Exposure to the Neurotoxic Dinoflagellate, Alexandrium catenella, Induces Apoptosis of the Hemocytes of the Oyster, Crassostrea gigas
Abstract
:1. Introduction
2. Results and Discussion
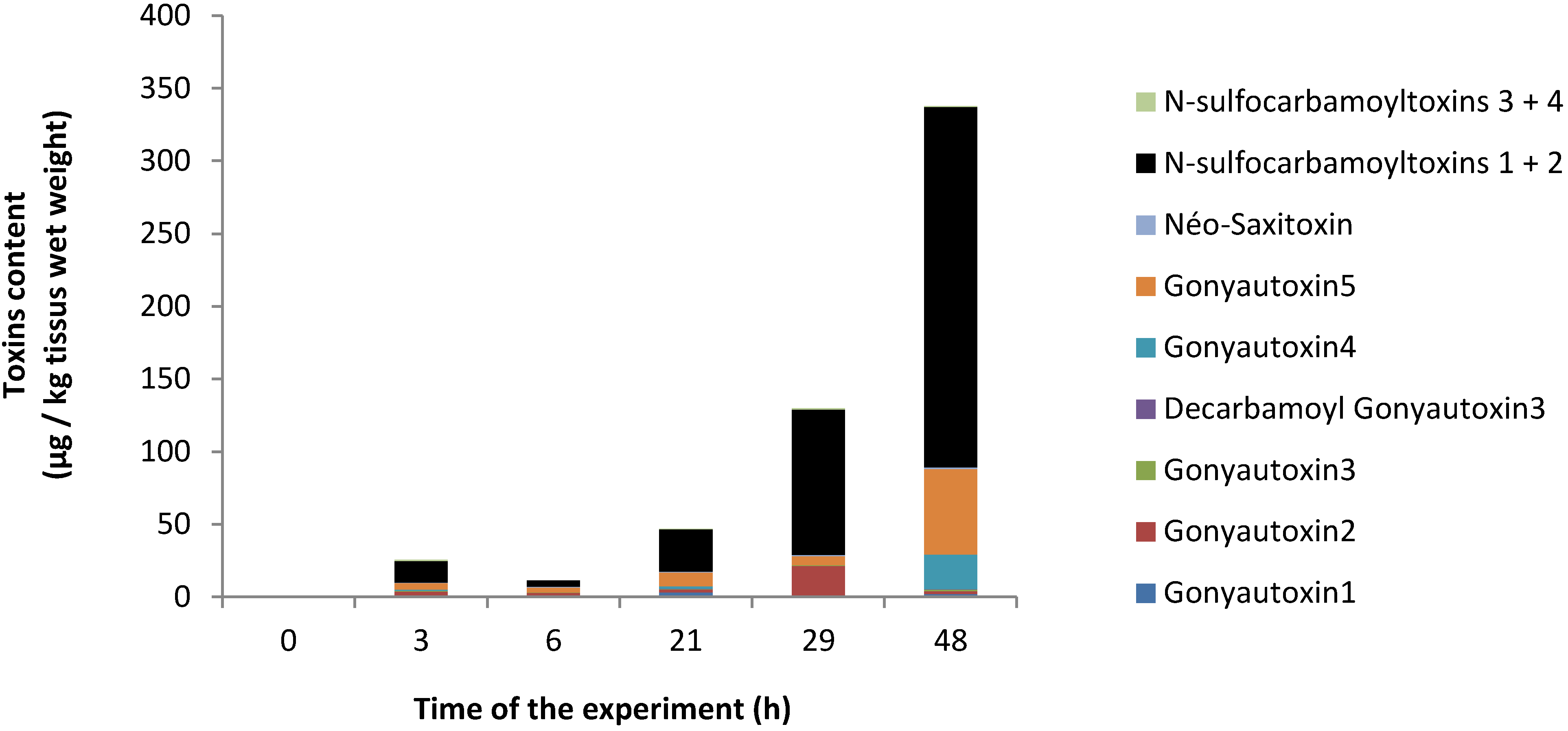
2.1. PSP Accumulation in Oyster Tissues
2.2. Level of Hemocytes in Apoptosis

2.3. Temporal Expression of the Genes Related to Apoptotic Processes
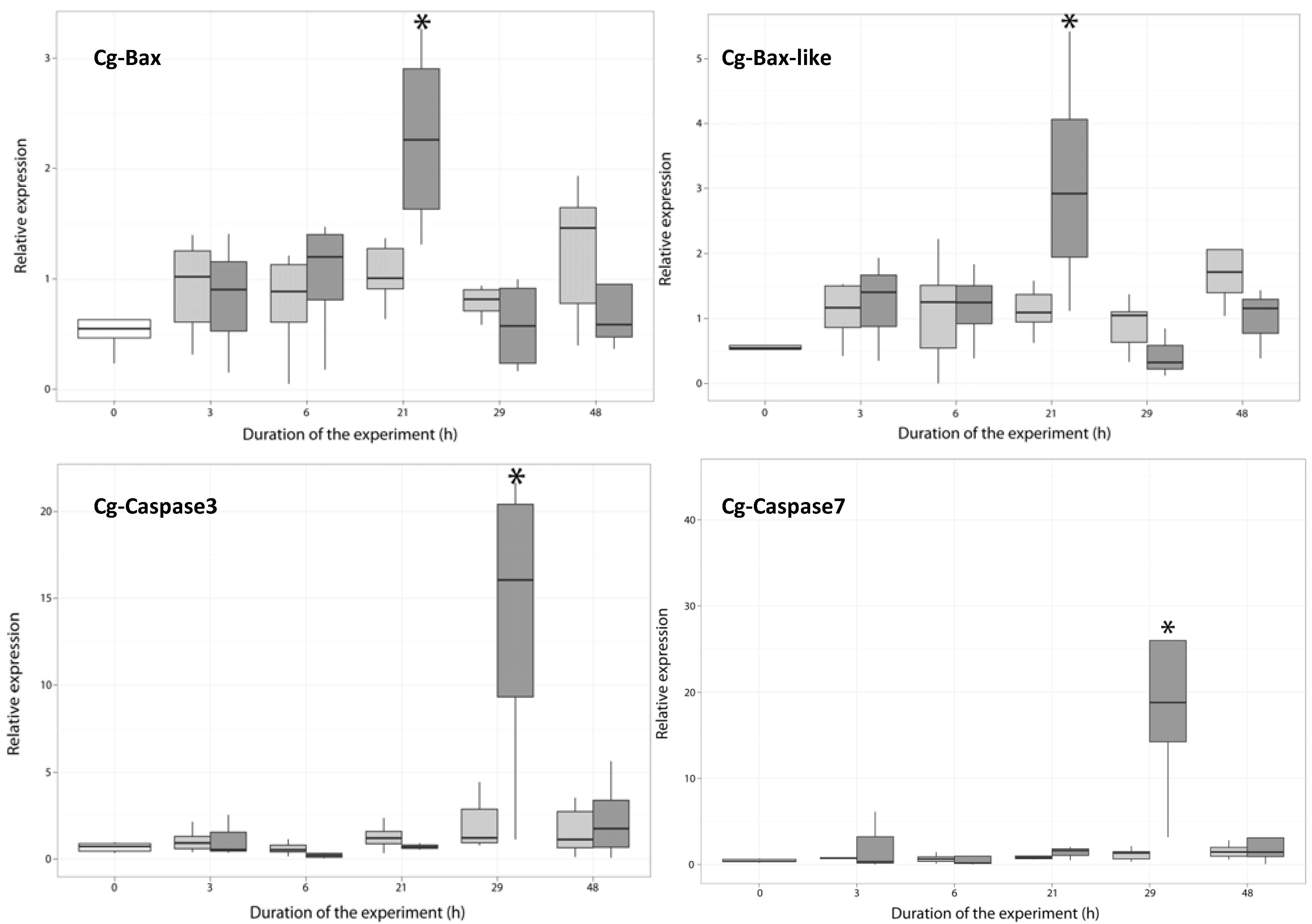
2.3.1. Expression of Apoptosis-Related Genes
2.3.2. Expression of Anti-Apoptosis-Related Genes

3. Experimental Section
3.1. Oysters and Microalgae
3.2. Experimental Exposures
3.3. Tissue Sampling
3.4. Chemical Analysis of PSP Toxin by Liquid Chromatography/Fluorescence Detection (LC/FD)
3.5. Expression Analysis of Putative Apoptotic-Related Genes
| Gene | Primers sequences 5'→3' | Product size (bp) | GenBank ID |
|---|---|---|---|
| Cg-FADD | AAGAGAAAGTGTCAACCGAC CTCTCAAAACATCAAGACGG | 134 | HQ425700 |
| Cg-Bax like | AGGATAGCACTCTATGCAGG TCAACTCCTAGCAACCATGG | 198 | AM855407 |
| Cg-Bax1 | TCCACTGGAATATGTTCGAG GAAAGTTTCATGGTTTGCAC | 124 | HS140552 |
| Cg-Bcl2 | CAACTGTGACAAACGAGATG AGTCTACTAACTGTGGCATG | 123 | EU678310 |
| Cg-BI-1 | AATGGGCTTCCTGAGGAAGG GCAACCAACAGCATCCAGTG | 134 | HS115415 |
| Cg-IAP1 | TCGAGCAGCAATTTAACGC GAGGAAGGAGCTTTACCAC | 160 | HQ425702 |
| Cg-IAP7B | CATTATGGAAGCAGATAGATC ATGATGTCATCTTCCTTTGTC | 249 | FP000296 |
| Cg-caspase-2 | ACAGGGGAAATACTGAAGGAC AGCTACAGCTGTCAGAAAACC | 162 | HQ425706 |
| Cg-caspase-3 | ATCACCAGGAAGGATCATGG GTTCATCCGAACACGACTCG | 139 | CU988427 |
| Cg-caspase-7 | ATTGGACCACAGAGACAACG TGTTGCCTTTGAAGGGCTCC | 125 | HQ425703 |
| Cg-Hsp27 | GGCAAAGACCCATTTGGTAA ACAGTCAAGTTCCGGTCCAC | 206 | AM862573 |
| Cg-Hsp70 | TCATCAAGTGGATGGACCAG CATTCCTCCAGGCATGCCA | 149 | AF144646 |
| Ribosomal protein F40 (RPL40) | AATCTTGCACCGTCATGCAG AATCAATCTCTGCTGATCTGG | 149 | FP004478 |
3.6. Determination of Hemocyte Apoptosis Levels
3.7. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sokolova, I.M. Apoptosis in molluscan immune defense. ISJ-Invertebr. Surv. J. 2009, 6, 49–58. [Google Scholar]
- Sunila, I.; LaBanca, J. Apoptosis in the pathogenesis of infectious diseases of the eastern oyster Crassostrea virginica. Dis. Aquat. Org. 2003, 56, 163–170. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Zhang, G. Gene discovery, comparative analysis and expression profile reveal the complexity of the Crassostrea gigas apoptosis system. Dev. Comp. Immunol. 2011, 35, 603–610. [Google Scholar] [CrossRef]
- Armstrong, J.S. The role of the mitochondrial permeability transition in cell death. Mitochondrion 2006, 6, 225–234. [Google Scholar] [CrossRef]
- Wolf, B.B.; Green, D.R. Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J. Biol. Chem. 1999, 274, 20049–20052. [Google Scholar] [CrossRef]
- Deveraux, Q.L.; Reed, J.C. IAP family proteins: Suppressors of apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef]
- Cory, S.; Huang, D.C.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef]
- Robertson, D.M.; Ladage, P.M.; Yamamoto, N.; Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Bcl-2 and Bax regulation of corneal homeostasis in genetically altered mice. Eye Contact Lens 2006, 32, 3–7. [Google Scholar] [CrossRef]
- Orme, M.; Meier, P. Inhibitor of apoptosis proteins in Drosophila: Gatekeepers of death. Apoptosis 2009, 14, 950–960. [Google Scholar] [CrossRef]
- Tatsushi, I.; Masayuki, M. Role of Bcl-2 family members in invertebrates. Biochim. Biophys. Acta 2004, 1644, 73–81. [Google Scholar] [CrossRef]
- Green, D.R.; Bissonnette, R.P.; Cotter, T.G. Apoptosis and cancer. Important Adv. Oncol. 1994, 11, 37–52. [Google Scholar]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; DenisLarose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 1997, 17, 5317–5327. [Google Scholar]
- Garrido, C.; Bruey, J.M.; Fromentin, A.; Hammann, A.; Arrigo, A.P.; Solary, E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999, 13, 2061–2070. [Google Scholar]
- Pandey, P.; Farber, R.; Nakazawa, A.; Kumar, S.; Bharti, A.; Nalin, C.; Weichselbaum, R.; Kufe, D.; Kharbanda, S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 2000, 19, 1975–1981. [Google Scholar] [CrossRef]
- Clark, R.F.; Williams, S.R.; Nordt, S.P.; Manoguerra, A.S. A review of selected seafood poisonings. Undersea Hyperb. Med. 1999, 26, 175–184. [Google Scholar]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Li, A.M.Y.; Yu, P.K.N.; Hsieh, D.P.H.; Wang, W.X.; Wu, R.S.S.; Lam, P.K.S. Uptake and depuration of paralytic shellfish toxins in the green lipped mussel, Perna viridis: A dynamic model. Environ. Toxicol. Chem. 2005, 24, 129–135. [Google Scholar] [CrossRef]
- Shumway, S.E. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacul. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- Bardouil, M.; Bohec, M.; Cormerais, M.; Bougrier, S.; Lassus, P. Experimental study of the effects of a toxic microalgal diet on feeding of the oyster Crassostrea gigas Thunberg. J. Shellfish Res. 1993, 12, 417–422. [Google Scholar]
- Wildish, D.; Lassus, P.; Martin, J.; Saulnier, A.; Bardouil, M. Effect of the PSP-causing dinoflagellate, Alexandrium sp. on the initial feeding response of Crassostrea gigas. Aquat. Living Resour. 1998, 11, 35–43. [Google Scholar] [CrossRef]
- Lassus, P.; Baron, R.; Garen, P.; Truquet, P.; Masselin, P.; Bardouil, M.; Leguay, D.; Amzil, Z. Paralytic shellfish poison outbreaks in the Penze estuary: Environmental factors affecting toxin uptake in the oyster, Crassostrea gigas. Aquat. Living Resour. 2004, 17, 207–214. [Google Scholar] [CrossRef]
- Rolland, J.L.; Pelletier, K.; Masseret, E.; Rieuvilleneuve, F.; Savar, V.; Santini, A.; Amzil, Z.; Laabir, M. Paralytic toxins accumulation and tissue Expression of α-amylase and lipase genes in the pacific oyster Crassostrea gigas fed with the neurotoxic dinoflagellate Alexandrium catenella. Mar. Drugs 2012, 10, 2519–2534. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Qiu, L.; Zhang, H. Bivalve Immunity. In Invertebrate Immunity; Soderhall, K., Ed.; Springer: New York, NY, USA, 2010; Volume 708, p. 316. [Google Scholar]
- Kiss, T. Apoptosis and its functional significance in mollusks. Apoptosis 2010, 15, 313–321. [Google Scholar] [CrossRef]
- Matozzo, V.; Rova, G.; Marin, M.G. Haemocytes of the cockle Cerastoderma glaucum: Morphological characterisation and involvement in immune responses. Fish Shellfish Immunol. 2007, 23, 732–746. [Google Scholar] [CrossRef]
- Ifremer Environnement Littoral. Available online: http://envlit.ifremer.fr/var/envlit/storage/documents/dossiers/toxines10ans/ (accessed on 18 November 2013).
- Kiss, T.; Osipenko, O.N. Toxic effects of heavy metals on ionic channels. Pharmacol. Rev. 1994, 46, 245–267. [Google Scholar]
- Molnar, G.; Salanki, J.; Kiss, T. Cadmium inhibits GABA-activated ion currents by increasing intracellular calcium level in snail neurons. Brain Res. 2004, 1008, 205–211. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Evans, S.; Hughes, F.M. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J. Exp. Biol. 2004, 207, 3369–3380. [Google Scholar] [CrossRef]
- Guo, B.; Yin, M.B.; Tóth, K.; Cao, S.; Azrak, R.G.L.; Rustum, Y.M. Dimerization of mitochondrial Bax is associated with increased drug response in Bax-transfected A253 cells. Oncol. Res. 1999, 11, 91–99. [Google Scholar]
- Jurgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef]
- Rautureau, G.J.P.; Day, C.L.; Hinds, M.G. Intrinsically disordered proteins in Bcl-2 regulated apoptosis. Int. J. Mol. Sci. 2010, 11, 1808–1824. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X-L during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672. [Google Scholar]
- Antonsson, B.; Montessuit, S.; Sanchez, B.; Martinou, J.-C. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001, 276, 11615–11623. [Google Scholar]
- Enari, M.; Talanian, R.V.; Wrong, W.W.; Nagata, S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature 1996, 380, 723–726. [Google Scholar] [CrossRef]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell. Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef]
- Salvesen, G.S.; Abrams, J.M. Caspase activation–stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene 2004, 23, 2774–2784. [Google Scholar] [CrossRef]
- Tran, S.E.; Meinander, A.; Eriksson, J.E. Instant decisions: Transcription-independent control of death-receptor-mediated apoptosis. Trends Biochem. Sci. 2004, 29, 601–608. [Google Scholar] [CrossRef]
- Guo, Y.; Srinivasula, S.M.; Druilhe, A.; Fernandes-Alnemri, T.; Alnemri, E.S. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J. Biol. Chem. 2002, 277, 13430–13437. [Google Scholar]
- Hanada, M.; Aimé-Sempé, C.; Sato, T.; Reed, J.C. Structure-function analysis of Bcl-2 protein identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 1995, 270, 11962–11969. [Google Scholar]
- Xu, Q.; Reed, J.C. Bax Inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1988, 1, 337–346. [Google Scholar]
- Hetz, C.; Vitte, P.A.; Bombrun, A.; Bombrun, A.; Rostovtseva, T.K.; Montessuit, S.; Hiver, A.; Schwarz, M.K.; Church, D.J.; Korsmeyer, S.J.; et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 2005, 280, 42960–42970. [Google Scholar] [CrossRef]
- Ahn, T.; Yun, C.H.; Kim, H.R.; Chae, H.J. Cardiolipin, phospha-tidylserine, and BH4 domain of Bcl-2 family regulate Ca2+/H+ antiporter activity of human Bax inhibitor-1. Cell Calcium 2010, 47, 387–396. [Google Scholar] [CrossRef]
- Dubrez-Daloz, L.; Dupoux, A.; Cartier, J. IAPs: More than just inhibitors of apoptosis proteins. Cell Cycle 2008, 7, 1036–1046. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Wang, J. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Wei, Y.; Fan, T.; Yu, M. Inhibitor of apoptosis proteins and apoptosis. Acta Biochim. Biophys. Sin. 2008, 40, 278–288. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Fabbri, E.; Valbonesi, P.; Franzellitti, S. HSP expression in bivalves. ISJ-Invertebr. Surviv. J. 2008, 5, 135–161. [Google Scholar]
- Boutet, I.; Tanguy, A.; Moraga, D. Response of the Pacific oyster Crassostrea gigas to hydrocarbon contamination under experimental conditions. Gene 2004, 329, 147–157. [Google Scholar] [CrossRef]
- Mello, D.F.; de Oliveira, E.S.; Vieira, R.C.; Simoes, E.; Trevisan, R.; Dafre, A.L.; Barracco, M.A. Cellular and transcriptional responses of Crassostrea gigas hemocytes exposed in vitro to brevetoxin (PbTx-2). Mar. Drugs 2012, 10, 583–597. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat shock genes–integrating cell survival and death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Tirard, C.T.; Grossfeld, R.M.; Volety, A.K.; Chu, F.L.E. Heat-shock proteins of the oyster parasite perkinsus-marinus. Dis. Aquat. Org. 1995, 22, 147–151. [Google Scholar] [CrossRef]
- Encomio, V.G.; Chu, F.L.E. Seasonal variation of heat shock protein 70 in eastern oysters (Crassostrea virginica) infected with Perkinsus marinus (Dermo). J. Shellfish Res. 2005, 24, 167–175. [Google Scholar]
- Boutet, I.; Tanguy, A.; Rousseau, S.; Auffret, M.; Moraga, D. Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Harrison, P.J.; Waters, R.E.; Taylor, F.J.R. A broad-spectrum artificial seawater medium for coastal and open ocean phytoplankton. J. Phycol. 1980, 16, 28–35. [Google Scholar]
- Laabir, M.; Jauzein, C.; Genovesi, B.; Masseret, E.; Grzebyk, D.; Cecchi, P.; Vaquer, A.; Perrin, Y.; Colos, Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011, 33, 1550–1563. [Google Scholar] [CrossRef]
- Oshima, Y. Chemical and Enzymatic Transformation of Paralytic Shellfish Toxins in Marine Organisms. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier/Intercept: Paris, France, 1995; pp. 475–480. [Google Scholar]
- Lacoste, A.; Jalabert, F.; Malham, S.; Cueff, A.; Gelebart, F.; Cordevant, C.; Lange, M.; Poulet, S.A. A vibrio splendidus strain is associated with summer mortality of juvenile oysters Crassostrea gigas in the bay of Morlaix (North Brittany, France). Dis. Aquat. Org. 2001, 46, 139–145. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Degremont, L. Evidence of herpesvirus (OsHV-1) resistance in juvenile Crassostrea gigas selected for high resistance to the summer mortality phenomenon. Aquaculture 2011, 317, 94–98. [Google Scholar] [CrossRef]
- Garnier, M.; Labreuche, Y.; Garcia, C.; Robert, A.; Nicolas, J.L. Evidence for the involvement of pathogenic bacteria in summer mortalities of the Pacific oyster Crassostrea gigas. Microb. Ecol. 2007, 53, 187–196. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Medhioub, W.; Ramondenc, S.; Vanhove, A.S.; Vergnes, A.; Masseret, E.; Savar, V.; Amzil, Z.; Laabir, M.; Rolland, J.L. Exposure to the Neurotoxic Dinoflagellate, Alexandrium catenella, Induces Apoptosis of the Hemocytes of the Oyster, Crassostrea gigas. Mar. Drugs 2013, 11, 4799-4814. https://doi.org/10.3390/md11124799
Medhioub W, Ramondenc S, Vanhove AS, Vergnes A, Masseret E, Savar V, Amzil Z, Laabir M, Rolland JL. Exposure to the Neurotoxic Dinoflagellate, Alexandrium catenella, Induces Apoptosis of the Hemocytes of the Oyster, Crassostrea gigas. Marine Drugs. 2013; 11(12):4799-4814. https://doi.org/10.3390/md11124799
Chicago/Turabian StyleMedhioub, Walid, Simon Ramondenc, Audrey Sophie Vanhove, Agnes Vergnes, Estelle Masseret, Veronique Savar, Zouher Amzil, Mohamed Laabir, and Jean Luc Rolland. 2013. "Exposure to the Neurotoxic Dinoflagellate, Alexandrium catenella, Induces Apoptosis of the Hemocytes of the Oyster, Crassostrea gigas" Marine Drugs 11, no. 12: 4799-4814. https://doi.org/10.3390/md11124799






