1. Introduction
Sargassum muticum (Yendo) Fensholt is an invasive macroalga living on the Western coasts that causes negative impacts on ecology, fishing and recreational activities. The unsuccessful eradication trials suggest a possible valorization of this alga biomass. The potential use of the whole alga as a low cost adsorbent for heavy metals’ removal was confirmed [
1]. Also,
S. muticum has been used as a substrate for hydrothermal processing, yielding a soluble fraction with antioxidant activity and a solid residue with potential as a fertilizer [
2].
Marine brown seaweeds contain alginate, laminaran and sulfated polysaccharides known as fucoidans. Fucoidans are made up of fucose, galactose, mannose, xylose, glucose, uronic acids, sulfate substituents, and acetyl groups; and may also contain some protein components. Algal fucoidans are highly heterogeneous in structure, oppositely to the fucan sulfates from marine invertebrates, which are composed mainly of sulfated
l-fucose and present a more simple and regular structure. Fucoidans may differ considerably in composition, molecular mass and structure, depending on the algal species considered [
3], geographic location, environmental conditions, harvest season, vegetative and generative life-stages [
4] or on the type of tissues sampled [
5]. On the other hand, the extraction and purification conditions may affect the polysaccharide composition and structure of the isolated compounds (including charge density, distribution, degree of sulfation, molecular mass and linkage pattern) [
6,
7].
Since fucoidans can interfere with molecular mechanisms of cell-to-cell recognition, they are potent blockers of some biological processes, showing a variety of activities, including antiviral, antiinflammatory, antiangiogenic, antiproliferative, antitumoral, anticoagulant, immunomodulating and antioxidant properties [
4,
8,
9,
10]. Due to their non-toxic character, fucoidans have been recently explored for medicinal properties, but their detailed structural analysis is complex [
11], and the structure–activity interrelationships are not yet clear [
6,
12].
Sargassum sp. is a potential source of fucose-containing sulfated polysaccharides [
6], whose biological properties have received attention recently [
13]. Some brown seaweed fucoidans have a backbone of 3-linked α-
l-fucopyranose, whereas in other cases the backbone presents alternating 3- and 4-linked α-
l-fucopyranose residues and sulfated galactofucans [
11]. These latter compounds are prominently found in various
Sargassum species [
14], and are mainly built of (1→6)-β-
d-galactose and/or (1→2)-β-
d-mannose units with branching points formed by (1→3) and/or (1→4)-α-
l-fucose, (1→4)-α-
d-glucuronic acid, terminal β-
d-xylose and sometimes (1→4)-α-
d-glucose [
14].
Crude fucoidans have been extracted from brown algae with water [
14], diluted acid [
4], diluted alkali, and 2% aqueous calcium chloride [
3] under mild conditions. The extraction conditions have to be controlled carefully, as fucoidans are sensitive to degradation [
6]. Since some methods cannot analyze large molecules, partial depolymerization of fucoidans has been carried out with acids [
4] or radicals [
12] before analysis. Low-chemical processes have been proposed for isolating pure, native fucoidans [
7,
9,
10] in order to obtain products with enhanced biological activities [
7,
12].
Hydrothermal processing (also called autohydrolysis) is an autocatalyzed reaction suitable for the fractionation of vegetal biomass [
15,
16,
17] that has been applied to alginate [
18] and for algae fractionation, looking at the manufacture of multisulfated oligosaccharides [
3] with structural features similar to the ones of the parent polymer [
4].
This study provides an assessment on the aqueous processing of raw and alginate extracted Sargassum muticum biomass, with an emphasis on the sugar composition of the solubilized fraction. The effects of maximal temperature achieved in non-isothermal treatments on the fucoidan extraction yield, as well as on both composition and radical scavenging properties of the resulting products were measured.
2. Results and Discussion
The extraction methodology and conditions affect the composition, structure and size of the fucoidan fractions, which determine their functional properties and bioactivity. Alginate is one of the major components of
Sargassum sp. [
19,
20], for which biological activities have been reported [
21]. The proximate composition of the algal biomass (Sm) and the corresponding alginate-depleted product (AESm) are summarized in
Table 1. Sm contained 26% minerals, 7% protein, 26% saccharides, and 20% acid insoluble residue. After removing alginate and extractives, the proportion of the other components in the AESm increased correspondently. Considering the yields of the process [
2], 3.52 g fucose were present in 88.8 g Sm (d. b.), but only 2.29 g fucose were recovered in AESm. Crude fucoidan accounted for 4 wt%, in comparison with less than 1 wt% reported for
Sargassum sp. [
20], 8 wt% for
S. muticum [
19], and 7–9 wt% for sterile and reproductive tissues of
S. pallidum [
5]. The streams and products obtained during processing of Sm and AESm are indicated in the flow diagram of
Figure 1.
Table 1.
Composition of the raw materials used in this study.
Table 1.
Composition of the raw materials used in this study.
| Component | Content (wt%) |
|---|
| | Sm | AESm |
| Glucose | 8.41 ± 0.19 | 12.15 ± 0.08 |
| Xylose | 0.88 ± 0.05 | 0.78 ± 0.02 |
| Galactose | 2.52 ± 0.11 | 2.13 ± 0.07 |
| Mannose | 0.33 ± 0.02 | 0.42 ± 0.04 |
| Fucose | 3.97 ± 0.07 | 4.02 ± 0.14 |
| AIR 1 | 20.28 ± 0.18 | 32.72 ± 3.79 |
| Acetyl groups | 0.22 ± 0.01 | 0.26 ± 0.02 |
| Alginate | 10.23 ± 0.75 | |
| Protein | 6.92 ± 0.08 | 8.38 ± 0.04 |
| Ash | 26.04 ± 0.07 | 30.66 ± 1.49 |
| Total phenolics 2 | 1.03 ± 0.11 | |
| Extractives | | |
| 96% Ethanol | 6.32 ± 0.24 | 0.16 ± 0.01 |
| Ethyl acetate | 5.88 ± 0.27 | 1.42 ± 0.09 |
| Hexane | 4.10 ± 0.99 | 2.39 ± 0.08 |
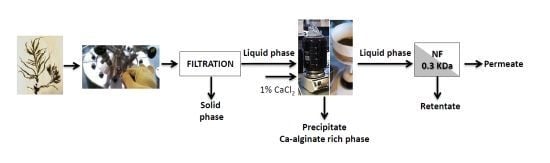
Figure 1.
Flow diagram for the processing of Sargassum muticum (Sm) and alginate-extracted S. muticum (AESm).
Figure 1.
Flow diagram for the processing of Sargassum muticum (Sm) and alginate-extracted S. muticum (AESm).
The effects of temperature on the pH of the reaction media is shown in
Figure 2a. The pH of AESm-containing media was higher than that obtained with Sm. In both cases, the minimum pH was reached operating at 200–210 °C, and a slight decrease after CaCl
2 addition was observed. The lower pH attained after processing the whole algae could be ascribed to the decomposition of alginate, as the hydrothermal treatment of alginate results in the formation of water soluble acids [
18]. The salt concentrations are shown in
Figure 2b. A maximum of 12 g CaCl
2 equivalents/L was found for liquors from Sm processing (stream L-Sm in
Figure 1), whereas a steady increase from 8 to 11 g/L was observed when the media made with AESm were heated from 170 to 240 °C (stream L-AESm in
Figure 1). Both types of reaction media showed higher salt concentrations when the autohydrolysis temperature increased, whereas slightly lower values were observed for the diafiltration retentates. The ash content varied slightly with the treatment temperature, being higher in L-Sm and in L-AESm than in H-Sm, H-AESm, R-Sm and R-AESm (
Figure 2c).
Figure 2.
Effect of autohydrolysis temperature on the (
a) pH, (
b) salt content, and (
c) ash content in samples from the streams (●) H-Sm, (○) H-AESm, (■) L-Sm, (□) L-AESm and (▲) R-Sm and (△) R-AESm generated according to the scheme for processing
Sargassum muticum (Sm) and alginate extracted
S. muticum (AESm) in
Figure 1.
Figure 2.
Effect of autohydrolysis temperature on the (
a) pH, (
b) salt content, and (
c) ash content in samples from the streams (●) H-Sm, (○) H-AESm, (■) L-Sm, (□) L-AESm and (▲) R-Sm and (△) R-AESm generated according to the scheme for processing
Sargassum muticum (Sm) and alginate extracted
S. muticum (AESm) in
Figure 1.
Figure 3 shows the influence of temperature on the total solubilization yield, on the non-volatile components (NVC) of streams H-Sm and H-AESm, on the recovery yields in streams H, L and R for Sm and AESm samples and on the NVC content of the liquors obtained from these streams after CaCl
2 precipitation, L-Sm and L-AESm and after diafiltration, R-Sm and R-AESm. The amount of solubilized products in streams H-Sm and H-AESm (measured as percentages with respect to the dry mass of materials subjected to hydrothermal processing) increased with temperatures in the range studied, reaching values of up to 61 wt% at 220–240 °C for Sm and 86 wt% for AESm. In this latter case, the overall extraction yield obtained in the process (including alginate removal and hydrothermal processing) was close to 97%. The extract content of NVC varied slightly with extraction temperature. The extracts with the highest NVC contents were obtained in liquors from AESm treatments.
Figure 3.
Effect of temperature on (a) the solubilization of Sm and AESm in the (♦) H-Sm and (◊) H-AESm streams and on the recovery of solutes in the liquors generated from processing of Sm and AESm: (●) H-Sm, (■) L-Sm and (▲) R-Sm streams (g Sm extract/100 g Sm) and (○) H-AESm, (□) L-AESm and (△) R-AESm streams (g AESm extract/100 g AESm), and on (b) the generation of non-volatile components (NVC) (g NVC/100 g liquor) found in streams (●) H-Sm and (○) H-AESm and in (▲) R-Sm and (△) R-AESm.
Figure 3.
Effect of temperature on (a) the solubilization of Sm and AESm in the (♦) H-Sm and (◊) H-AESm streams and on the recovery of solutes in the liquors generated from processing of Sm and AESm: (●) H-Sm, (■) L-Sm and (▲) R-Sm streams (g Sm extract/100 g Sm) and (○) H-AESm, (□) L-AESm and (△) R-AESm streams (g AESm extract/100 g AESm), and on (b) the generation of non-volatile components (NVC) (g NVC/100 g liquor) found in streams (●) H-Sm and (○) H-AESm and in (▲) R-Sm and (△) R-AESm.
The extraction percentages of the various constituent sugars, measured in the streams H-Sm and H-AESm, in respect to the amount of the corresponding units of the starting materials (Sm or AESm) are shown in
Figure 4. The same figure presents the data concerning the recovery percentages in the streams obtained after precipitation and diafiltration (R-Sm and R-AESm). The maximum recovery of fucose units in soluble products H-Sm and H-AESm occurred at 170 °C, accounting for 85.6%–88.0% of the amount present in substrates. Considering the recovery values in streams R-Sm and R-AESm, up to 55%–72% was obtained. Higher recovery of xylose and galactose structural units in the soluble products was observed, with values higher than 90%. At temperatures above 200 °C, the recovery of sugars forming part of the soluble saccharides decreased markedly. In all experiments, the recoveries of glucosyl, mannosyl and uronyl units in oligosaccharides were limited, no matter the operational conditions and type of substrates.
Figure 4.
Effects of temperature on the recovery percentage of structural components of saccharides in the liquors from autohydrolysis (a) H-Sm and (b) H-AESm and in purified streams (c) R-Sm and (d) R-AESm, expressed as percentage of the respective amounts present in the starting materials (Sm or AESm). Percentage conversion to soluble products of (▲) fucose, (X) galactose, (■) xylose, (●) glucose, (◊) mannose and (_) uronic acids.
Figure 4.
Effects of temperature on the recovery percentage of structural components of saccharides in the liquors from autohydrolysis (a) H-Sm and (b) H-AESm and in purified streams (c) R-Sm and (d) R-AESm, expressed as percentage of the respective amounts present in the starting materials (Sm or AESm). Percentage conversion to soluble products of (▲) fucose, (X) galactose, (■) xylose, (●) glucose, (◊) mannose and (_) uronic acids.
In a previous study, the temperature range 190–200 °C was identified as optimal for AESm processing, based on the results achieved for total yield, yield in phenolics and ABTS radical scavenging capacity of isolates [
2]. The solubilization yields decreased with temperatures in the range tested, a fact ascribed to the instability of fucose-containing compounds under harsh conditions [
6].
Similar fucoidan extraction yields have been reported for mild acid treatments of other
Sargassum sp. In most cases, the algae were extracted with organic solvents to remove pigments and phenol compounds before acidic processing. Fucose-containing polysaccharides were obtained at 5.2% yield from
S. horneri subjected to acid extraction [
22], at 5% yield from
Sargassum trichophyllum refluxed with water [
10], and at 7%–9% yield from
Sargassum sp. [
6] and
S. pallidum [
5]. The reported fucoidan extraction yields for other brown algae lie in a wider range than for
Sargasssum sp. (up to 20% for
Pelvetia or to 11% for
Ascophyllum nodosum) [
9].
The recovery of uronyl groups in oligosaccharides was higher in experiments with AESm than in assays with Sm. Under typical conditions for hydrothermal processing, the alginate structural units (mannuronic and guluronic acid), are much more reactive than cellulose and cellulose-derived saccharides, due to their hydrophilic character and the self-catalytic nature of carboxylic groups. Alginate decomposition has been proposed to proceed through an acid hydrolysis pathway at 22–80 °C, influenced by the presence of phenolic compounds susceptible to oxidation, transition metal ions, oxygen and the pH of the solution [
23]. Under typical conditions for hydrothermal processing [
18] and under subcritical conditions, very short timeframes are required to decompose alginate [
24].
Under non isothermal processing (180–240 °C) in an inert atmosphere, alginate depolymerized into oligosaccharides, monosaccharides and decomposition products (lactic acid and glycolic acid and low molecular weight products). The decomposition of alginate is promoted by temperature and probably took place by releasing mannuronic acid at short reaction times, followed by the release of guluronic acid. Mannuronic acid is rapidly decomposed into water soluble acids, solids and gas [
18]. Uronic acids were identified as the most abundant structural units of the soluble saccharides. Both fucoidans and residual alginate contain uronic acids as structural units. This behaviour was confirmed in the Fourier transform infrared spectroscopy (FT-IR) spectra of the alginate rich fraction A-Sm (
Figure 5), with two IR bands at approximately 1100 and 1025 cm
−1, assigned to mannuronic and guluronic units, respectively [
8,
21]. The presence of a strong band at 1025 cm
−1 and a small shoulder at 1080–1100 cm
−1 in samples from Sm treatments at 170 or 190 °C has been ascribed to the presence of small amounts of mannuronic acid in the homoguluronan-enriched alginate fraction [
21]. No signal appeared at 1025 cm
−1 in AESm processed at temperatures above 200 °C; whereas a progressive darkening of the media was observed, as reported for hydrothermally treated alginate solutions [
18].
The FT-IR spectra of the alginate rich products, A-Sm and A-AESm, presented characteristic bands at 2923.7–2928.9 cm
−1 (C–H stretching), 1616.7–1652.5 cm
−1 (carbohydrate O–C–O asymmetric stretching vibrations), 1419.1–1429.6 cm
−1 (C–OH deformation vibration), 1321.3–1326.3 cm
−1 (C–C–H, and O–C–H deformation), 1074.2–1083.4 cm
−1 (C–O stretching vibrations) and 1033 cm
−1 (C–O and C–C stretching vibrations) of pyranose rings, at 946 cm
−1 (C–O stretching vibration uronic acid), 900 (α-
l-gulopyranuronic asymmetric ring vibration) and 815 cm
−1 (mannuronic residues) [
25].
Figure 5.
FT-IR profiles of the alginate rich fractions from (
a) A-Sm and (
b) A-AESm extracted and recovered according to the scheme in
Figure 1.
Figure 5.
FT-IR profiles of the alginate rich fractions from (
a) A-Sm and (
b) A-AESm extracted and recovered according to the scheme in
Figure 1.
Bands were observed at 821–823 cm
−1 for A-Sm samples and at 816–820 cm
−1 for A-AESm at temperatures below 200 °C. These bands have been ascribed to mannuronic acid, although sulfation of an equatorial primary hydroxyl group attached to the pyranose ring of hexoses shows an absorption band at 820 cm
−1, attributable to the C–O–S vibration of equatorial sulfate groups [
3,
26,
27]. Samples from Sm and from AESm showed shoulders or small shoulders at 1257.4–1263.4 cm
−1, at the characteristic band of sulfate groups in polysaccharides, corresponding to the S=O stretching vibration of the sulfate group common to all the sulfate esters bands between the regions 1240–1272 cm
−1 [
3,
14,
25,
26]. Some of these samples presented a very light band close to 622 and 583 cm
−1, attributed to the asymmetric and symmetric O=S=O deformation of sulfates [
28].
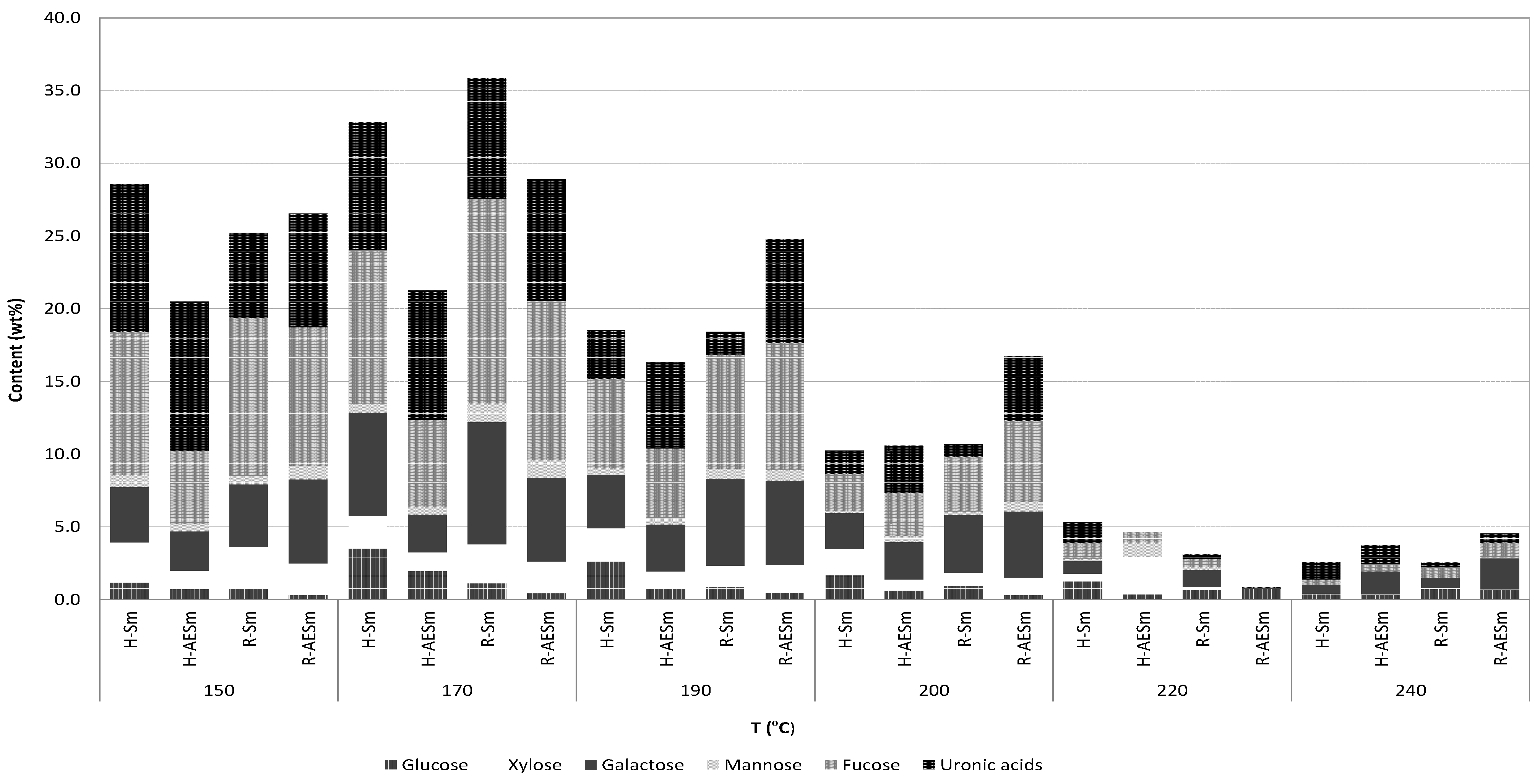
The refined products obtained from streams R-Sm and R-AESm by precipitation with calcium chloride and diafiltration were freeze-dried and characterized. HPLC analysis showed the presence of structural units of saccharides containing fucose, galactose, xylose, glucuronic acid and mannose, in agreement with fucan or heterofucan parent polymers. Monosaccharides were not found in the liquors from hydrothermal processing of AESm and Sm. The relative amount of the structural units making part of the saccharides obtained at the various treatment temperatures are presented in
Figure 6 as a function of the treatment temperature. As a general pattern, the maximal concentrations of constituent sugars were achieved in treatments performed under low- or medium-severity conditions, harsher autohydrolysis conditions resulted in decreased amounts of soluble saccharides. At temperatures above 200 °C, the degradation of sugars was evident and the saccharidic fraction accounted for less than 10% of the solubilized fraction. Fucose was the major structural component of soluble saccharides, except in H-AESm, followed by uronic acids and galactose. Galactose content was lower in the H-AESm streams. Operating at the optimal temperature (170 °C), the soluble saccharides accounted for 30%–35% of the dried extracts, and the product showed a mass ratio of the constituent sugars fucose:galactose:glucose:xylose:mannose of 1:0.67:0.33:0.21:0.06 for H-Sm and 1:0.44:0.33:0.22:0.09 for H-AESm, 1:0.60:0.08:0.19:0.09 for R-Sm and 1:0.53:0.04:0.20:0.11 for R-AESm.
Figure 6.
Relative proportions of sugars making part of soluble saccharides obtained after drying (H-Sm and H-AESm) and after CaCl2 precipitation and diafiltration (R-Sm and R-AESm), expressed as weight percentage.
Figure 6.
Relative proportions of sugars making part of soluble saccharides obtained after drying (H-Sm and H-AESm) and after CaCl2 precipitation and diafiltration (R-Sm and R-AESm), expressed as weight percentage.
Related values have been reported for extracts from
Sargassum sp. For example, a fucoidan from
S.
stenophylum obtained by extraction with water and purified by ethanol and cetylpyridinium chloride precipitation was made up of fucose, galactose and xylose, with smaller amounts of mannose, glucose and glucuronic acid [
14]. Fucose and galactose were the most abundant constituents of a fucoidan from
S. trichophyllum [
10] and also in the crude extract isolated from the reproductive tissue of
S. pallidum by acidic processing and dialysis [
5], whereas a crude
S. tenerrimum fucoidan containing fucose, xylose, galactose and glucose was isolated by acidic processing, dialysis and freeze-drying [
21].
Sargassum filipendula heterofucans obtained by proteolytic digestion and acetone precipitation were composed of fucose, glucose, glucuronic acid, galactose and sulfate; in some fractions mannose and glucuronic acids were not detected [
13]. Rhamnose, polyphenols and low amounts of xylose were also found in polysaccharides from
Sargassum sp. obtained by ethanol extraction followed by acidic processing, concentration, dialysis, and fractionation [
20]. Heterofucans isolated from other
Sargassum sp. were composed of fucose, galactose, xylose, glucuronic acid and mannose [
29].
Owing to the resistance of glucuronic acid to hydrolytic degradation, the products extracted at short reaction times presented comparatively high fucose contents, whereas longer times resulted in increased extraction yields, but also in products with lower contents of fucose and sulfate and higher proportions of glucuronic acid [
6,
14]. It has been suggested that the fraction resistant to acidic hydrolysis present in brown algae contained a core of glucuronic acid and mannose residues [
4]. Some brown seaweed sulfated polysaccharides have a backbone consisting of 3- linked α-
l-fucopyranose, while in others, as in
Sargassum species, the backbone is made up of alternating 3- and 4-linked α-
l-fucopyranose residues and sulfated galactofucans [
11,
14].
Figure 7 shows the HPSEC profiles determined for standards and retentates from hydrothermal liquors. Expectedly, higher temperatures resulted in increased depolymerization of the polysaccharides, generating polymers with molecular masses higher than 80 kDa. Low molecular weight polymers with molar masses in the range 5–12 kDa were obtained in experiments at 190 °C or higher. In literature, low temperature operation (30–50 °C) led to fractions with molar mass ≥10 kDa after 72 h of treatment, whereas severer conditions could cause degradation [
12,
30]. The production of fucoidans at 30 °C and pH 3 in media containing 0.2 M H
2O
2 resulted in the production of compounds with molar masses above 100 kDa after 6 h, which were reduced to approximately 10 kDa after 72 h. In comparison, the molar masses of the products obtained at 90 °C for 4 h were below 1 kDa [
12].
Figure 7.
HPSEC elution profiles determined for the samples R-Sm and R-AESm. The MW (♦) of dextran standards are also presented. (- - -) 150 °C, (—) 170 °C, (-----) 190 °C, (····) 200 °C, (— ) 220 °C and (− . − .) 240 °C.
Figure 7.
HPSEC elution profiles determined for the samples R-Sm and R-AESm. The MW (♦) of dextran standards are also presented. (- - -) 150 °C, (—) 170 °C, (-----) 190 °C, (····) 200 °C, (— ) 220 °C and (− . − .) 240 °C.
The biological properties of fucoidans depend on a number of factors, including the relative abundance of sulfate groups, structural features, and molar mass distribution. As fucoidans must permeate the biological membranes to exert their effects
in vivo, depolymerization of high molar mass compounds to fragments below 30 kDa results in more active fractions [
7,
12]. Low-molecular-weight, sulfated polysaccharides are effective radical scavengers [
12], whereas compounds of 26–35 kDa from
S. tenerrimum and
S. plagiophyllum showed activity as antiviral agents and as inhibitors of carcinogen metabolic activation compounds, respectively [
21].
A number of properties have been reported for polysaccharides from
Sargassum sp., including reducing, chelating, and scavenging activities against DPPH, hydroxyl and superoxide radicals [
13,
29]. The antioxidant action of samples from autohydrolysis of
S. muticum were evaluated in this work by measuring their scavenging capacity against the ABTS radical, and expressed as Trolox equivalent antioxidant capacity (TEAC assay). This method was selected due to its operational simplicity, and because the TEAC values of related compounds are available. Additionally, the results of this assay often correlates with the ones of the methods FRAP, DPPH and Folin, due to similarities in the chemical fundament. The ABTS radical scavenging capacity of the extracts, higher for H-Sm and L-Sm samples, increased with temperature in the studied range (
Table 2), in agreement with data reported for non diafiltered samples [
2]. The antioxidant activity of the target products was moderate under all the operational conditions. Typically, one gram of the extracts was equivalent to less than 0.25 g of Trolox. Related results have been reported for crude fucoidans and fractions [
13]. The antioxidant activity of the crude extracts can be ascribed to the effects of different compounds, including phenolic compounds and fucoidan. Both the phenolic extraction yield and the content of the extracts, measured as gallic acid equivalents, showed a steady increase from 170 to 220 °C, whereas the sugar and fucoidan content decreased progressively with the temperature of hydrothermal processing (
Figure 6). The total phenolic contents of extracts coming from the nanofiltration (streams R-Sm and R-AESm) showed maximum values at 190 °C, with up to 6% for extracts from AESm. Higher values were reported for the H-AESm stream [
2], suggesting that some small phenolic components could have permeated the membrane.
Table 2.
(a) Phenolic extraction yield of H-Sm, L-Sm and R-Sm streams (g GAE/100 g Sm) and of H-AESm, L-AESm and R-AESm streams (g GAE/100 g AESm); (b) phenolic content of extracts (g GAE/100 g extract); and (c) TEAC value (g Trolox equivalents/g extract), for H-Sm, L-Sm, R-Sm, H-AESm, L-AESm and R-AESm extracts.
Table 2.
(a) Phenolic extraction yield of H-Sm, L-Sm and R-Sm streams (g GAE/100 g Sm) and of H-AESm, L-AESm and R-AESm streams (g GAE/100 g AESm); (b) phenolic content of extracts (g GAE/100 g extract); and (c) TEAC value (g Trolox equivalents/g extract), for H-Sm, L-Sm, R-Sm, H-AESm, L-AESm and R-AESm extracts.
| H-Sm | H-AESm | L-Sm | L-AESm | R-Sm | R-AESm |
|---|
| T (°C) | a | b | c | a | b | c | a | b | c | a | b | c | a | b | c | a | b | c |
|---|
| 150 | 1.13 | 6.42 | 0.18 | 1.58 | 4.61 | 0.12 | 0.71 | 2.59 | 0.06 | 0.34 | 1.95 | 0.05 | 1.39 | 11.94 | 0.10 | 0.26 | 2.84 | - |
| 170 | 1.49 | 5.87 | 0.14 | 2.04 | 5.35 | 0.13 | 1.30 | 3.24 | 0.07 | 0.44 | 2.30 | 0.05 | - | - | - | 0.25 | 2.60 | 0.06 |
| 190 | 1.87 | 7.52 | 0.18 | 3.59 | 6.76 | 0.16 | 1.65 | 4.14 | 0.09 | 1.31 | 4.20 | 0.10 | - | - | - | 0.84 | 6.01 | 0.15 |
| 200 | 2.34 | 9.00 | 0.21 | 3.05 | 5.17 | 0.22 | 1.92 | 4.65 | 0.10 | 1.72 | 5.01 | 0.11 | 0.74 | 5.86 | 0.10 | 0.84 | 5.49 | 0.15 |
| 220 | 2.31 | 9.73 | 0.23 | 3.10 | 5.69 | 0.26 | 2.24 | 5.04 | 0.12 | 2.76 | 5.13 | 0.11 | 1.42 | 9.15 | 0.14 | 1.28 | 5.98 | 0.16 |
| 240 | 2.19 | 9.76 | 0.24 | 2.96 | 6.78 | 0.24 | 2.13 | 5.12 | 0.11 | 2.00 | 4.13 | 0.09 | 0.80 | 7.71 | 0.19 | 0.89 | 5.64 | 0.13 |