Cytotoxic Effects of Fucoidan Nanoparticles against Osteosarcoma
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxic Effects of Fucoidan on Osteosarcoma Cells

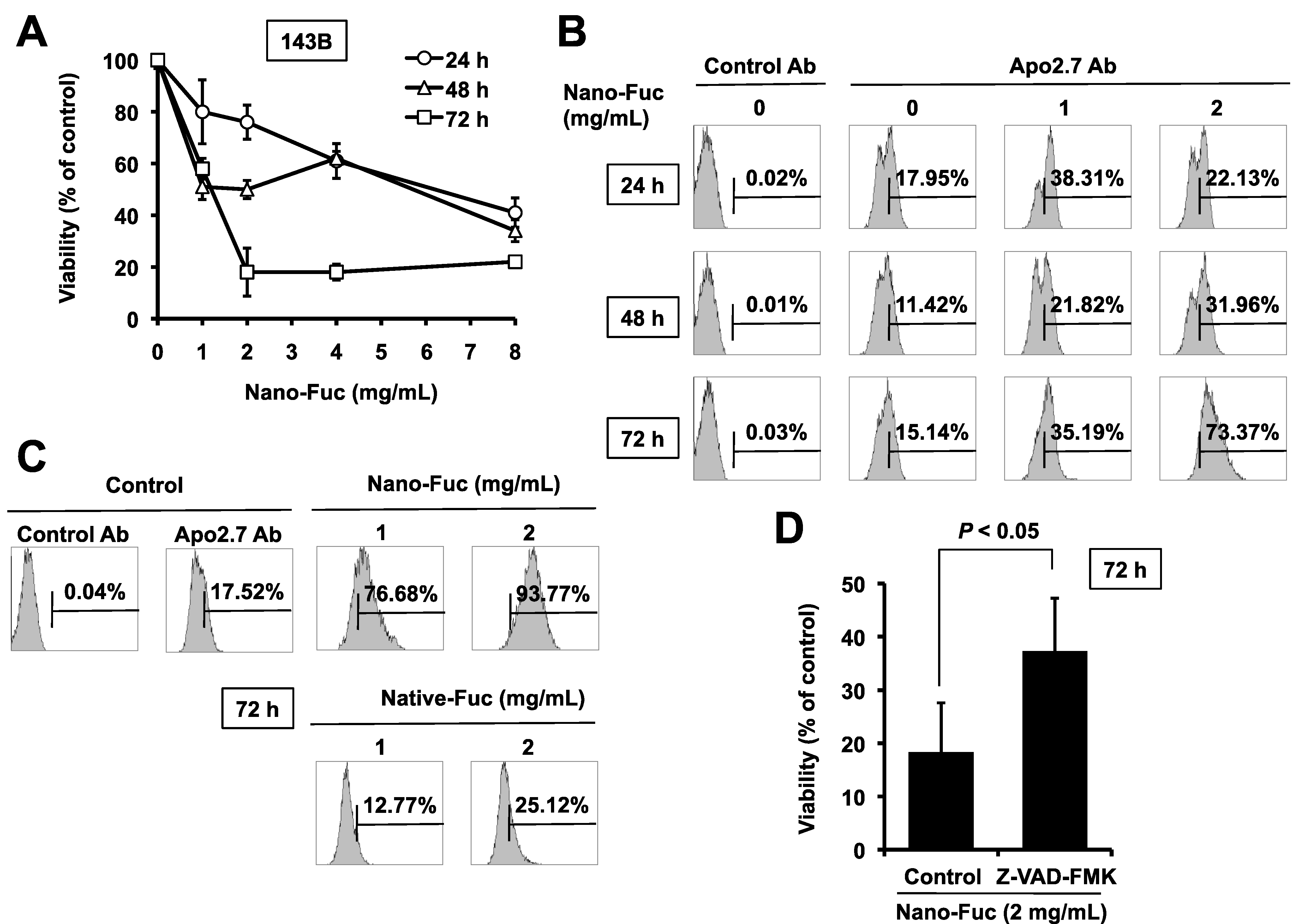
2.2. Effects of Fucoidan on Tumorigenesis of Osteosarcoma Cells in Mice
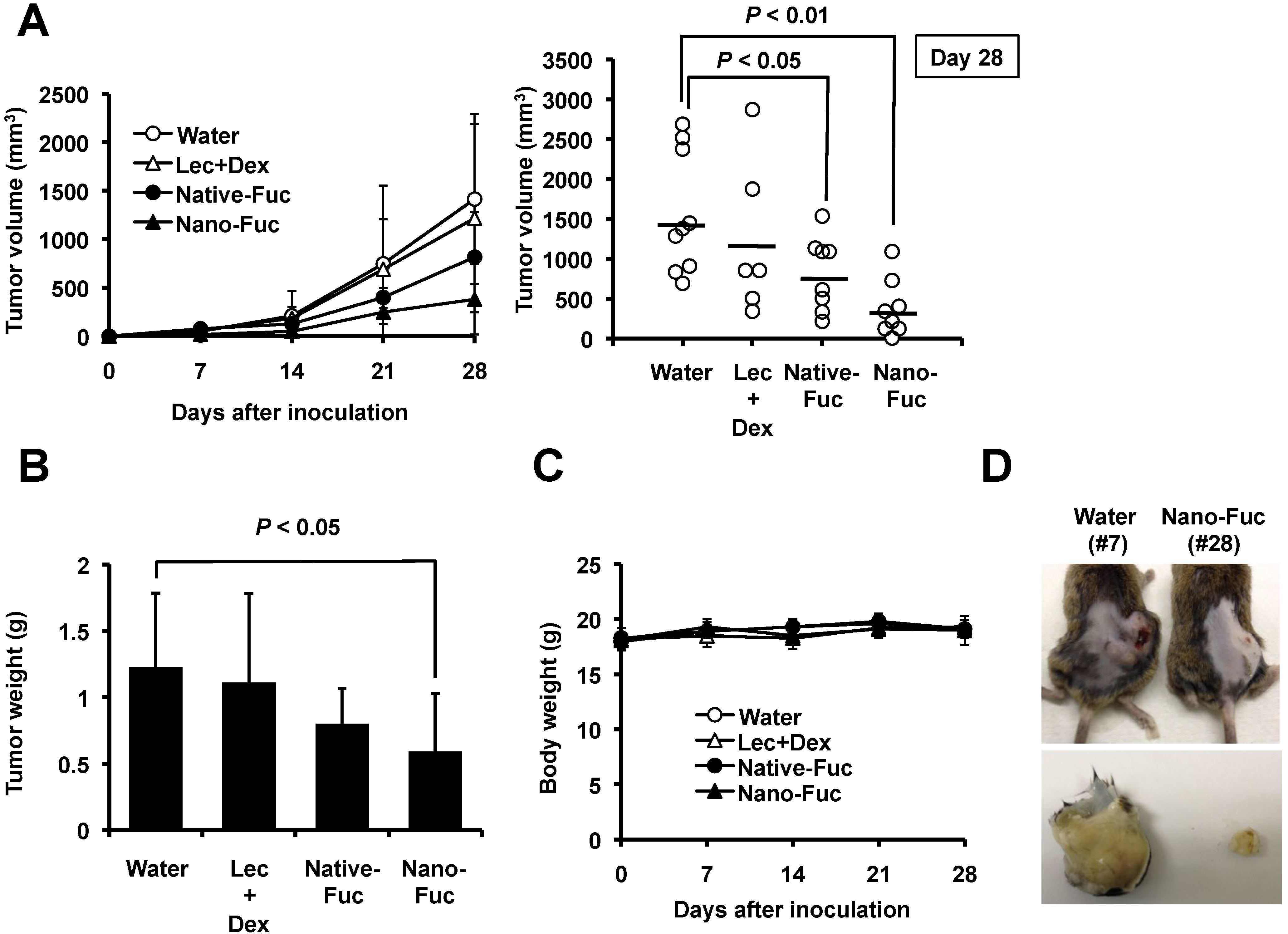
2.3. Fucoidan Induces Apoptosis in Osteosarcoma Xenograft Model
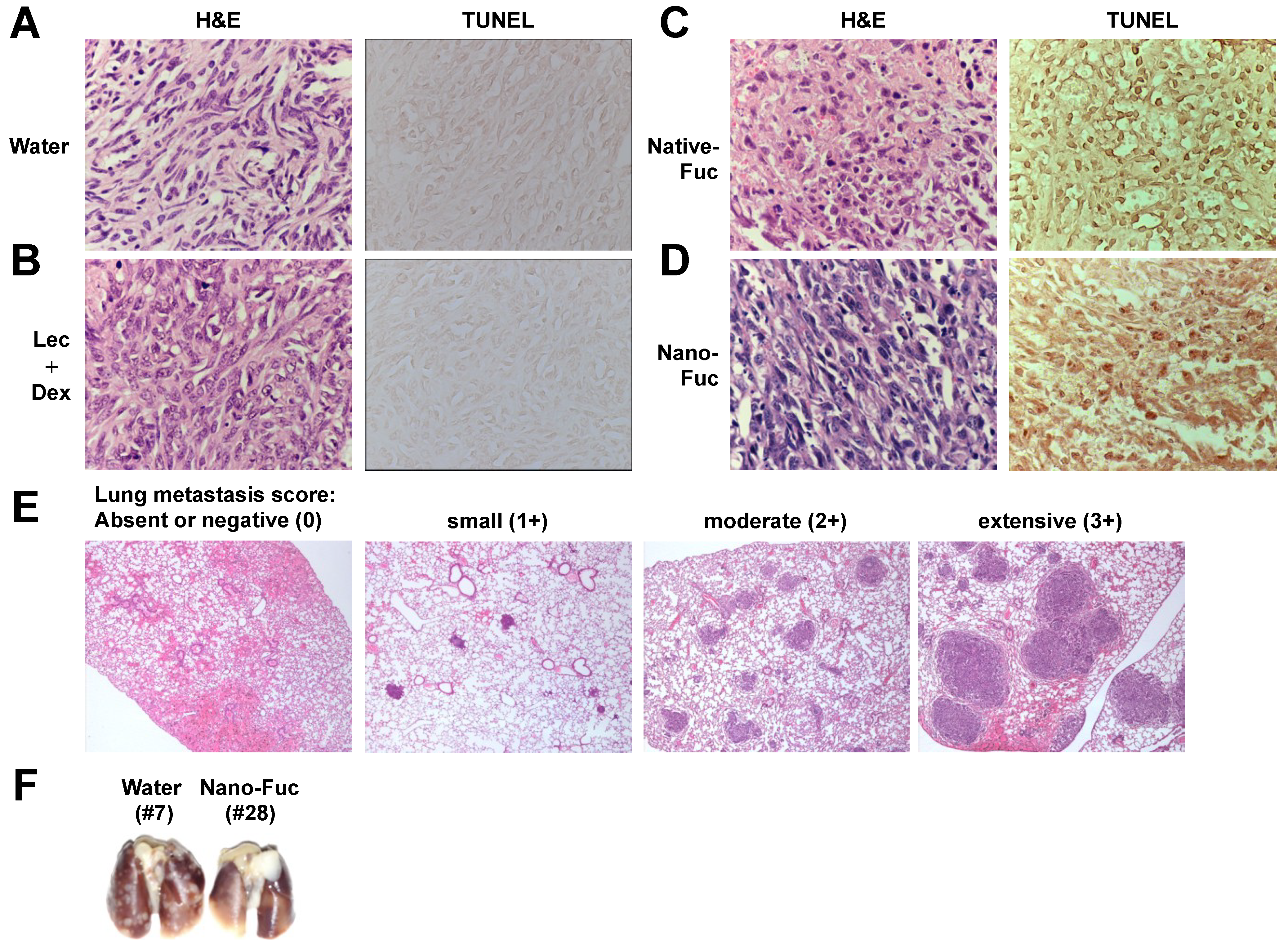
2.4. Fucoidan Inhibits Metastasis of Mouse Osteosarcoma Cells to the Lung
2.5. Cell Permeation of Fucoidan
| Sample | Amount of Permeated Fucoidan (µg/cm2/h) | Permeation Rate of Fucoidan (%) |
|---|---|---|
| Native fucoidan | 0.034 ± 0.03 | 0.001 ± 0.001 |
| Nanoparticle fucoidan | 5.298 ± 0.341 | 0.229 ± 0.015 |
3. Experimental Section
3.1. Cell Lines and Reagents
3.2. Preparation of Native Fucoidan from Seaweed
3.3. Preparation of Liposome Encapsulating Fucoidan
3.4. Assessment of Cell Viability and Apoptosis
3.5. Preparation of Sarcoma Animal Model
3.6. Permeation across Caco-2 Monolayers
3.7. Statistical Analysis
4. Conclusions
Conflict of Interest
References
- Morya, V.K.; Kim, J.; Kim, E.-K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; Multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Araya, N.; Takahashi, K.; Sato, T.; Nakamura, T.; Sawa, C.; Hasegawa, D.; Ando, H.; Aratani, S.; Yagishita, N.; Fujii, R.; et al. Fucoidan therapy decreases the proviral load in patients with human T-lymphotropic virus type-1-associated neurological disease. Antivir. Ther. 2011, 16, 89–98. [Google Scholar] [CrossRef]
- Takeda, K.; Tomimori, K.; Kimura, R.; Ishikawa, C.; Nowling, T.K.; Mori, N. Anti-tumor activity of fucoidan is mediated by nitric oxide released from macrophages. Int. J. Oncol. 2012, 40, 251–260. [Google Scholar]
- Mori, N.; Nakasone, K.; Tomimori, K.; Ishikawa, C. Beneficial effects of fucoidan in patients with chronic hepatitis C virus infection. World J. Gastroenterol. 2012, 18, 2225–2230. [Google Scholar] [CrossRef]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Salyers, A.A.; Vercellotti, J.R.; West, S.E.; Wilkins, T.D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl. Environ. Microbiol. 1977, 33, 319–322. [Google Scholar]
- Yang, C.; Chung, D.; Shin, I.-S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Shimizu, J.; Wada-Funada, U.; Mano, H.; Matahira, Y.; Kawaguchi, M.; Wada, M. Proportion of murine cytotoxic T cells is increased by high molecular-weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus). J. Health Sci. 2005, 51, 394–397. [Google Scholar] [CrossRef]
- Azuma, K.; Ishihara, T.; Nakamoto, H.; Amaha, T.; Osaki, T.; Tsuka, T.; Imagawa, T.; Minami, S.; Takashima, O.; Ifuku, S.; et al. Effects of oral administration of fucoidan extracted from Cladosiphon okamuranus on tumor growth and survival time in a tumor-bearing mouse model. Mar. Drugs 2012, 10, 2337–2348. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Savage, S.; Mirabello, L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma 2011, 2011, 548151. [Google Scholar]
- Anninga, J.K.; Gelderblom, H.; Fiocco, M.; Kroep, J.R.; Taminiau, A.H.M.; Hogendoorn, P.C.W.; Egeler, R.M. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur. J. Cancer 2011, 47, 2431–2445. [Google Scholar] [CrossRef]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary bone osteosarcoma in the pediatric age: State of the art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef]
- Zhang, C.; Ao, Z.; Seth, A.; Schlossman, S.F. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J. Immunol. 1996, 157, 3980–3987. [Google Scholar]
- Konopleva, M.; Zhao, S.; Xie, Z.; Segall, H.; Younes, A.; Claxton, D.F.; Estrov, Z.; Kornblau, S.M.; Andreeff, M. Apoptosis. Molecules and mechanisms. Adv. Exp. Med. Biol. 1999, 457, 217–236. [Google Scholar]
- Takahashi, M.; Inafuku, K.; Miyagi, T.; Oku, H.; Wada, K.; Imura, T.; Kitamoto, D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. J. Oleo Sci. 2007, 56, 35–42. [Google Scholar] [CrossRef]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kimura, R.; Rokkaku, T.; Takeda, S.; Senba, M.; Mori, N. Cytotoxic Effects of Fucoidan Nanoparticles against Osteosarcoma. Mar. Drugs 2013, 11, 4267-4278. https://doi.org/10.3390/md11114267
Kimura R, Rokkaku T, Takeda S, Senba M, Mori N. Cytotoxic Effects of Fucoidan Nanoparticles against Osteosarcoma. Marine Drugs. 2013; 11(11):4267-4278. https://doi.org/10.3390/md11114267
Chicago/Turabian StyleKimura, Ryuichiro, Takayoshi Rokkaku, Shinji Takeda, Masachika Senba, and Naoki Mori. 2013. "Cytotoxic Effects of Fucoidan Nanoparticles against Osteosarcoma" Marine Drugs 11, no. 11: 4267-4278. https://doi.org/10.3390/md11114267




