Crambescidin-816 Acts as a Fungicidal with More Potency than Crambescidin-800 and -830, Inducing Cell Cycle Arrest, Increased Cell Size and Apoptosis in Saccharomyces cerevisiae
Abstract
:1. Introduction
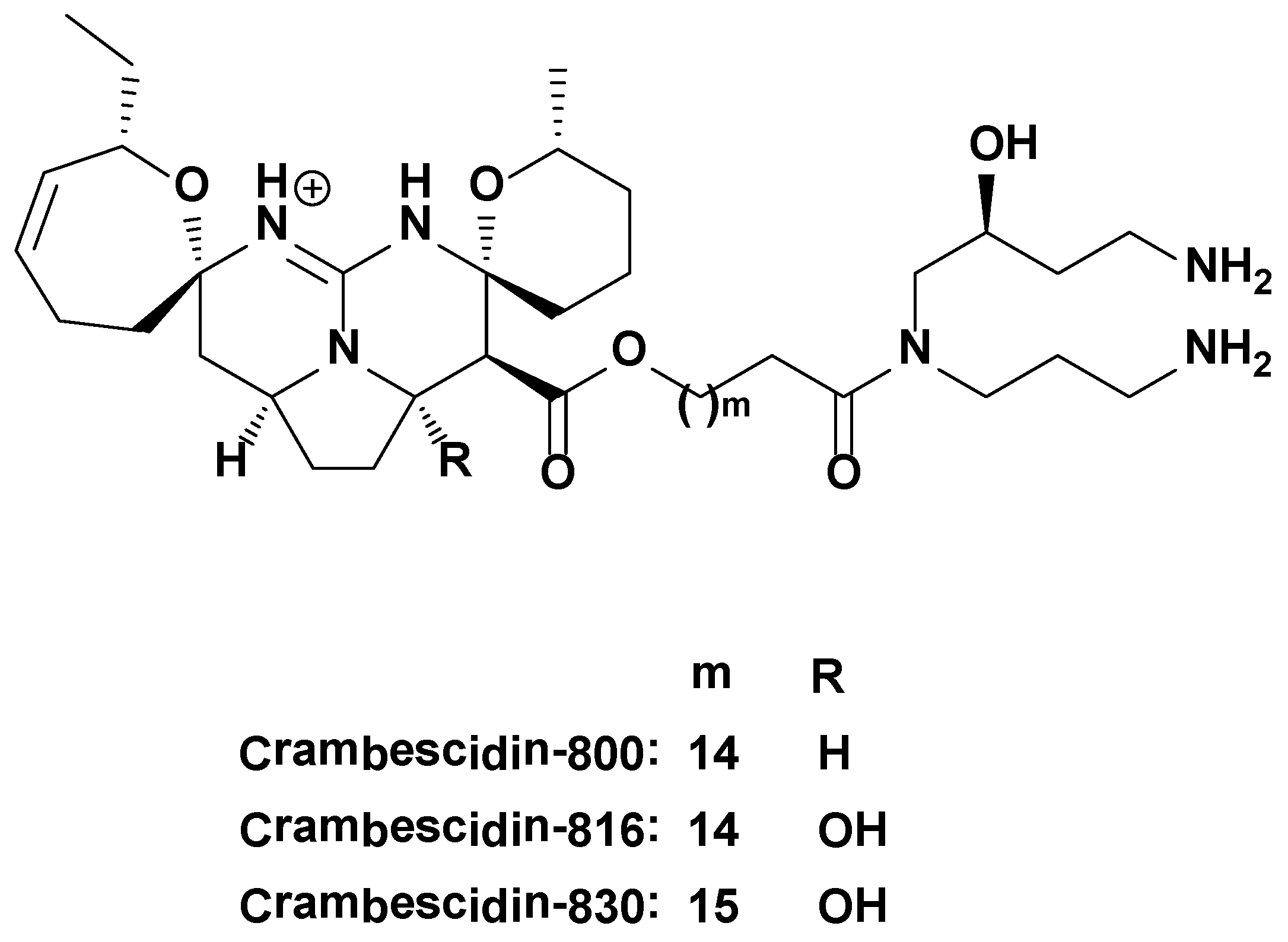
2. Results and Discussion
2.1. C816 Is More Potent on S. cerevisiae than C800 and C830, and Has a Fungicidal Effect
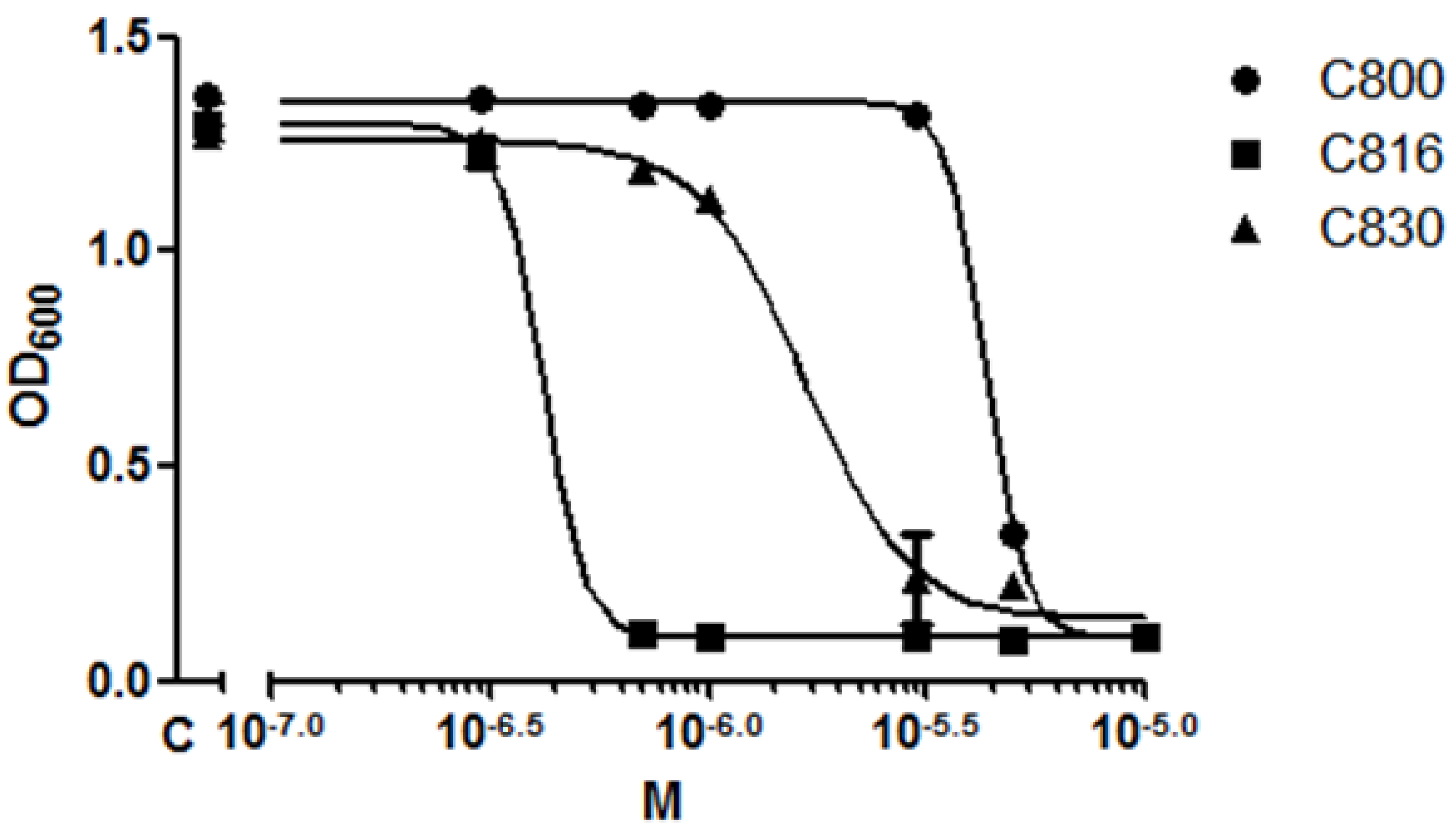

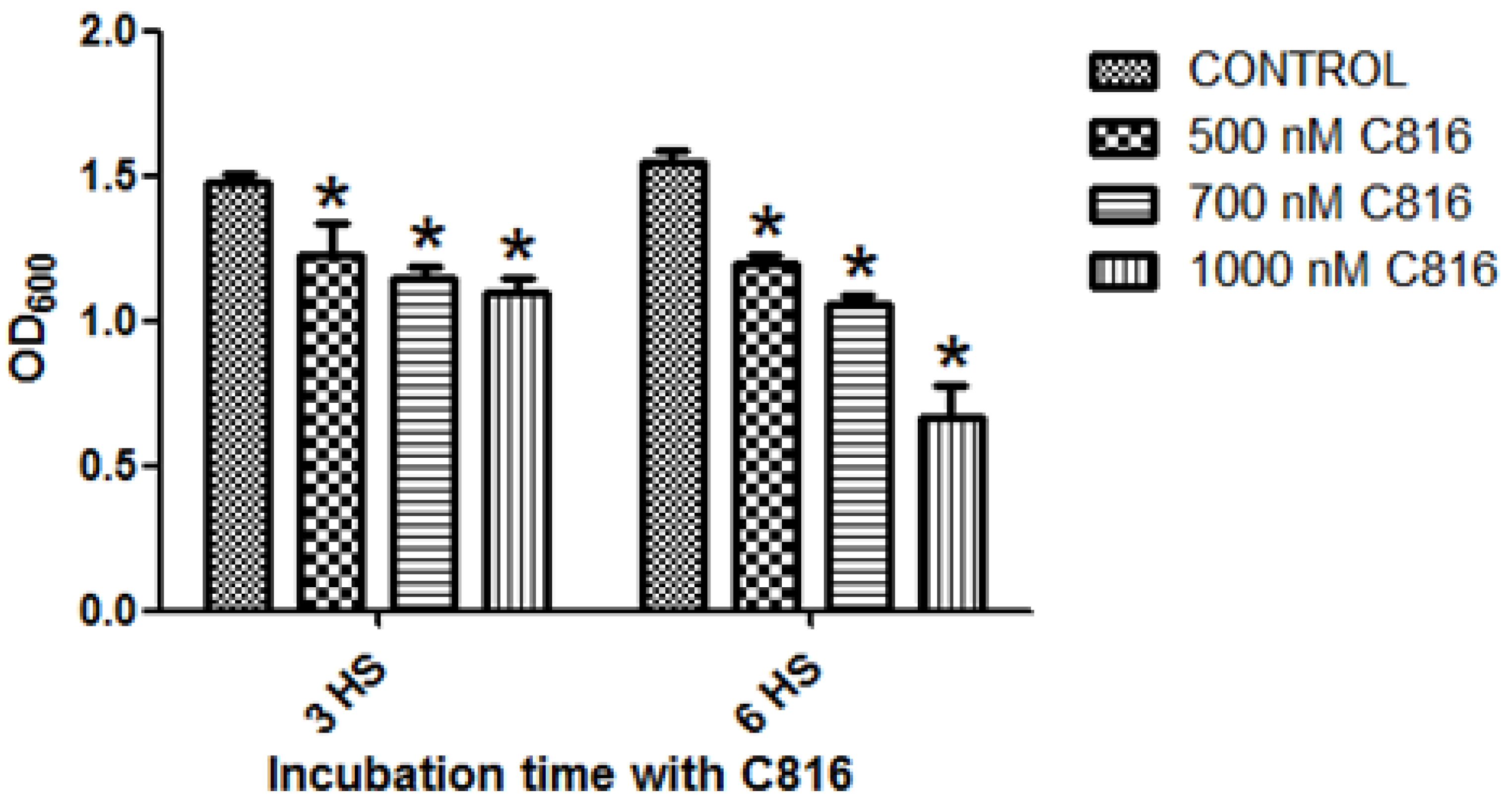
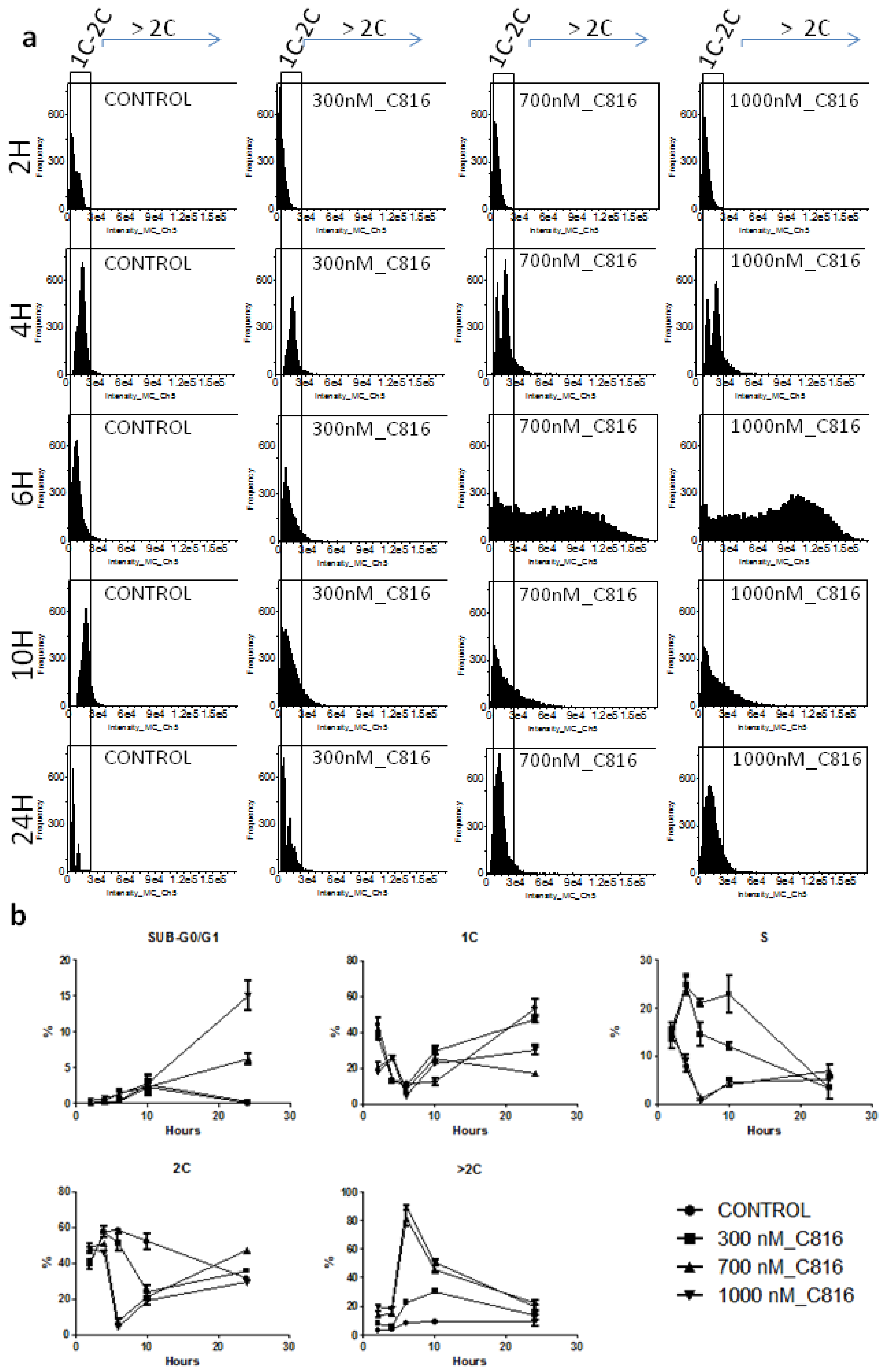
2.2. C816 Induces Cell Cycle Arrest and an Increase in S. cerevisiae DNA Content
2.3. C816 Induces an Increase in Cell Size
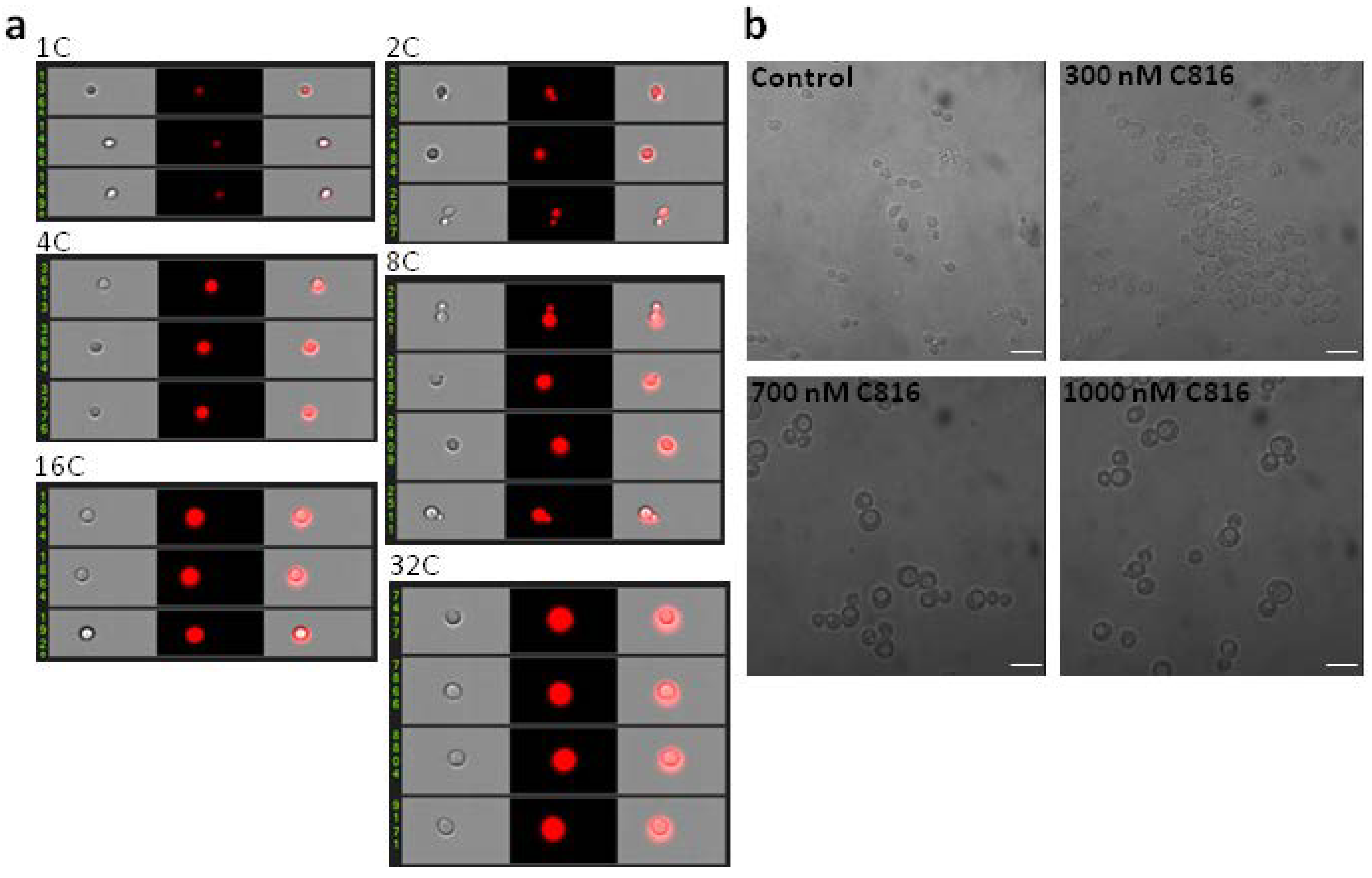
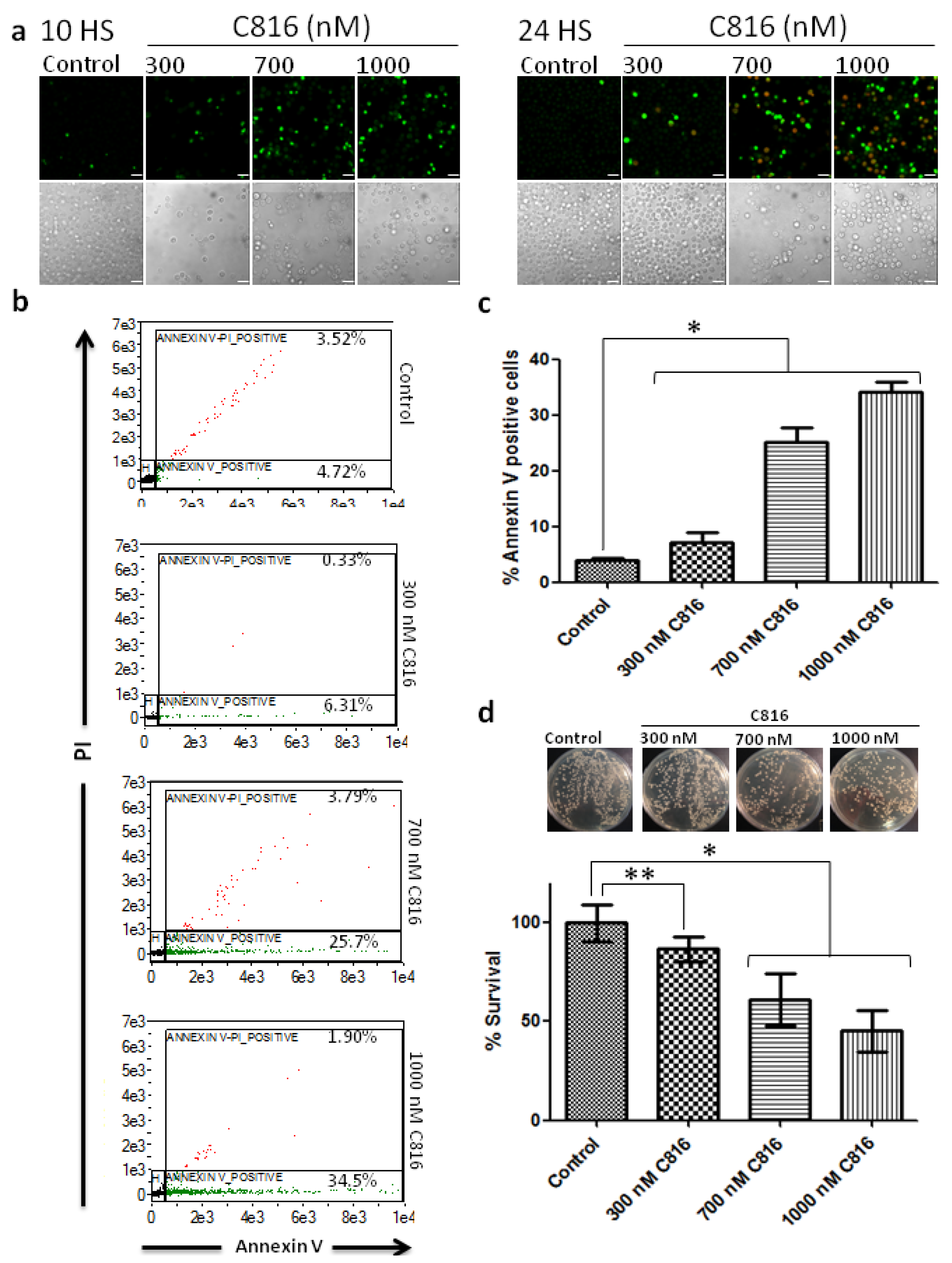
2.4. C816 Induces Apoptosis in S. cerevisiae
2.5. C816 Disrupts the Mitochondrial Membrane Potential (ΔΨm) in S. cerevisiae

3. Experimental Section
3.1. Molecules Tested
3.2. Cell Strain and Culture
3.3. Cell Growth Inhibition Determination
3.4. Determination of Fungicidal Effect
3.5. Cell Cycle Assay by Flow Cytometry
3.6. Determination of Apoptosis by Annexin V and Propidium Iodide (PI) Staining
3.7. Determination of Mitochondrial Transmembrane Potential (ΔΨm)
3.8. Statistics
4. Conclusion
Acknowledgments
Conflicts of Interest
References
- Laport, M.S.; Santos, O.C.; Muricy, G. Marine sponges: potential sources of new antimicrobial drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Marine Compounds and their Antimicrobial Activities. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2011. [Google Scholar]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 265–286. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2003–4: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 145, 553–581. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [CrossRef]
- Buscema, M.; Van de Vyver, G. Cytotoxic rejection of xenografts between marine sponges. J. Exp. Zool. 1985, 235, 297–308. [Google Scholar] [CrossRef]
- Burkholder, P.R.; Ruetzler, K. Antimicrobial activity of some marine sponges. Nature 1969, 222, 983–984. [Google Scholar] [CrossRef]
- Uriz, M.J.; Beccero, M.A.; Tur, J.M.; Turon, X. Location of toxicity within the Mediterranean sponge Crambe crambe. Mar. Biol. 1996, 124, 583–590. [Google Scholar] [CrossRef]
- Aron, Z.D.; Pietraszkiewicz, H.; Overman, L.E.; Valeriote, F.; Cuevas, C. Synthesis and anticancer activity of side chain analogs of the crambescidin alkaloids. Bioorg. Med. Chem. Lett. 2004, 14, 3445–3449. [Google Scholar] [CrossRef]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res. 2004, 24, 2325–2330. [Google Scholar]
- Gassner, N.C.; Tamble, C.M.; Bock, J.E.; Cotton, N.; White, K.N.; Tenney, K.; St Onge, R.P.; Proctor, M.J.; Giaever, G.; Nislow, C.; et al. Accelerating the discovery of biologically active small molecules using a high-throughput yeast halo assay. J. Nat. Prod. 2007, 70, 383–390. [Google Scholar] [CrossRef]
- Berlinck, R.G.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Ferri, S.; Spampinato, S.; Speroni, E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca++ channel blocker activity of crambescidin 816. J. Nat. Prod. 1993, 56, 1007–1015. [Google Scholar] [CrossRef]
- Martin, V.; Vale, C.; Bondu, S.; Thomas, O.P.; Vieytes, M.R.; Botana, L.M. Differential Effects of Crambescins and Crambescidin 816 in Voltage-Gated Sodium, Potassium and Calcium Channels in Neurons. Chem. Res. Toxicol. 2013, in press. [Google Scholar]
- Hughes, T.R. Yeast and drug discovery. Funct. Integr. Genomics 2002, 2, 199–211. [Google Scholar] [CrossRef]
- Pichler, S.; Piatti, S.; Nasmyth, K. Is the yeast anaphase promoting complex needed to prevent re-replication during G2 and M phases? EMBO J. 1997, 16, 5988–5997. [Google Scholar] [CrossRef]
- Endo, K.; Mizuguchi, M.; Harata, A.; Itoh, G.; Tanaka, K. Nocodazole induces mitotic cell death with apoptotic-like features in Saccharomyces cerevisiae. FEBS Lett. 2010, 584, 2387–2392. [Google Scholar] [CrossRef]
- Hartwell, L.H. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 1971, 69, 265–276. [Google Scholar] [CrossRef]
- Cid, V.J.; Adamikova, L.; Cenamor, R.; Molina, M.; Sanchez, M.; Nombela, C. Cell integrity and morphogenesis in a budding yeast septin mutant. Microbiology 1998, 144, 3463–3474. [Google Scholar] [CrossRef]
- Longtine, M.S.; DeMarini, D.J.; Valencik, M.L.; Al-Awar, O.S.; Fares, H.; De Virgilio, C.; Pringle, J.R. The septins: Roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 1996, 8, 106–119. [Google Scholar] [CrossRef]
- Howell, A.S.; Lew, D.J. Morphogenesis and the cell cycle. Genetics 2012, 190, 51–77. [Google Scholar] [CrossRef]
- Lew, D.J.; Reed, S.I. Cell cycle control of morphogenesis in budding yeast. Curr. Opin. Genet. Dev. 1995, 5, 17–23. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; López-Alonso, H.; Roel, M.; Thomas, O.P.; Ternon, E.; Vieytes, M.R.; Vega, F.V.; Botana, L.M. Mechanism of cytotoxic action of crambescidin-816 on liver-derived tumour cell lines. Brit. J. Pharm. 2013. submitted for publication. [Google Scholar]
- Wysocki, R.; Kron, S.J. Yeast cell death during DNA damage arrest is independent of caspase or reactive oxygen species. J. Cell Biol. 2004, 166, 311–316. [Google Scholar] [CrossRef]
- Madeo, F.; Herker, E.; Wissing, S.; Jungwirth, H.; Eisenberg, T.; Frohlich, K.U. Apoptosis in yeast. Curr. Opin. Microbiol. 2004, 7, 655–660. [Google Scholar] [CrossRef]
- van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Almeida, B.; Silva, A.; Mesquita, A.; Sampaio-Marques, B.; Rodrigues, F.; Ludovico, P. Drug-induced apoptosis in yeast. Biochim. Biophys. Acta 2008, 1783, 1436–1448. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef]
- Desagher, S.; Osen-Sand, A.; Nichols, A.; Eskes, R.; Montessuit, S.; Lauper, S.; Maundrell, K.; Antonsson, B.; Martinou, J.C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999, 144, 891–901. [Google Scholar] [CrossRef]
- Korper, S.; Nolte, F.; Rojewski, M.T.; Thiel, E.; Schrezenmeier, H. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34+ cell line KG-1a. Exp. Hematol. 2003, 31, 815–823. [Google Scholar] [CrossRef]
- Narita, M.; Shimizu, S.; Ito, T.; Chittenden, T.; Lutz, R.J.; Matsuda, H.; Tsujimoto, Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 14681–14686. [Google Scholar]
- Giannattasio, S.; Atlante, A.; Antonacci, L.; Guaragnella, N.; Lattanzio, P.; Passarella, S.; Marra, E. Cytochrome c is released from coupled mitochondria of yeast en route to acetic acid-induced programmed cell death and can work as an electron donor and a ROS scavenger. FEBS Lett. 2008, 582, 1519–1525. [Google Scholar] [CrossRef]
- Ludovico, P.; Rodrigues, F.; Almeida, A.; Silva, M.T.; Barrientos, A.; Corte-Real, M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell 2002, 13, 2598–2606. [Google Scholar] [CrossRef]
- Pereira, C.; Camougrand, N.; Manon, S.; Sousa, M.J.; Corte-Real, M. ADP/ATP carrier is required for mitochondrial outer membrane permeabilization and cytochrome c release in yeast apoptosis. Mol. Microbiol. 2007, 66, 571–582. [Google Scholar] [CrossRef]
- Sapienza, K.; Bannister, W.; Balzan, R. Mitochondrial involvement in aspirin-induced apoptosis in yeast. Microbiology 2008, 154, 2740–2747. [Google Scholar] [CrossRef]
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D., Jr.; Chen, L.B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef]
- Barns, S.M.; Lane, D.J.; Sogin, M.L.; Bibeau, C.; Weisburg, W.G. Evolutionary relationships among pathogenic Candida species and relatives. J. Bacteriol. 1991, 173, 2250–2255. [Google Scholar]
- Bondu, S.; Genta-Jouve, G.; Leirόs, M.; Vale, C.; Guigonis, J.-M.; Botana, L.M.; Thomas, O.P. Additional bioactive guanidine alkaloids from the Mediterranean sponge Crambe crambe. RSC Adv. 2012, 2, 2828–2835. [Google Scholar] [CrossRef]
- Tani, N.; Rahnasto-Rilla, M.; Wittekindt, C.; Salminen, K.A.; Ritvanen, A.; Ollakka, R.; Koskiranta, J.; Raunio, H.; Juvonen, R.O. Antifungal activities of novel non-azole molecules against S. cerevisiae and C. albicans. Eur. J. Med. Chem. 2012, 47, 270–277. [Google Scholar] [CrossRef]
- Madeo, F.; Frohlich, E.; Frohlich, K.U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997, 139, 729–734. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Costa-Oliveira, S.; Tavares, C.; Martinez-de-Oliveira, J. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clin. Microbiol. Infect. 2001, 7, 609–618. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rubiolo, J.A.; Ternon, E.; López-Alonso, H.; Thomas, O.P.; Vega, F.V.; Vieytes, M.R.; Botana, L.M. Crambescidin-816 Acts as a Fungicidal with More Potency than Crambescidin-800 and -830, Inducing Cell Cycle Arrest, Increased Cell Size and Apoptosis in Saccharomyces cerevisiae. Mar. Drugs 2013, 11, 4419-4434. https://doi.org/10.3390/md11114419
Rubiolo JA, Ternon E, López-Alonso H, Thomas OP, Vega FV, Vieytes MR, Botana LM. Crambescidin-816 Acts as a Fungicidal with More Potency than Crambescidin-800 and -830, Inducing Cell Cycle Arrest, Increased Cell Size and Apoptosis in Saccharomyces cerevisiae. Marine Drugs. 2013; 11(11):4419-4434. https://doi.org/10.3390/md11114419
Chicago/Turabian StyleRubiolo, Juan A., Eva Ternon, Henar López-Alonso, Olivier P. Thomas, Félix V. Vega, Mercedes R. Vieytes, and Luis M. Botana. 2013. "Crambescidin-816 Acts as a Fungicidal with More Potency than Crambescidin-800 and -830, Inducing Cell Cycle Arrest, Increased Cell Size and Apoptosis in Saccharomyces cerevisiae" Marine Drugs 11, no. 11: 4419-4434. https://doi.org/10.3390/md11114419





