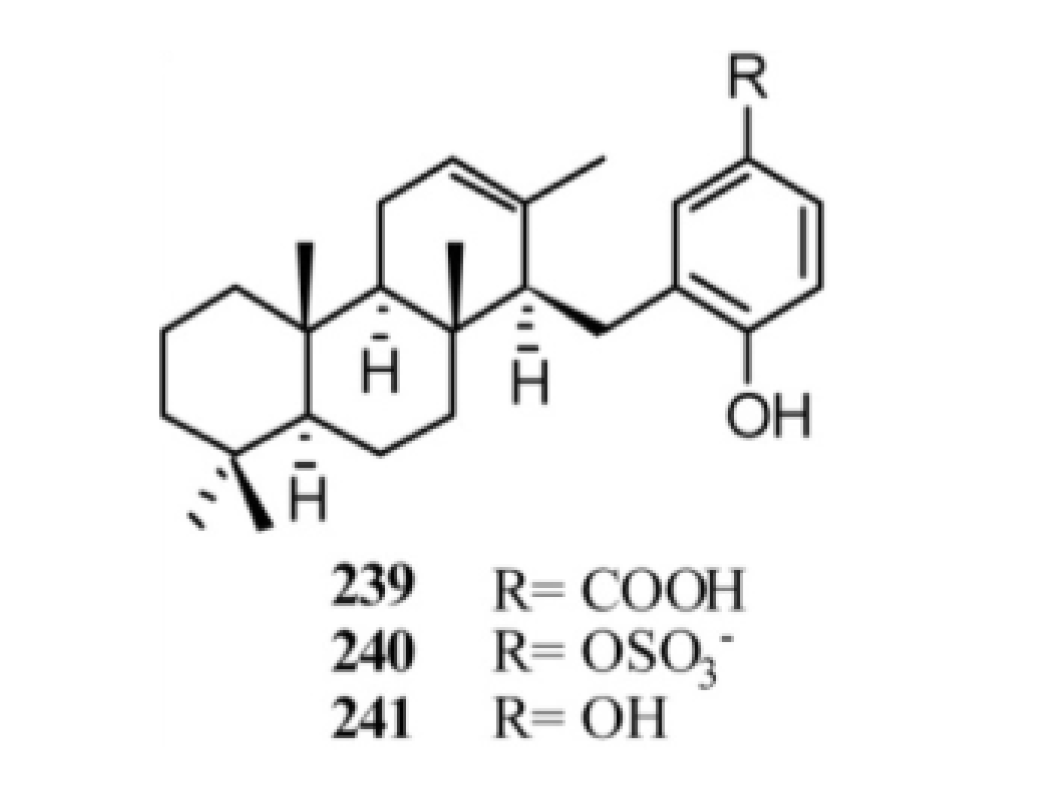
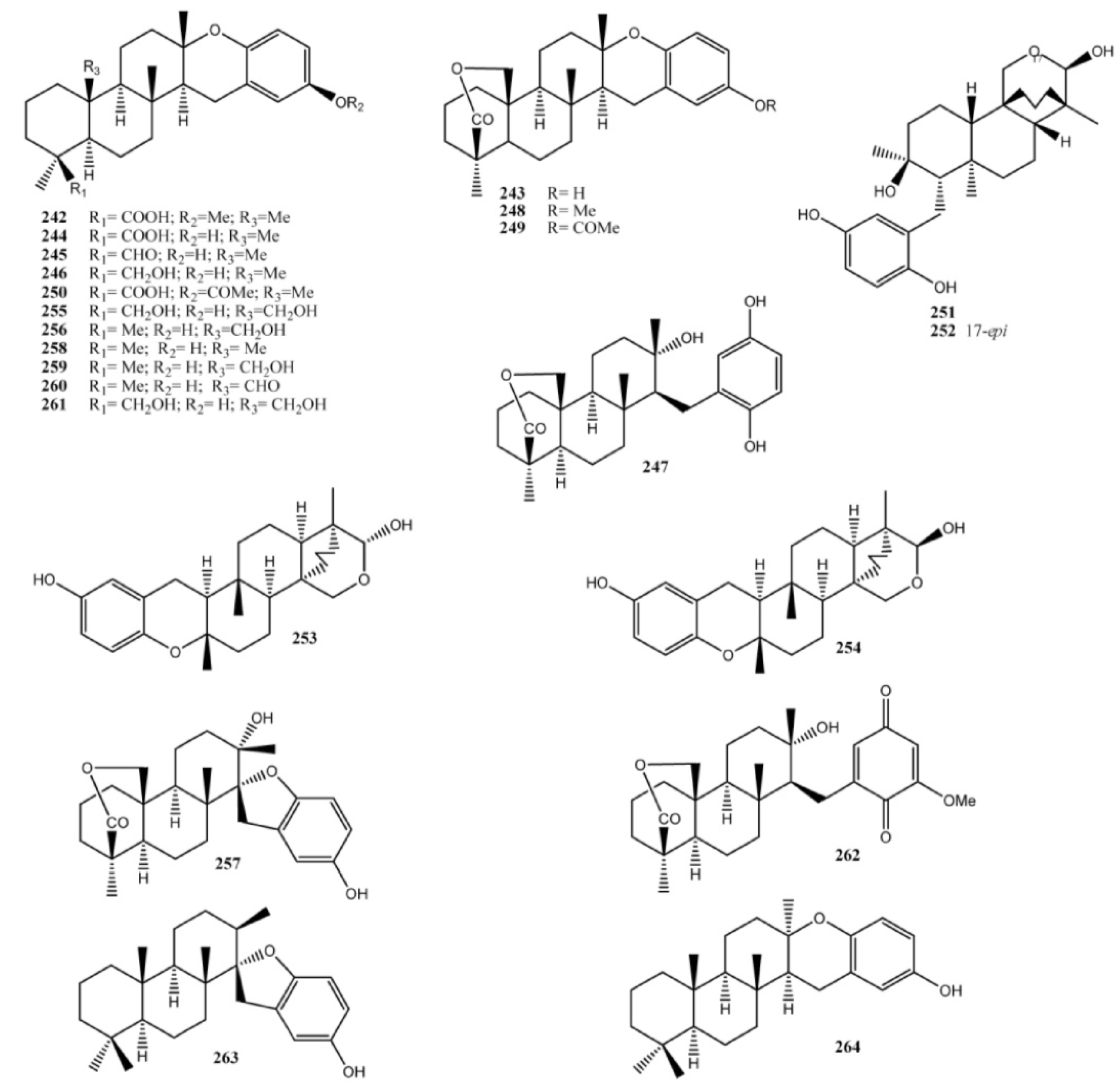
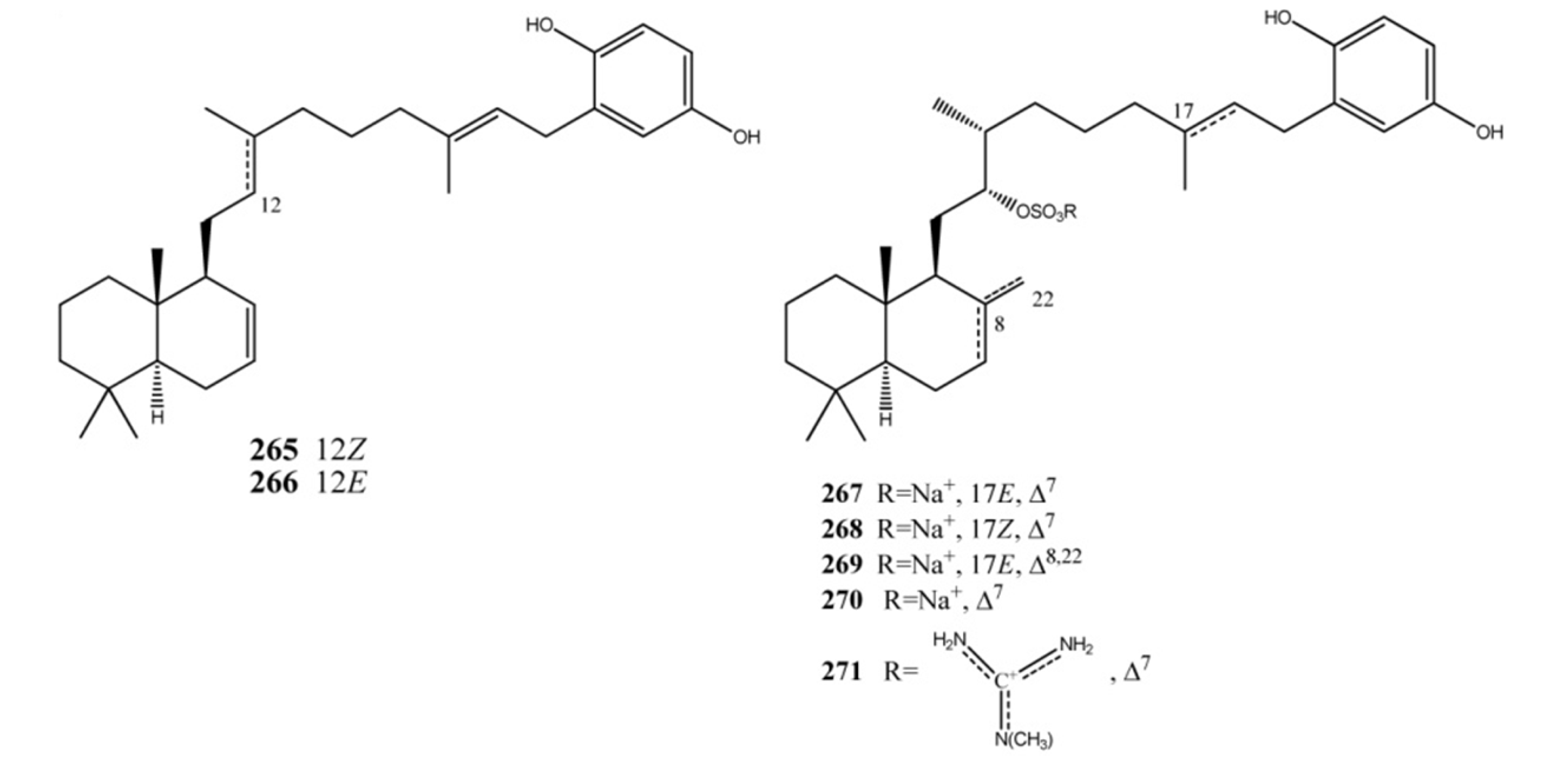

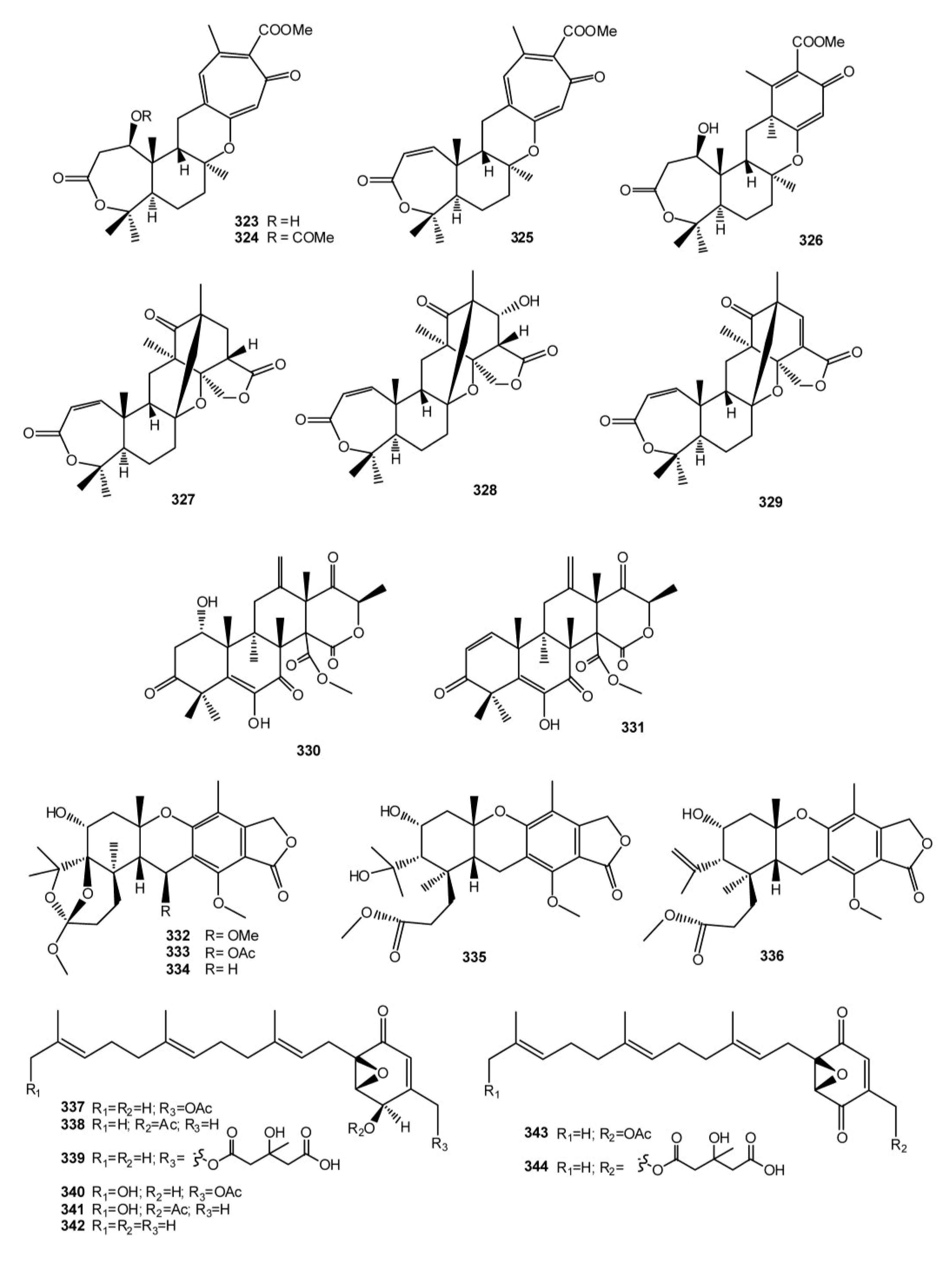
4. Meroterpenes from Soft Corals

5. Conclusive Remarks
Acknowledgments
Conflict of Interest
References
- Thomson, R.H. Naturally Occurring Quinones, 2nd ed.; Academic Press: London, UK, 1971; pp. 93–197. [Google Scholar]
- Pennock, J.F. Terpenoids in Plants; Pridham, J.B., Ed.; Academic Press: London, UK, 1967; pp. 129–146. [Google Scholar]
- Monks, T.J.; Hanzlik, R.P.; Cohen, G.M.; Ross, D.; Graham, D.G. Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 1992, 112, 2–16. [Google Scholar] [CrossRef]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Zubìa, E.; Ortega, M.; Salvà, J. Natural products chemistry in marine ascidians of the genus Aplidium. Mini Rev. Org. Chem. 2005, 2, 546–564. [Google Scholar]
- Menna, M.; Fattorusso, E.; Imperatore, C. Alkaloids from marine ascidians. Molecules 2011, 16, 8694–8732. [Google Scholar] [CrossRef] [Green Version]
- Menna, M.; Aiello, A. The Chemistry of Marine Tunicates. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglialatela-Scafati, O., Eds.; Springer Science + Business Media: New York, NY, USA, 2012; Volume 1, pp. 295–385. [Google Scholar]
- Fenical, W. Geranyl hydroquinone, a cancer-protective agent from the tunicate Aplidium species. Food Drugs Sea Proc. 1976, 4, 388–394. [Google Scholar]
- Sato, A.; Shindo, T.; Kasanuki, N.; Hasegawa, K. Antioxidant metabolites from the tunicate Amaroucium multiplicatum. J. Nat. Prod. 1989, 52, 975–981. [Google Scholar] [CrossRef]
- Aknin, M.; Dayan, T.L.A.; Rudi, A.; Kashman, Y.; Gaydou, E.M. Hydroquinone antioxidants from the Indian Ocean tunicate Aplidium savignyi. J. Agric. Food Chem. 1999, 47, 4175–4177. [Google Scholar] [CrossRef]
- Garrido, L.; Zubía, E.; Ortega, M.J.; Salvá, J. New meroterpenoids from the ascidian Aplidium conicum. J. Nat. Prod. 2002, 65, 1328–1331. [Google Scholar] [CrossRef]
- Shubina, L.K.; Fedorov, S.N.; Radchenko, O.S.; Balaneva, N.N.; Kolesnikova, S.A.; Dmitrenok, P.S.; Bode, A.; Dong, Z.; Stonik, V.A. Desmethylubiquinone Q2 from the far-eastern ascidian Aplidium glabrum: Structure and synthesis. Tetrahedron Lett. 2005, 46, 559–562. [Google Scholar] [CrossRef]
- Chan, S.T.S.; Pearce, A.N.; Januario, A.H.; Page, M.J.; Kaiser, M.; McLaughlin, R.J.; Harper, J.L.; Webb, V.L.; Barker, D.; Copp, B.R. Anti-inflammatory and antimalarial meroterpenoids from the New Zealand ascidian Aplidium scabellum. J. Org. Chem. 2011, 76, 9151–9156. [Google Scholar]
- Guella, G.; Mancini, I.; Pietra, F. Verapliquinones: Novel diprenylquinones from an Aplidium sp. (Ascidiacea) of Ile-Verte waters, Brittany. Helv. Chim. Acta 1987, 70, 621–626. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef]
- Howard, B.M.; Clarkson, K. Simple prenylated hydroquinone derivatives from the marine urochordate Aplidium californicum natural anticancer and antimutagenic agents. Tetrahedron Lett. 1979, 46, 4449–4452. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Bode, A.M.; Stonik, V.A.; Dong, Z. Evaluation of cancer-preventive activity and structure-activity relationship of 3-demethylubiquinone Q2, isolated from the ascidian Aplidium glabrum and its synthetic analogs. Pharm. Res. 2006, 23, 70–81. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Agafonova, I.G.; Bode, A.M.; Jin, J.O.; Kwak, J.Y.; Dong, Z.; Stonik, V. Anticancer activity of 3-demethylubiquinone Q2. In vivo experiments and probable mechanism of action. Anticancer Res. 2008, 28, 927–932. [Google Scholar]
- Laird, D.W.; Poole, R.; Wikstroem, M.; van Altena, I.A. Pycnanthuquinone C, an unusual 6,6,5-tricyclic geranyltoluquinone from the western australian brown alga Cystophora harveyi. J. Nat. Prod. 2007, 70, 671–674. [Google Scholar] [CrossRef]
- Lumb, J.P.; Trauner, D. Pericyclic reactions of prenylated naphthoquinones: Biomimetic syntheses of mollugin and microphyllaquinone. Org. Lett. 2005, 7, 5865–5868. [Google Scholar] [CrossRef]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar]
- Simon-Levert, A.; Arrault, A.; Bontemps-Subielos, N.; Canal, C.; Banaigs, B. Meroterpenes from the ascidian Aplidium aff. densum. J. Nat. Prod. 2005, 68, 1412–1415. [Google Scholar] [CrossRef]
- Simon-Levert, A.; Aze, A.; Bontemps-Subielos, N.; Banaigs, B.; Genevière, A.M. Antimitotic activity of methoxyconidiol, a meroterpene isolated from an ascidian. Chem. Biol. Interact. 2007, 168, 106–116. [Google Scholar] [CrossRef]
- Targett, N.M.; Keeran, W.S. A terpenehydroquinone from the marine ascidian Aplidium constellatum. J. Nat. Prod. 1984, 47, 556–557. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Metzger, R.; Hobbs, L.; Capon, R.J. New chromenols from a southern Australian tunicate Aplidium solidum. Aust. J. Chem. 1996, 49, 1217–1219. [Google Scholar]
- Carrol, A.R.; Bowden, B.F.; Coll, J.C. Studies of Australian ascidians. III. A new tetrahydrocannabinol derivative from the ascidian Synoicum costellatum. Aust. J. Chem. 1993, 46, 1079–1083. [Google Scholar] [CrossRef]
- Choi, H.; Hwang, H.; Chin, J.; Kim, E.; Lee, J.; Nam, S.J.; Lee, B.C.; Rho, B.J.; Kang, H. Tuberatolides, potent FXR antagonists from the korean marine tunicate Botryllus tuberatus. J. Nat. Prod. 2011, 74, 90–94. [Google Scholar]
- Davis, R.A.; Carroll, A.R.; Quinn, R.J. Longithorols C–E. Three new macrocyclic sesquiterpene hydroquinone metabolites from the Australian ascidian Aplidium longithorax. J. Nat. Prod. 1999, 62, 1405–1409. [Google Scholar] [CrossRef]
- Carbone, M.; Nunez-Pons, L.; Paone, M.; Castelluccio, F.; Avila, C.; Gavagnin, M. Rossinone-related meroterpenes from the Antarctic ascidian Aplidium fuegiense. Tetrahedron 2012, 68, 3541–3544. [Google Scholar] [CrossRef]
- Fort, D.M.; Ubillas, R.P.; Mendez, C.D.; Jolad, S.D.; Inman, W.D.; Carney, J.R.; Chen, J.L.; Ianiro, T.T.; Hasbun, C.; Bruening, R.C.; et al. Novel antihyperglycemic terpenoid-quinones from Pycnanthus angolensis. J. Org. Chem. 2000, 65, 6534–6539. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Yang, Z.; Tang, Y. Rapid biomimetic total synthesis of (±)-rossinone B. Org. Lett. 2010, 12, 5554–5557. [Google Scholar] [CrossRef]
- Fu, X.; Hossain, M.B.; van der Helm, D.; Schmitz, F.J. Longithorone A: Unprecedented dimeric prenylated quinone from the Tunicate Aplidium longithorax. J. Am. Chem. Soc. 1994, 116, 12125–12126. [Google Scholar] [CrossRef]
- Fu, X.; Hossain, M.B.; Schmitz, F.J.; van der Helm, D. Longithorones, unique prenylated para- and metacyclophane type quinones from the Tunicate Aplidium longithorax. J. Org. Chem. 1997, 62, 3810–3819. [Google Scholar] [CrossRef]
- Davis, R.A.; Carroll, A.R.; Quinn, R.J. Longithorones J and K, two new cyclofarnesylated quinone derived metabolites from the Australian ascidian Aplidium longithorax. J. Nat. Prod. 1999, 62, 158–160. [Google Scholar] [CrossRef]
- Fu, X.; Ferreira, M.L.G.; Schmitz, F.J. Longithorols A and B, novel prenylated paracyclophane- and metacyclophane-tipe hydroquinones from the Tunicate Aplidium longithorax. J. Nat. Prod. 1999, 62, 1306–1310. [Google Scholar] [CrossRef]
- Kotha, S.; Shirbhate, M.E. Diversity-oriented approach to macrocyclic cyclophane derivatives via ring-closing metathesis. Synlett 2012, 23, 2183–2188. [Google Scholar] [CrossRef]
- Issa, H.H.; Tanaka, J.; Rachmat, R.; Higa, T. Floresolides, new metacyclophane hydroquinone lactones from an ascidian, Aplidium sp. Tetrahedron Lett. 2003, 44, 1243–1245. [Google Scholar]
- Layton, M.E.; Morales, C.A.; Shair, M.D. Biomimetic synthesis of (−)-longithorone A. J. Am. Chem. Soc. 2002, 124, 773–775. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Menna, M.; Esposito, G.; Iuvone, T.; Pala, D. Conicaquinones A and B, two novel cytotoxic terpene quinones from the Mediterranean ascidian Aplidium conicum. Eur. J. Org. Chem. 2003, 2003, 898–900. [Google Scholar]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Mangoni, A.; Menna, M. Isolation and structure determination of aplidinones A–C from the Mediterranean ascidian Aplidium conicum: A successful regiochemistry assignment by quantum mechanical 13C NMR chemical shift Calculations. Eur. J. Org. Chem. 2005, 2005, 5024–5030. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Macho, A.; Menna, M.; Muñoz, E. Antitumor effects of two novel naturally occurring terpene quinones isolated from the Mediterranean ascidian Aplidium conicum. J. Med. Chem. 2005, 48, 3410–3416. [Google Scholar] [CrossRef]
- Carbone, A.; Lucas, C.L.; Moody, C.J. Biomimetic synthesis of the apoptosis-inducing thiazinoquinone thiaplidiaquinone A. J. Org. Chem. 2012, 77, 9179–9189. [Google Scholar]
- Khalil, I.M.; Barker, D.; Copp, B.R. Biomimetic synthesis of thiaplidiaquinones A and B. J. Nat. Prod. 2012, 75, 2256–2260. [Google Scholar]
- Aiello, A.; Fattorusso, E.; Luciano, P.; Menna, M.; Calzado, M.A.; Muñoz, E.; Bonadies, F.; Guiso, M.; Sanasi, M.F.; Cocco, G.; et al. Synthesis of structurally simplified analogues of aplidinone A, a pro-apoptotic marine thiazinoquinone. Bioorg. Med. Chem. 2010, 18, 719–727. [Google Scholar] [CrossRef]
- De Rosa, S.; de Giulio, A.; Iodice, C. Biological effects of prenylated hydroquinones: Structure-activity relationship studies in antimicrobial, brine shrimp, and fish lethality assays. J. Nat. Prod. 1994, 57, 1711–1716. [Google Scholar] [CrossRef]
- Muller, W.E.G.; Maidhof, A.; Zahn, R.K.; Schroder, H.C.; Gasic, M.J.; Heidemann, D.; Bernd, A.; Kurelec, B.; Eich, E.; Seibert, G. Potent antileukemic activity of the novel cytostatic agent avarone and its analogs in vitro and in vivo. Cancer Res. 1985, 45, 4822–4826. [Google Scholar]
- Kondracki, M.L.; Guyot, M. Biologically active quinone and hydroquinone sesquiterpenoids from the sponge Smenospongia sp. Tetrahedron 1989, 45, 1995–2004. [Google Scholar] [CrossRef]
- Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M. Biogenetically diverse, bioactive constituents of a sponge, order Verongida: Bromotyramines and sesquiterpene-shikimate derived metabolites. J. Org. Chem. 1993, 58, 6565–6569. [Google Scholar] [CrossRef]
- Alvi, K.A.; Diaz, M.C.; Crews, P.; Slate, D.L.; Lee, R.H.; Moretti, R. Evaluation of new sesquiterpene quinones from two Dysidea sponge species as inhibitors of protein tyrosine kinase. J. Org. Chem. 1992, 57, 6604–6607. [Google Scholar] [CrossRef]
- Loya, S.; Tal, R.; Kashman, Y.; Hizi, A. Illimaquinone, a selective inhibitor of the RNase H activity of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1990, 34, 2009–2012. [Google Scholar] [CrossRef]
- Gordaliza, M. Cytotoxic terpene quinones from marine sponges. Mar. Drugs 2010, 8, 2849–2870. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Rivera, F.; Rojo, L.; Campos, N.; Pedro, M.; Nascimento, M.S.J. Studies on quinones. Part 42: Synthesis of furylquinone and hydroquinones with antiproliferative activity against human tumor cell lines. Bioorg. Med. Chem. 2008, 16, 862–868, and all previous parts. [Google Scholar] [CrossRef]
- Ishibashi, M.; Ohizumi, Y.; Cheng, J.-F.; Nakamura, H.; Hirata, Y.; Sasaki, T.; Kobayashi, J. Metachromins A and B, Novel antineoplastic sesquiterpenoids from the okinawan sponge Hippospongia cf. metachromia. J. Org. Chem. 1988, 53, 2855–2858. [Google Scholar] [CrossRef]
- Kobayashi, J.; Murayama, T.; Ohizumi, Y.; Ohta, T.; Nozoe, S.; Sasaki, T. Metachromin C, a new cytotoxic sesquiterpenoid from the okinawan marine sponge Hippospongia metachromia. J. Nat. Prod. 1989, 52, 1173–1176. [Google Scholar] [CrossRef]
- Kobayashi, J.; Naitoh, N.; Sasaki, T.; Shigemori, H. Metachromins D–H, new cytotoxic sesquiterpenoids from the okinawan marine sponge Hippospongia metachromia. J. Org. Chem. 1992, 57, 5773–5776. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, T.; Fromont, J.; Kobayashi, J. Metachromins L–Q, new sesquiterpenoid quinones with an aminoacid residue from sponge Spongia sp. Tetrahedron 2007, 63, 8770–8773. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamada, M.; Kubota, T.; Fromont, J.; Kobayashi, J. Metachromins R–T, new sesquiterpenoids from marine sponge Spongia sp. Chem. Pharm. Bull. 2007, 55, 1731–1733. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Metachromins U–W: Cytotoxic merosesquiterpenoids from an Australian specimen of the sponge Thorecta reticulata. J. Nat. Prod. 2011, 74, 1335–1338. [Google Scholar] [CrossRef]
- Minale, L.; Riccio, R.; Sodano, G. Avarol, a novel sesquiterpenoid hydroquinone with a rearranged drimane skeleton from the sponge Disidea avara. Tetrahedron Lett. 1974, 38, 3401–3404. [Google Scholar] [CrossRef]
- Cimino, G.; de Rosa, S.; de Stefano, S.; Cariello, L.; Zanetti, L. Structure of two biologically active sesquiterpenoid amino-quinones from the marine sponge Dysidea avara. Experientia 1982, 38, 896. [Google Scholar] [CrossRef]
- De Rosa, S.; Minale, L.; Riccio, R.; Sodano, G. The absolute configuration of avarol, a rearranged sesquiterpenoid hydroquinone from a marine sponge. J. Chem. Soc. Perkin Trans. 1 1976, 1408–1414. [Google Scholar] [CrossRef]
- Giordano, F.; Puliti, R. Structure of the 2,5-dimethyl ether of avarol, a sesquiterpenoid hydroquinone from the marine sponge Dysidea avara. Acta Cryst. 1987, C43, 985–988. [Google Scholar]
- Müller, W.E.G.; Sobel, C.; Sachsse, W.; Diehl-Seifert, B.; Zahn, R.K.; Eich, E.; Kljajić, Z.; Schröder, H.C. Biphasic and differential effects of the cytostatic agents avarone and avarol on DNA metabolism of human and murine T and B lymphocytes. Eur. J. Cancer Clin. Oncol. 1986, 22, 473–476. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Sobel, C.; Diehl-Seifert, B.; Maidhof, A.; Schöder, H.C. Influence of the antileukemic and anti-human immunodeficiency virus agent avarol on selected immune responses in vitro and in vivo. Biochem. Pharmacol. 1987, 36, 1489–1494. [Google Scholar] [CrossRef]
- Cozzolino, R.; de Giulio, A.; de Rosa, S.; Strazzullo, G.; Gašič, M.J.; Sladić, D.; Zlatović, M. Biological activities of avarol derivatives, 1. Amino derivatives. J. Nat. Prod. 1990, 53, 699–702. [Google Scholar] [CrossRef]
- Sarin, P.S.; Sun, D.; Thornton, A.; Müller, W.E.G. Inhibition of replication of the etiologic agent of acquired immune deficiency syndrome (human T-lymphotropic retrovirus/lymphadenopathy-associated virus) by avarol and avarone. J. Natl. Cancer Inst. 1987, 78, 663–666. [Google Scholar]
- Schroeder, H.C.; Sarin, P.S.; Rottmann, M.; Wenger, R.; Maidhof, A.; Renneisen, K.; Muller, W.E.G. Differential modulation of host cell and HIV gene expression by combinations of Avarol and AZT in vitro. Biochem. Pharmacol. 1988, 37, 3947–3952. [Google Scholar] [CrossRef]
- Schroeder, H.C.; Wenger, R.; Gerner, H.; Reuter, P.; Kuchino, Y.; Sladic, D.; Muller, W.E.G. Suppression of the modulatory effects of the antileukemic and anti-human immunodeficiency virus compound avarol on gene expression by tryptophan. Cancer Res. 1989, 49, 2069–2076. [Google Scholar]
- Schatton, W.; Schatton, M.; Pietschmamm, R. Method for the Preparation of Compositions with High Avarol Content from Sponge and Use for the Prevention and Treatment of Psoriasis and Tumors. Eur. Pat. Appl. EP 1391197 A1, 25 February 2004. [Google Scholar]
- Iguchi, K.; Sahashi, A.; Khono, J.; Yamada, Y. New sesquiterpenoid hydroquinone and quinones from the Okinawan marine sponge (Dysidea sp.). Chem. Pharm. Bull. 1990, 38, 1121–1123. [Google Scholar] [CrossRef]
- Hirsch, S.; Rudi, A.; Kashman, Y.; Loya, Y. New avarone and avarol derivatives from the marine sponge Dysidea cinerea. J. Nat. Prod. 1991, 54, 92–97. [Google Scholar] [CrossRef]
- Crispino, A.; de Giulio, A.; de Rosa, S.; Strazzullo, G. A new bioactive derivative of avarol from the marine sponge Dysidea avara. J. Nat. Prod. 1989, 52, 646–648. [Google Scholar] [CrossRef]
- De Giulio, A.; de Rosa, S.; di Vincenzo, G.; Strazzullo, G. Further bioactive derivative of avarol from Dysidea avara. Tetrahedron 1990, 46, 7971–7976. [Google Scholar] [CrossRef]
- Loya, S.; Tal, R.; Hizi, A.; Issacs, S.; Kashman, Y.; Loya, Y. Hexaprenoid hydroquinones, novel inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. J. Nat. Prod. 1993, 56, 2120–2125. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Lakshmi, V.; Powell, D.R.; van der Helm, D. Arenarol and arenarone: Sesquiterpenoids with rearranged drimane skeletons from the marine sponge Dysidea arenaria. J. Org. Chem. 1984, 49, 241–244. [Google Scholar] [CrossRef]
- Luibrand, R.T.; Erdman, T.R.; Vollmer, J.J.; Scheuer, P.J.; Finer, J.; Clardy, J. Ilimaquinone, a sesquiterpenoid quinone from a marine sponge. Tetrahedron 1979, 35, 609–612. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Namikoshi, M.; Kobayashi, H.; Yao, X.; Cai, G. Sesquiterpene quinones from a marine sponge Hippospongia sp. that inhibit maturation of starfish oocytes and induce cell cycle arrest with HepG2 cells. Pharm. Biol. 2006, 44, 522–527. [Google Scholar] [CrossRef]
- Cartè, B.; Rose, C.B.; Faulkner, D.J. 5-Epi-Ilimaquinone, a metabolite of the sponge Fenestraspongia sp. J. Org. Chem. 1985, 50, 2785–2787. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Warren, R.G.; Wells, R.J.; Blount, J.F. New quinones from a dictyoceratid sponge. Aust. J. Chem. 1978, 31, 2685–2697. [Google Scholar] [CrossRef]
- Rodriguez, J.; Quinoa, H.; Riguera, R.; Peters, B.M.; Abrell, L.M.; Crews, P. The structures and stereochemistry of cytotoxic sesquiterpene quinones from Dactylospongia elegans. Tetrahedron 1992, 48, 6667–6680. [Google Scholar] [CrossRef]
- Capon, R.J.; MacLeod, J.K. Revision of the absolute stereochemistry of ilimaquinone. J. Org. Chem. 1987, 52, 5059–5060. [Google Scholar] [CrossRef]
- Nambiar, M.P.; Wu, H.C. Ilimaquinone inhibits the cytotoxicities of ricin, diphtheria toxin, and other protein toxins in Vero cells. Exp. Cell Res. 1995, 219, 671–678. [Google Scholar] [CrossRef]
- Takizawa, P.A.; Yucel, J.K.; Veit, B.; Faulkner, D.J.; Deerinck, T.; Soto, G.; Ellisman, M.; Malhotra, V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell 1993, 73, 1079–1090. [Google Scholar] [CrossRef]
- Casaubon, R.L.; Snapper, M.L. S-adenosylmethionine reverses ilimaquinone’s vesiculation of the Golgi apparatus: A fluorescence study on the cellular interactions of ilimaquinone. Bioorg. Med. Chem. Lett. 2001, 11, 133–136. [Google Scholar] [CrossRef]
- Lu, P.-H.; Chueh, S.-C.; Kung, F.-L.; Pan, S.-L.; Shen, Y.-C.; Guh, J.-H. Illimaquinone, a marine sponge metabolite, displays anticancer activity via GADD153-mediated pathway. Eur. J. Pharmacol. 2007, 556, 45–54. [Google Scholar] [CrossRef]
- Guzmán, F.S.; Copp, B.R.; Mayne, C.L.; Concepcion, G.P.; Mangalindan, G.C.; Barrows, L.R.; Ireland, C.M. Bolinaquinone: A novel cytotoxic sesquiterpene hydroquinone from Philippine Dysidea sponge. J. Org. Chem. 1998, 63, 8042–804. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Shen, X.; Guo, Y. A novel sesquiterpene quinone from Hainan sponge Dysidea villosa. Bioorg. Med. Chem. Lett. 2009, 19, 390–392. [Google Scholar] [CrossRef]
- Jankam, A.; Somerville, M.J.; Hooper, J.N.A.; Brecknell, D.J.; Suksamrarna, A.; Garson, M.J. Dactylospongiaquinone, a new meroterpenoid from the Australian marine sponge Dactylospongia n. sp. Tetrahedron 2007, 63, 1577–1582. [Google Scholar] [CrossRef]
- Yong, K.W.L.; Jankam, A.; Hooper, J.N.A.; Suksamrarn, A.; Garson, M.J. Stereochemical evaluation of sesquiterpene quinones from two sponges of the genus Dactylospongia and the implication for enantioselective processes in marine terpene biosynthesis. Tetrahedron 2008, 64, 6341–6348. [Google Scholar] [CrossRef]
- Utkina, N.K.; Veselova, M.V. New sesquiterpene quinones from marine sponges of the order Dictyoceratida. Chem. Nat. Compd. 1990, 26, 37–40. [Google Scholar] [CrossRef]
- Swersey, J.C.; Barrows, L.R.; Ireland, C.M. Mamanuthaquinone: An antimicrobial and cytotoxic metabolite of Fasciospongia sp. Tetrahedron Lett. 1991, 32, 6687–6690. [Google Scholar] [CrossRef]
- Talpir, R.; Rudi, A.; Kashman, Y.; Loya, Y.; Hizi, A. Three new sesquiterpene hydroquinones from marine origin. Tetrahedron 1994, 50, 4179–4184. [Google Scholar] [CrossRef]
- Poigny, S.; Huor, T.; Guyot, M.; Samadi, M. Synthesis of (−)-hyatellaquinone and revision of absolute configuration of naturally occurring (+)-hyatellaquinone. J. Org. Chem. 1999, 64, 9318–9320. [Google Scholar] [CrossRef]
- Bernet, A.; Schröder, J.; Seifert, K. Total synthesis of the marine sesquiterpene quinones hyatellaquinone and spongiaquinone. Helv. Chim. Acta 2003, 86, 2009–2020. [Google Scholar] [CrossRef]
- McNamara, C.E.; Larsen, L.; Perry, N.B.; Harper, J.L.; Berridge, M.V.; Chia, E.W.; Kelly, M.; Webb, V.L. Anti-inflammatory sesquiterpene-quinones from the New Zealand sponge Dysidea cf. cristagalli. J. Nat. Prod. 2005, 68, 1431–1433. [Google Scholar]
- Coval, S.J.; Conover, M.A.; Mierzwa, R.; King, A.; Puar, M.S.; Phife, D.W.; Pai, J.K.; Burrier, R.E.; Ahn, H.S. Wiedendiol A and B, cholesteryl esther transfer protein inhibitors from marine sponge Xetospongia widenmayeri. Bioorg. Med. Chem. Lett. 1995, 5, 605–610. [Google Scholar] [CrossRef]
- Laube, T.; Bernet, A.; Dahse, H.-M.; Jacobsen, I.D.; Seifert, K. Synthesis and pharmacological activities of some sesquiterpene quinones and hydroquinones. Bioorg. Med. Chem. 2009, 17, 1422–1427. [Google Scholar] [CrossRef]
- Venkateswarlu, Y.; Faulkner, D.J.; Steiner, J.L.R.; Corcoran, E.; Clardy, J. Smenochromenes, unusual macrocyclic sesquiterpene hydroquinone derivatives from a Seychelles sponge of the genus Smenospongia. J. Org. Chem. 1991, 56, 6271–6274. [Google Scholar] [CrossRef]
- Goclik, E.; Konig, G.M.; Wright, A.D.; Kaminsky, R. Pelorol from the tropical marine sponge Dactylospongia elegans. J. Nat. Prod. 2000, 63, 1150–1152. [Google Scholar] [CrossRef]
- Sullivan, B.; Djura, P.; McIntyre, D.E.; Faulkner, D.J. Antimicrobial constituents of the sponge Siphonodictyon coralliphagum. Tetrahedron 1981, 37, 979–982. [Google Scholar] [CrossRef]
- Sullivan, B.; Faulkner, D.J.; Matsumoto, G.K.; He, C.H.; Clardy, J. Metabolites of the burrowing sponge Siphonodictyon coralliphagum. J. Org. Chem. 1986, 51, 4568–4573. [Google Scholar] [CrossRef]
- Grube, A.; Assmann, M.; Lichte, E.; Sasse, F.; Pawlik, J.R.; Koeck, M. Bioactive metabolites from the caribbean sponge Aka coralliphagum. J. Nat. Prod. 2007, 70, 504–509. [Google Scholar] [CrossRef]
- Kondracki, M.L.; Guyot, M. Smenospongine: A cytotoxic and antimicrobial aminoquinone isolated from Smenospongia sp. Tetrahedron Lett. 1987, 28, 5815–5818. [Google Scholar] [CrossRef]
- Kwak, J.H.; Schmitz, F.J.; Kelly, M. Sesquiterpene quinols/quinones from the Micronesian sponge Petrosaspongia metachromia. J. Nat. Prod. 2000, 63, 1153–1156. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Smenospongine, a spongean sesquiterpene aminoquinone, induces erythroid differentiation in K562 cells. Anticancer Drugs 2004, 15, 363–369. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Rachmat, R.; Kobayashi, M. M. Sesquiterpene aminoquinones, from a marine sponge, induce erythroid differentiation in human chronic myelogenous leukemia, K562 cells. Chem. Pharm. Bull. 2004, 52, 935–937. [Google Scholar] [CrossRef]
- Ovenden, S.P.B.; Nielson, J.L.; Liptrot, C.H.; Willis, R.H.; Tapiolas, D.M.; Wright, A.D.; Motti, C.A. Sesquiterpene benzoxazoles and sesquiterpene quinones from the marine sponge Dactylospongia elegans. J. Nat. Prod. 2011, 74, 65–68. [Google Scholar] [CrossRef]
- Augustijns, P.; Annaert, P.; Heylen, P.; van den Mooter, G.; Kinget, R. Drug absorption studies of prodrug esters using the Caco-2 model: Evaluation of ester hydrolysis and transepithelial transport. Int. J. Pharm. 1998, 166, 45–53. [Google Scholar] [CrossRef]
- Giannini, C.; Debitus, C.; Lucas, R.; Ubeda, A.; Paya, M.; Hooper, J.N.A.; D’Auria, M.V. New sesquiterpene derivatives from the sponge Dysidea species with a selective inhibitor profile against human phospholipase A2 and other leukocyte functions. J. Nat. Prod. 2001, 64, 612–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Guo, Y.; Jiang, H.; Shen, X. A sesquiterpene quinone, dysidine, from the sponge Dysidea villosa, activates the insulin pathway through inhibition of PTPas. Acta Pharmacol. Sin. 2009, 30, 333–345. [Google Scholar] [CrossRef]
- Diaz-Marrero, A.R.; Austin, P.; van Soest, R.; Matainaho, T.; Roskelley, C.D.; Roberge, M.; Andersen, R.J. Avinosol, a meroterpenoid-nucleoside conjugate with antiinvasion activity isolated from the marine sponge Dysidea sp. Org. Lett. 2006, 8, 3749–3752. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Yoshida, W.Y.; Scheuer, P.J. Popolohuanone A and B. Two new sesquiterpenoid aminoquinones from Pacific sponge Dysidea sp. Tetrahedron 1990, 46, 8025–8030. [Google Scholar]
- Carney, J.R.; Scheuer, P.J. Popolohuanone E, a topoisomerase II inhibitor eith selective lung citotoxicity from Pohnpei sponge Dysidea sp. Tetrahedron Lett. 1993, 34, 3727–3730. [Google Scholar] [CrossRef]
- Utkina, N.K.; Denisenko, V.A.; Krasokhin, V.B. Sesquiterpenoidd aminoquinones from marine sponge Dysidea sp. J. Nat. Prod. 2010, 73, 788–791. [Google Scholar] [CrossRef]
- Shigemori, H.; Madono, T.; Sasaki, T.; Mikami, Y.; Kobayashi, J. Nakijiquinones A and B, new antifungal sesquiterpenoid quinones with an amino acid residue from an Okinawan marine sponge. Tetrahedron 1994, 50, 8347–8354. [Google Scholar] [CrossRef]
- Kobayashi, J.; Madono, T.; Shigemori, H. Nakijiquinones C and D, new sesquiterpenoid quinones with a hydroxy amino acid residue from a marine sponge inhibiting c-erbB-2 kinase. Tetrahedron 1995, 51, 10867–10874. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, T.; Kobayashi, J. Nakijiquinones E and F, new dimeric sesquiterpenoid quinones from marine sponge. Bioorg. Med. Chem. 2009, 17, 2185–2188. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kubota, T.; Ito, J.; Mikami, Y.; Fromont, J.; Kobayashi, J. Nakijiquinones G–I, new sesquiterpenoid quinones from marine sponge. Bioorg. Med. Chem. 2008, 16, 7561–7564. [Google Scholar]
- Takahashi, Y.; Ushio, M.; Kubota, T.; Yamamoto, S.; Fromont, J.; Kobayashi, J. Nakijiquinones J–R, sesquiterpenoid quinones with an amine residue from Okinawan marine sponges. J. Nat. Prod. 2010, 73, 467–471. [Google Scholar] [CrossRef]
- Stahl, P.; Kissau, L.; Mazitschek, R.; Huwe, A.; Furet, P.; Giannis, A.; Waldmann, H. Total synthesis and biological evaluation of the nakijiquinones. J. Am. Chem. Soc. 2001, 123, 11586–11593. [Google Scholar]
- Mitome, H.; Nagasawa, T.; Miyaoka, H.; Yamada, Y.; van Soest, R.W.M. Dactyloquinones A and B, new sesquiterpenoid quinones from the okinawanmarine sponge Dactylospongia elegans. J. Nat. Prod. 2001, 64, 1506–1508. [Google Scholar] [CrossRef]
- Mitome, H.; Nagasawa, T.; Miyaoka, H.; Yamada, Y.; van Soest, R.W.M. Dactyloquinones C, D and E novel sesquiterpenoid quinones, from the Okinawan marine sponge, Dactylospongia elega. Tetrahedron 2002, 58, 1693–1696. [Google Scholar] [CrossRef]
- Djura, P.; Stierle, D.B.; Sullivan, B.; Faulkner, D.J.; Arnold, E.V.; Clardy, J. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (≡Polyfibrospongia) echina. J. Org. Chem. 1980, 45, 1435–1441. [Google Scholar] [CrossRef]
- Wright, A.E.; Rueth, S.A.; Cross, S.S. An antiviral sesquiterpene hydroquinone from the marine sponge Strongylophora hartmani. J. Nat. Prod. 1991, 54, 1108–1111. [Google Scholar] [CrossRef]
- Cimino, G.; de Stefano, S.; Minale, L. Chromazonarol, a chroman-sesquiterpenoid from sponge Dysidea pallenscens. Experientia 1975, 31, 1117. [Google Scholar] [CrossRef]
- Barrero, A.J.; Alvarez-Manzaneda, E.J.; Herrador, M.M.; Chahboun, R.; Galera, P. Synthesis and antitumoral activities of marine ent-chromazonarol and related compounds. Bioorg. Med. Chem. Lett. 1999, 9, 2325–2328. [Google Scholar] [CrossRef]
- Song, F.; Fan, X.; Xu, X.; Wang, S.; Li, S.; Yang, Y.; Shi, J. Studies on chemical constituents of the brown alga Dictyopteris divaricata. Zhongguo Zhong Yao Za Zhi 2006, 31, 125–128. [Google Scholar]
- Hideaki, I.; Kazuaki, I.; Hisashi, Y. A new artificial cyclase for poliprenoids: Enantioselective total synthesis of (−)-chromazonarol, (+)-8-epi-puupehedione and (−)-11′-deoxytaondiol methyl ether. J. Am. Chem. Soc. 2004, 126, 11133–11123. [Google Scholar]
- Ravi, B.N.; Perzanowski, H.P.; Ross, R.A.; Erdman, T.R.; Scheuer, P.J.; Finer, J.; Clardy, J. Recent research in marine natural products: The puupehenones. Pure Appl. Chem. 1979, 51, 1893–1900. [Google Scholar] [CrossRef]
- Nasu, S.S.; Yeung, B.K.S.; Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M.; Goins, K. Puupehenone-related metabolites from two Hawaiian sponges, Hyrtios spp. J. Org. Chem. 1995, 60, 7290–7292. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Debitus, C.; Guyot, M. Dipuupehedione, a cytotoxic new red dimer from a New Caledonian marine sponge Hyrtios sp. Tetrahedron Lett. 1996, 37, 3861–3864. [Google Scholar] [CrossRef]
- Bourguet-Kondracki, M.L.; Lacombe, F.; Guyot, M. Methanol adduct of puupehenone, a biologically active derivative from the marine sponge Hyrtios species. J. Nat. Prod. 1999, 62, 1304–1305. [Google Scholar] [CrossRef]
- Piña, I.C.; Sanders, M.L.; Crews, P. Puupehenone congeners from an Indo-Pacific Hyrtios sponge. J. Nat. Prod. 2003, 66, 2–6. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Gresa, M.P.L.; Gavagnin, M.; Romero, V.; Melck, D.; Manzo, E.; Guo, Y.W.; van Soest, R.; Cimino, G. Studies on puupehenone-metabolites of a Dysidea sp.: Structure and biological activity. Tetrahedron 2007, 63, 1380–1384. [Google Scholar] [CrossRef]
- Robinson, S.J.; Hoobler, E.K.; Riener, M.; Loveridge, S.T.; Tenney, K.; Valeriote, F.A.; Holman, T.R.; Crews, P. Using enzyme assays to evaluate the structure and bioactivity of sponge-derived meroterpenes. J. Nat. Prod. 2009, 72, 1857–1863. [Google Scholar] [CrossRef]
- Xu, W.H.; Ding, Y.; Jacob, M.R.; Agarwal, A.K.; Clark, A.M.; Ferreira, D.; Liang, Z.S.; Li, X.C. Puupehanol, a sesquiterpene-dihydroquinone derivative from the marine sponge Hyrtios sp. Bioorg. Med. Chem. Lett. 2009, 19, 6140–6143. [Google Scholar] [CrossRef]
- Amade, P.; Chevelot, L.; Perzanowski, H.P.; Scheuer, P.J. A dimer of puupehenone. Helv. Chim. Acta 1983, 66, 1672–1675. [Google Scholar]
- Castro, M.E.; González-Iriarte, M.; Barrero, A.F.; Salvador-Tormo, N.; Muñoz-Chápuli, R.; Medina, M.A.; Quesada, A.R. Study of puupehenone and related compounds as inhibitors of angiogenesis. Int. J. Cancer 2004, 110, 31–38. [Google Scholar]
- El Sayed, K.A.; Bartyzel, P.; Shen, X.; Perry, T.L.; Zjawiony, J.K.; Hamann, M.T. Marine natural products as antituberculosis agents. Tetrahedron 2000, 56, 949–953. [Google Scholar]
- Douat-Casassus, C.; Marchand-Geneste, N.; Diez, E.; Aznar, C.; Picard, P.; Geoffre, S.; Huet, A.; Bourguet-Kondracki, M.L.; Gervois, N.; Jotereau, F.; et al. Covalent modification of a melanoma-derived antigenic peptide with a natural quinone methide. Preliminary chemical, molecular modelling and immunological evaluation studies. Mol. BioSyst. 2006, 2, 240–249. [Google Scholar] [CrossRef]
- Utkina, N.K.; Denisenko, V.A.; Krasokhin, V.B. Diplopuupehenone, a new unsymmetrical puupehenone-related dimer from the marine sponge Dysidea sp. Tetrahedron Lett. 2011, 52, 3765–3768. [Google Scholar]
- Quideau, S.; Lebon, M.; Lamidey, A.M. Enantiospecific synthesis of the antituberculosis marine sponge metabolite (+)-puupehenone. The arenol oxidative activation route. Org. Lett. 2002, 4, 3975–3978. [Google Scholar] [CrossRef]
- Chan, J.A.; Freyer, A.J.; Carté, B.K.; Hemling, M.E.; Hofmann, G.H.; Mattern, M.R.; Mentzer, M.A.; Westley, J.W. Protein kinase C inhibitors: Novel spitosequiterpene aldehydes from a marine sponge Aka (=Siphonodictyon) coralliphagum. J. Nat. Prod. 1994, 57, 1543–1548. [Google Scholar] [CrossRef]
- Utkina, N.K.; Denisenko, V.A.; Scholokova, O.V.; Virovaya, M.V.; Prokof’eva, N.G. Cyclosmenospongine, a new sesquiterpenoid aminoquinone from an Australian marine sponge Spongia sp. Tetrahedron Lett. 2003, 44, 101–102. [Google Scholar] [CrossRef]
- Utkina, N.K.; Denisenko, V.A.; Scholokova, O.V.; Makarchenko, A.E. Determination of the absolute stereochemistry of cyclosmenospongine. J. Nat. Prod. 2003, 66, 1263–1265. [Google Scholar] [CrossRef]
- Mitome, H.; Nagasawa, T.; Miyaoka, H.; Yamada, Y.; van Soest, R.W.M. A new sesquiterpenoid quinone and other related compounds from theokinawan marine sponge Dactylospongia elegans. J. Nat. Prod. 2003, 66, 46–50. [Google Scholar] [CrossRef]
- Killday, K.B.; Wright, A.E. Bis(Sulfato)-cyclosiphonodictyol A, a new disulfated sesquiterpene-hydroquinone from a deep water collection of the marine sponge Siphonodictyon coralliphagum. J. Nat. Prod. 1995, 58, 958–960. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Killmer, L.; Offen, P.; Carte, B.; Jurewicz, A.J.; Johnson, R.K. Frondosins, five new sesquiterpene hydroquinone derivatives with novel skeletons from the sponge Dysidea frondosa: Inhibitors of interleukin-8 receptors. Tetrahedron 1997, 53, 5047–5060. [Google Scholar] [CrossRef]
- Jiao, W.-H.; Huang, X.-J.; Yang, J.-S.; Yang, F.; Piao, S.-J.; Gao, H.; Li, J.; Ye, W.-C.; Yao, X.; Chen, W.-S.; et al. Dysidavarones A–D, new sesquiterpene quinones from the marine sponge Dysidea avara. Org. Lett. 2012, 14, 202–205. [Google Scholar] [CrossRef]
- Nakamura, H.; Kobayashi, J.; Kobayashi, M.; Ohizumi, Y.; Hirata, Y. Xestoquinone. A novel cardiotonic marine natural product isolated from the Okinawan sea sponge Xestospongia sapra. Chem. Lett. 1985, 6, 713–716. [Google Scholar]
- Roll, D.M.; Scheuer, P.J. Halenaquinone, a pentacyclic polyketide from a marine sponge. J. Am. Chem. Soc. 1983, 105, 6177–6178. [Google Scholar] [CrossRef]
- Cao, S.; Foster, C.; Brisson, M.; Lazo, J.S.; Kingston, D.G.I. Halenaquinone and xestoquinone derivatives, inhibitors of Cdc25B phosphatase from a Xestospongia sp. Bioorg. Med. Chem. 2005, 13, 999–1003. [Google Scholar] [CrossRef]
- Concepcion, G.P.; Foderaro, T.A.; Eldredge, G.S.; Lobkovsky, E.; Clardy, J.; Barrows, L.R.; Ireland, C.M. Topoisomerase II-mediated DNA Cleavage by adocia- and xestoquinones from the philippine sponge Xestospongia sp. J. Med. Chem. 1995, 38, 4503–4507. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Bloor, S.J. Xesto- and halenaquinone derivatives from a sponge, Adocia sp., from truk lagoon. J. Org. Chem. 1988, 53, 3922–3925. [Google Scholar] [CrossRef]
- Desoubzdanne, D.; Marcourt, L.; Raux, R.; Chevalley, S.; Dorin, D.; Doerig, C.; Valentin, A.; Ausseil, F.; Debitus, C. Alisiaquinones and alisiaquinol, dual inhibitors of plasmodium falciparum enzyme targets from a new caledonian deep water sponge. J. Nat. Prod. 2008, 71, 1189–1192. [Google Scholar] [CrossRef]
- Zhang, H.; Khalil, Z.G.; Capon, R.J. Fascioquinols A–F: Bioactive meroterpenes from a deep-water southern Australian marine sponge Fasciospongia sp. Tetrahedron 2011, 67, 2591–2595. [Google Scholar] [CrossRef]
- Standish, A.J.; Salim, A.A.; Zhang, H.; Capon, R.J.; Morona, R. Chemical inhibition of bacterial protein tyrosine phosphatase suppresses capsule production. PLoS One 2012, 7, e36312. [Google Scholar]
- Standish, A.J.; Salim, A.A.; Capon, R.J.; Morona, R. Dual inhibition of DNA polymerase PolC and protein tyrosine phosphatase CpsB uncovers a novel antibiotic target. Biochem. Biophys. Res. Commun. 2013, 430, 167–172. [Google Scholar] [CrossRef]
- Tasdemir, D.; Concepción, G.P.; Mangalindan, G.C.; Harper, M.K.; Hajduc, E.; Ireland, C.M. New terpenoids from a Cacospongia sp. from the Philippines. Tetrahedron 2000, 56, 9025–9030. [Google Scholar] [CrossRef]
- Murray, L.M.; Johnson, A.; Diaz, M.C.; Crews, P. Geographic variation in the tropical marine sponge Jaspis cf. johnstoni: An unexpected source of new terpene-benzenoids. J. Org. Chem. 1997, 62, 5638–5641. [Google Scholar] [CrossRef]
- Rubio, B.K.; van Soest, R.W.M.; Crews, P. Extending the record of meroditerpenes from Cacospongia marine sponges. J. Nat. Prod. 2007, 70, 628–631. [Google Scholar] [CrossRef]
- Loll, P.J.; Axelsen, P.H. The structural biology of molecular recognition by vancomycin. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 265–289. [Google Scholar] [CrossRef]
- Carroll, J.; Jonsson, E.N.; Ebel, R.; Hartman, M.S.; Holman, T.R.; Crews, P. Probing sponge-derived terpenoids for human 15-lipoxygenase inhibitors. J. Org. Chem. 2001, 66, 6847–6851. [Google Scholar] [CrossRef]
- Williams, D.E.; Telliez, J.B.; Liu, J.; Tahir, A.; van Soest, R.; Andersen, R.J. Meroterpenoid MAPKAP (MK2) inhibitors isolated from the Indonesian marine sponge Acanthodendrilla sp. J. Nat. Prod. 2004, 67, 2127–2129. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Kenyon, V.A.; Whitman, S.; Morales, N.M.; Arguello, J.F.; Holman, T.R.; Crews, P. Redox inactivation of human 15-lipoxygenase by marine-derived meroditerpenes and synthetic chromanes: Archetypes for a unique class of selective and recyclable inhibitors. J. Am. Chem. Soc. 2004, 126, 14910–14920. [Google Scholar]
- Braekman, J.C.; Daloze, D.; Hulot, G.; Tursch, B.; Declercq, J.P.; Germain, G.; van Meerssche, M. Chemical studies of marine invertebrates. XXXVII. Three novel meroditerpenoids from the sponge Strongylophora durissima. Bull. Soc. Chim. Belg. 1978, 87, 917–926. [Google Scholar]
- Salva, J.; Faulkner, D.J. Metabolites of the sponge Strongylophora durissima from Maricaban Island, Philippines. J. Org. Chem. 1990, 55, 1941–1943. [Google Scholar] [CrossRef]
- Balbin-Oliveros, M.; Edrada, R.; Proksch, P.; Wray, V.; Witte, W.M.; van Soest, R.W.M. A new meroditerpenoid dimer from an undescribed Philippine marine sponge of the genus Strongylophora. J. Nat. Prod. 1998, 61, 948–952. [Google Scholar] [CrossRef]
- Liu, H.; Namikoshi, M.; Akano, K.; Kobayashi, H.; Nagai, H.; Yao, X. Seven new meroditerpenoids, from the marine sponge Strongylophora strongylata, that inhibited the maturation of starfish oocytes. J. Asian Nat. Prod. Res. 2005, 7, 661–670. [Google Scholar] [CrossRef]
- Warabi, K.; Patrick, B.O.; Austin, P.; Roskelley, C.D.; Roberge, M.; Andersen, R.J. Strongylophorine-26, an inhibitor of cancer cell invasion: SAR revealed by synthesis of analogues. J. Nat. Prod. 2007, 70, 736–740. [Google Scholar] [CrossRef]
- McHardy, L.M.; Warabi, K.; Andersen, R.J.; Roskelley, C.D.; Roberge, M. Strongylophorine-26, a Rho-dependent inhibitor of tumor cell invasion that reduces actin stress fibers and induces nonpolarized lamellipodial extensions. Mol. Cancer Ther. 2005, 4, 772–778. [Google Scholar] [CrossRef]
- Shen, Y.C.; Prakash, C.V. Two new acetylenic derivatives and a new meroditerpenoid from a Taiwanese marine sponge Strongylophora durissima. J. Nat. Prod. 2000, 63, 1686–1688. [Google Scholar] [CrossRef]
- Warabi, K.; McHardy, L.M.; Matainaho, L.; van Soest, R.W.M.; Roskelley, C.D.; Roberge, M.; Andersen, R.J. Strongylophorine-26, a new meroditerpenoid isolated from the marine sponge Petrosia (Strongylophora) corticata that exhibits anti-invasion activity. J. Nat. Prod. 2004, 67, 1387–1389. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Jadulco, R.C.; Bugni, T.S.; Harper, M.K.; Sturdy, M.; Ireland, C.M. Strongylophorines: Natural product inhibitors of hypoxia-inducible factor-1 transcriptional pathway. J. Med. Chem. 2008, 51, 1402–1405. [Google Scholar] [CrossRef]
- Sasaki, S.; Tozawa, T.; van Wagoner, R.M.; Ireland, C.M.; Harper, M.K.; Satoh, T. Strongylophorine-8, a pro-electrophilic compound from the marine sponge Petrosia (Strongylophora) corticata, provides neuroprotection through Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2011, 415, 6–10. [Google Scholar] [CrossRef]
- Bae, J.M.; Jeon, J.E.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, K.B.; Shin, J.H. Sesterterpenes from the tropical sponge Coscinoderma sp. J. Nat. Prod. 2011, 74, 1805–1811. [Google Scholar] [CrossRef]
- Alea, G.V.; Carroll, A.R.; Bowden, B.F. Coscinoquinol, a new cytotoxic sesterterpene from a dictyoceratid sponge, Coscinoderma sp. Aust. J. Chem. 1994, 47, 191–194. [Google Scholar] [CrossRef]
- Kernan, M.R.; Faulkner, D.J. Sesterterpene sulfates from a sponge of the family Halichondriidae. J. Org. Chem. 1988, 53, 4574–4578. [Google Scholar] [CrossRef]
- Loukaci, A.; Le Saout, I.; Samadi, M.; Leclerc, S.; Damiens, E.; Meijer, L.; Debitus, C.; Guyot, M. Coscinosulfate, a CDC25 phosphatase inhibitor from the sponge Coscinoderma mathewsi. Bioorg. Med. Chem. 2001, 9, 3049–3054. [Google Scholar] [CrossRef]
- Kimura, J.; Ishizuka, E.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borges, M. Isolation of 1-methylherbipoline salts of halisulfate-1 and of suvanine as serine protease inhibitors from a marine sponge, Coscinoderma mathewsi. J. Nat. Prod. 1998, 61, 248–250. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, T.H.; Yang, S.H.; Shin, H.J.; Shin, J.; Oh, K.B. Sesterterpene sulfates as isocitrate lyase inhibitors from tropical sponge Hippospongia sp. Bioorg. Med. Chem. Lett. 2007, 17, 2483–2486. [Google Scholar] [CrossRef]
- Shin, D.S.; Lee, T.H.; Lee, H.S.; Shin, J.; Oh, K.B. Inhibition of infection of the rice blast fungus by halisulfate 1, an isocitrate lyase inhibitor. FEMS Microbiol. Lett. 2007, 272, 43–47. [Google Scholar] [CrossRef]
- De Marino, S.; Festa, C.; D’Auria, M.V.; Bourguet-Kondracki, M.-L.; Petek, S.; Debitus, C.; Andres, R.M.; Terencio, M.C.; Paya, M.; Zampella, A. Coscinolactams A and B: New nitrogen-containing sesterterpenoids from the marine sponge Coscinoderma mathewsi exerting anti-inflammatory properties. Tetrahedron 2009, 65, 2905–2909. [Google Scholar] [CrossRef]
- Baratte, B.; Meijer, L.; Galaktionov, K.; Beach, D. Screening for antimitotic compounds using the cdc25 tyrosine phosphatase, an activator of the mitosis-inducing p34cdc2/cyclin Bcdc13 protein kinase. Anticancer Res. 1992, 12, 873–880. [Google Scholar]
- Isaacs, S.; Kashman, Y. Shaagrockol B and C; two hexaprenylhydroquinone disulfates from the Red Sea sponge Toxiclona toxius. Tetrahedron Lett. 1992, 33, 2227–2230. [Google Scholar] [CrossRef]
- Isaacs, S.; Hizi, A.; Kashman, Y. Toxicols A–C and toxiusol—New bioactive hexaprenoid hydroquinones from Toxiclona toxius. Tetrahedron 1993, 49, 4275–4282. [Google Scholar] [CrossRef]
- Blackburn, C.L.; Hopmann, C.; Sakowicz, R.; Berdelis, M.S.; Goldstein, L.S.B.; Faulkner, D.J. Adociasulfates 1–6, inhibitors of kinesin motor proteins from the sponge Haliclona (aka Adocia) sp. J. Org. Chem. 1999, 64, 5565–5570. [Google Scholar] [CrossRef]
- Blackburn, C.L.; Faulkner, D.J. Adociasulfate 10, a new merohexaprenoid sulfate from the sponge Haliclona (aka Adocia) sp. Tetrahedron 2000, 56, 8429–8432. [Google Scholar] [CrossRef]
- Kalaitzis, J.A.; Leone, P.A.; Harris, L.; Butler, M.S.; Ngo, A.; Hooper, J.N.A.; Quinn, R.J. Adociasulfates 1, 7, and 8: New bioactive hexaprenoid hydroquinones from the marine sponge Adocia sp. J. Org. Chem. 1999, 64, 5571–5574. [Google Scholar] [CrossRef]
- West, L.M.; Faulkner, D.J. Hexaprenoid hydroquinones from the sponge Haliclona (aka Adocia) sp. J. Nat. Prod. 2006, 69, 1001–1004. [Google Scholar] [CrossRef]
- Kalaitzis, J.A.; Quinn, R.J. Adociasulfate-9, a new hexaprenoid hydroquinone from the Great Barrier Reef sponge Adocia aculeata. J. Nat. Prod. 1999, 62, 1682–1684. [Google Scholar] [CrossRef]
- Barton, N.R.; Goldstein, L.S.B. Going mobile: Microtubule motors and chromosome segregation. Proc. Natl. Acad. Sci.USA 1996, 93, 1735–1742. [Google Scholar] [CrossRef]
- Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 1998, 279, 519–526. [Google Scholar] [CrossRef]
- Crews, P.; Harrison, B. New triterpene-ketides (merotriterpenes), haliclotriol A and B, from an indo-pacific Haliclona sponge. Tetrahedron 2000, 56, 9039–9046. [Google Scholar] [CrossRef]
- Trianto, A.; Hermawan, I.; de Voogd, N.J.; Tanaka, J. Halioxepine, a new meroditerpene from an Indonesian sponge Haliclona sp. Chem. Pharm. Bull. 2011, 59, 1311–1313. [Google Scholar] [CrossRef]
- Fisch, K.M.; Böhm, V.; Wright, A.D.; König, G.M. Antioxidative meroterpenoids from the brown alga Cystoseira crinite. J. Nat. Prod. 2003, 66, 968–975. [Google Scholar] [CrossRef]
- Williams, D.E.; Steinø, A.; de Voogd, N.J.; Mauk, A.G.; Andersen, R.J. Halicloic acids A and B isolated from the marine sponge Haliclona sp. collected in the Philippines inhibit indoleamine 2,3-dioxygenase. J. Nat. Prod. 2012, 75, 1451–1458. [Google Scholar] [CrossRef]
- Fukami, A.; Ikeda, Y.; Kondo, S.; Naganawa, H.; Takeuchi, T.; Furuya, S.; Hirabayashi, Y.; Shimoike, K.; Hosaka, S.; Watanabe, Y.; Umezawa, K. Akaterpin, a novel bioactive triterpene from the marine sponge Callyspongia sp. Tetrahedron Lett. 1997, 38, 1201–1202. [Google Scholar] [CrossRef]
- Gray, C.A.; de Lira, S.P.; Silva, M.; Pimenta, E.F.; Thiemann, O.H.; Oliva, G.; Hajdu, E.; Andersen, R.J.; Berlinck, R.G.S. Sulfated meroterpenoids from the Brazilian sponge Callyspongia sp. are inhibitors of the antileishmaniasis target adenosine phosphoribosyl transferase. J. Org. Chem. 2006, 71, 8685–8690. [Google Scholar] [CrossRef]
- Hooper, G.J.; Davies-Coleman, M.T. Sesquiterpene hydroquinones from the South African soft coral Alcyonium fauri. Tetrahedron Lett. 1995, 36, 3265–3268. [Google Scholar] [CrossRef]
- Sheu, J.H.; Su, J.H.; Sung, P.J.; Wang, G.H.; Dai, C.F. Novel meroditerpenoid-related metabolites from the formosan soft coral Nephthea chabrolii. J. Nat. Prod. 2004, 67, 2048–2052. [Google Scholar] [CrossRef]
- Su, J.H.; Ahmed, A.F.; Sung, P.J.; Wu, Y.C.; Sheu, J.H. Terpenoid-related metabolites from a formosan soft coral Nephthea Chabrolii. J. Nat. Prod. 2005, 68, 1651–1655. [Google Scholar] [CrossRef]
- Su, J.H.; Dai, C.F.; Huang, H.H.; Wu, Y.C.; Sung, P.J.; Hsu, C.H.; Sheu, J.H. Terpenoid-related metabolites from a Formosan soft coral Nephthea chabrolii. Chem. Pharm. Bull. 2007, 55, 594–597. [Google Scholar] [CrossRef]
- Chen, J.W.; Luo, Y.L.; Hwang, M.J.; Peng, F.C.; Ling, K.H.J. Territrem B, a tremorgenic mycotoxin that inhibits acetylcholinesterase with a noncovalent yet irreversible binding mechanism. Biol. Chem. 1999, 274, 34916–34923. [Google Scholar]
- Cueto, M.; MacMillan, J.B.; Jensen, P.R.; Fenical, W. Tropolactones A–D, four meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry 2006, 67, 1826–1831. [Google Scholar] [CrossRef]
- Cohen, E.; Koch, L.; Myint Thu, K.; Rahamim, Y.; Aluma, Y.; Ilan, M.; Yarden, O.; Carmeli, S. Novel terpenoids of the fungus Aspergillus insuetus isolated from the Mediterranean sponge Psammocinia sp. collected along the coast of Israel. Bioorg. Med. Chem. 2011, 19, 6587–6593. [Google Scholar] [CrossRef]
- Lopez-Gresa, M.P.; Cabedo, N.; Gonzalez-Mas, M.C.; Ciavatta, M.L.; Avila, C.; Primo, J. Terretonins E and F, inhibitors of the mitochondrial respiratory chain from the marine-derived fungus Aspergillus insuetus. J. Nat. Prod. 2009, 72, 1348–1351. [Google Scholar] [CrossRef]
- Zhou, Y.; Mándi, A.; Debbab, A.; Wray, V.; Schulz, B.; Müller, W.E.G.; Lin, W.H.; Proksch, P.; Kurtán, T.; Aly, A.H. New austalides from the sponge-associated fungus Aspergillus sp. Eur. J. Org. Chem. 2011, 2011, 6009–6019. [Google Scholar]
- Bugni, T.S.; Abbanat, D.; Bernan, V.S.; Malese, W.M.; Greenstein, M.; van Wagoner, R.; Ireland, C.M. Yanuthones: Novel metabolites from a marine isolate of Aspergillus niger. J. Org. Chem. 2000, 65, 7195–7200. [Google Scholar] [CrossRef]
- Sanchez, J.F.; Somoza, A.D.; Kellerc, N.P.; Wang, C.C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat. Prod. Rep. 2012, 29, 351–371. [Google Scholar] [CrossRef]
- Lo, H.; Entwistle, R.; Guo, C.; Ahuja, M.; Szewczyk, E.; Hung, J.; Chiang, Y.; Oakley, B.R.; Wang, C.C.C. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. J. Am. Chem. Soc. 2012, 134, 4709–4720. [Google Scholar] [CrossRef]
- Guo, C.; Knox, B.P.; Chiang, Y.; Lo, H.; Sanchez, J.F.; Lee, K.; Oakley, B.R.; Bruno, K.S.; Wang, C.C.C. Molecular genetic characterization of a cluster in A. terreus for biosynthesis of the meroterpenoid Terretonin. Org. Lett. 2012, 14, 5684–5687. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Menna, M.; Imperatore, C.; D'Aniello, F.; Aiello, A. Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications. Mar. Drugs 2013, 11, 1602-1643. https://doi.org/10.3390/md11051602
Menna M, Imperatore C, D'Aniello F, Aiello A. Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications. Marine Drugs. 2013; 11(5):1602-1643. https://doi.org/10.3390/md11051602
Chicago/Turabian StyleMenna, Marialuisa, Concetta Imperatore, Filomena D'Aniello, and Anna Aiello. 2013. "Meroterpenes from Marine Invertebrates: Structures, Occurrence, and Ecological Implications" Marine Drugs 11, no. 5: 1602-1643. https://doi.org/10.3390/md11051602





