Advances in the Study of the Structures and Bioactivities of Metabolites Isolated from Mangrove-Derived Fungi in the South China Sea
Abstract
:1. Introduction
2. Metabolites Derived from the Mangrove Fungi in the South China Sea
| Source | Compound | Activity | Ref. |
|---|---|---|---|
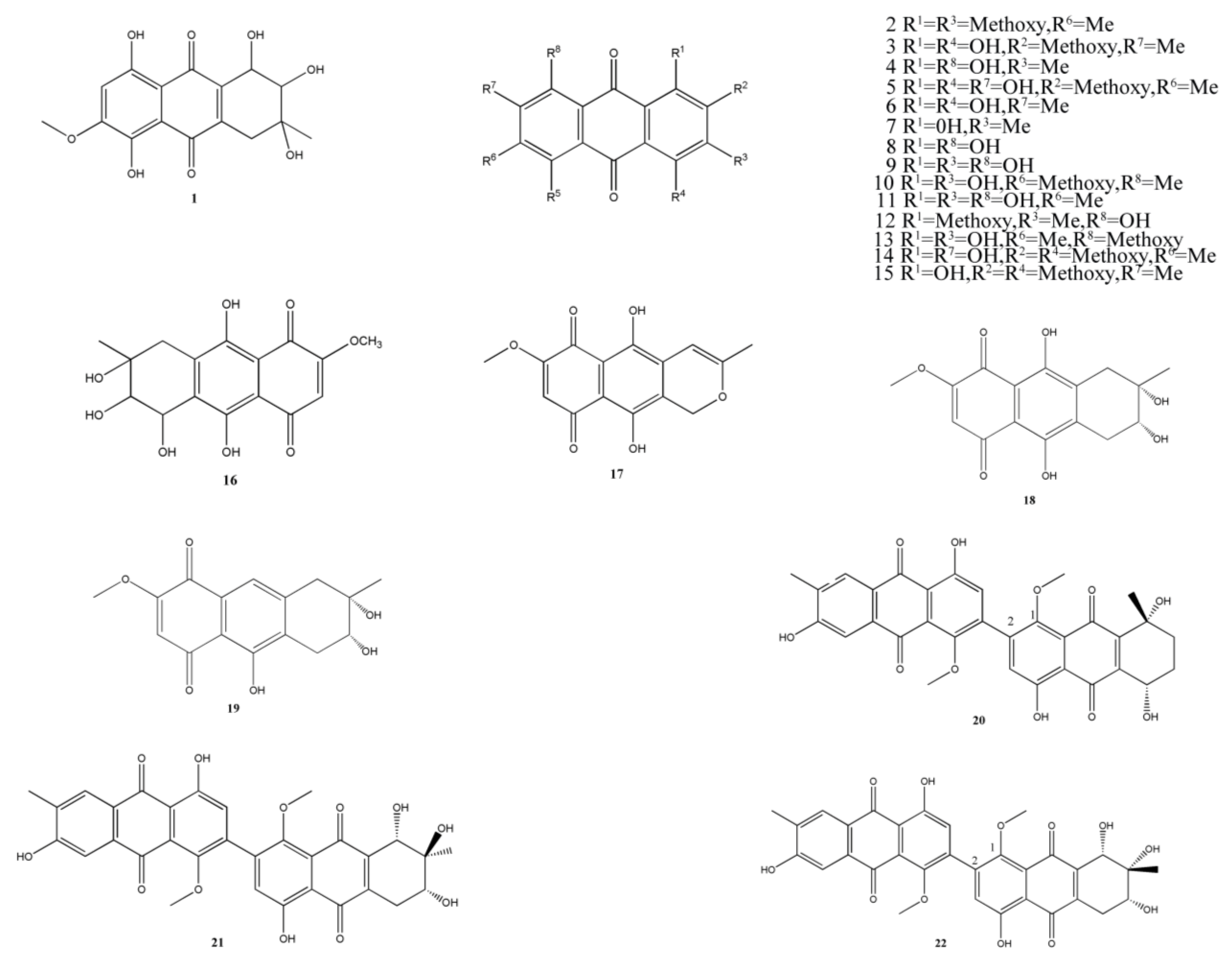
| Halorosellinia sp. (No. 1403) | SZ-685C (1) | Cytotoxic | [13] |
| Bostrycin (16) | Cytotoxic | [14] | |
| Halorosellinia sp. (No. 1403) and Guignardia sp. (No. 4382) | Compounds (2–15) | Cytotoxic (6) | [15] |
| Nigrospora sp. | 4-Deoxybostrycin (18) | Anti-mycobacteria | [16] |
| Nigrosporin (19) | Anti-mycobacteria | ||
| Alternaria sp. (ZJ9-6B) | Alterporriol K (20), L (21) and M (22) | Cytotoxic (20,21) | [17] |
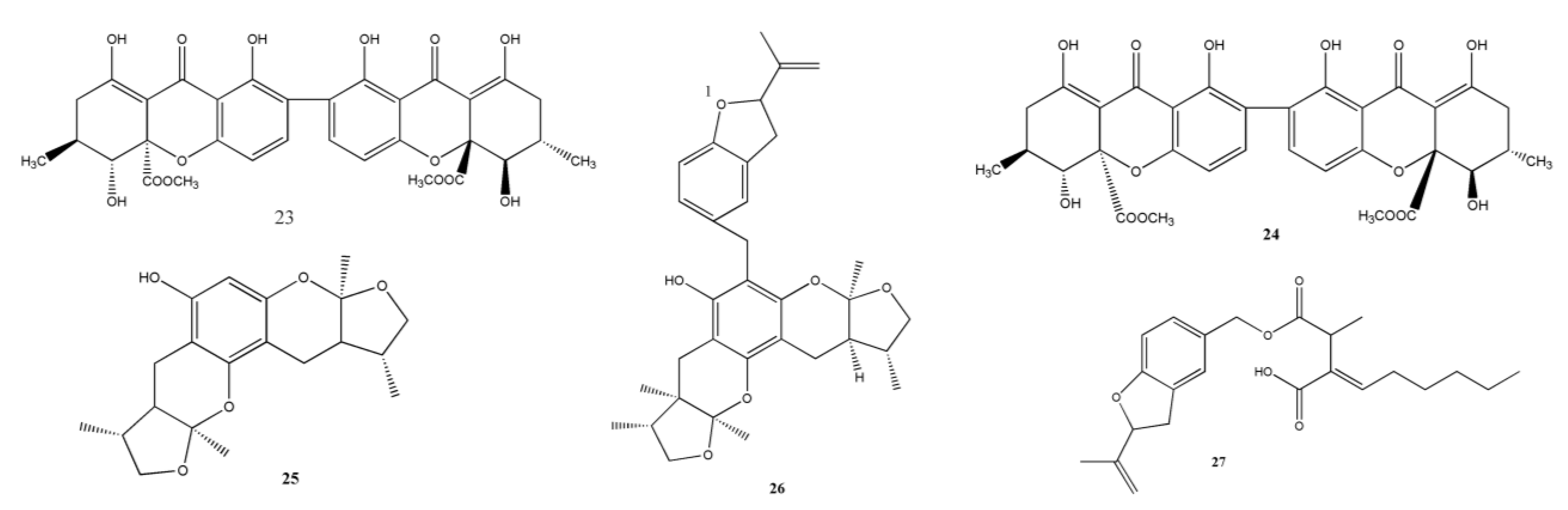
| Paecilomyces sp. | Secalonic acid A (23) | Cytotoxic | [18] |
| Paecilomyces sp. (tree 1–7) and endophytic fungus No. ZSU44 | Secalonic acid D (24) | Cytotoxic | [19,20,21] |
| Xylaria sp. (No. 2508) | Xyloketal B (25) | Protects Human umbilical vein endothelial cells from oxidized LDL-induced oxidative injury | [22] |
| Xyloketal J (26) | [23] | ||
| Xyloester A (27) | |||
| Xylaria sp. BL321 | Eremophilane sesquiterpenes (33–35) | [24] | |
| 07H239-A (36) | Effect on α-glucosidase | ||
| Aspergillus sp. | (+)-methyl sydowate (28) | Antibacterial | [25] |
| 7-deoxy-7,8-didehydrosydonic acid (29) | |||
| 7-deoxy-7,14-didehydrosydonic acid (30) | |||
| (+)-sydonic acid (31) | Antibacterial | ||
| (+)-sydowic acid (32) | Antibacterial | ||
| Aspergillus sp. (16-5c) | Asperterpenoid A (37) | Anti-Mycobacterium tuberculosis | [26] |
| Aspergillus terreus Gwq-48 | Isoaspulvinone E (58), pulvic acid (59) and aspulvinone E (60) | Anti-influenza A H1N1 virus | [27] |
| Diaporthe sp. | Diaporols A–I (38–46) | [28] | |
| Pestalotiopsis sp. | Pestalotiopsones A–F (47–52) | Cytotoxic (52) | [29] |
| Cytosporones J–N (68–72) | [30] | ||
| Dothiorelone B (73) | |||
| Pestalasins A–E (74–78) | |||
| 3-hydroxymethyl-6,8-dimethoxycoumarin (56) | |||
| 7-hydroxy-2-(2-hydroxypropyl)-5-methylchromone (53) | |||
| Phomopsis sp. (ZZF08) | Phomopsin A (61) | Cytotoxic | [31] |
| Cytochalasin H (62) | Cytotoxic | ||
| Glucosylceramide (63) | |||
| Phomopsis sp. (ZSU-H76) | Phomopsin A (61), B (64), C (65) | [32] | |
| Cytosporone B (66) and C (67) | Antifungal | ||
| Phomopsis sp. (No. SK7RN3G1) | 2,6-dihydroxy-3-methyl-9-oxoxanthene-8-carboxylic acid methyl ester (88) | [33] | |
| Penicillium sp. (091402) | (3 R*, 4S*)-6,8-dihydroxy-3,4,7-trimethylisocoumarin (79) | Cytotoxic | [34] |
| (3 R, 4S)-6,8- dihydroxy-3,4,5-trimethylisocoumarin (80) | |||
| (3 R, 4S)-6,8- dihydroxy-3,4,5,7-tetramethylisocoumarin (81) | |||
| ( S)-3-(3′,5′-dhydroxy-2′,4′-methlphenyl)butan-2-one (82) | |||
| Phenol A (83) | Cytotoxic | ||
| Penicillium sp. (ZZF 32#) | Dimethyl 8-methoxy-9-oxo-9H-xanthene-1, 6-dicarboxylate (86) | [35] | |
| 8-(methoxycarbonyl)-1-hydroxy-9-oxo-9 H-xanthene-3-carboxylic acid (87) | Antifungal | ||
| Talaromyces flavus | Talaperoxides A–D (89–92) | Cytotoxic | [36] |
| Steperoxide B (93, or merulin A) | Cytotoxic | ||
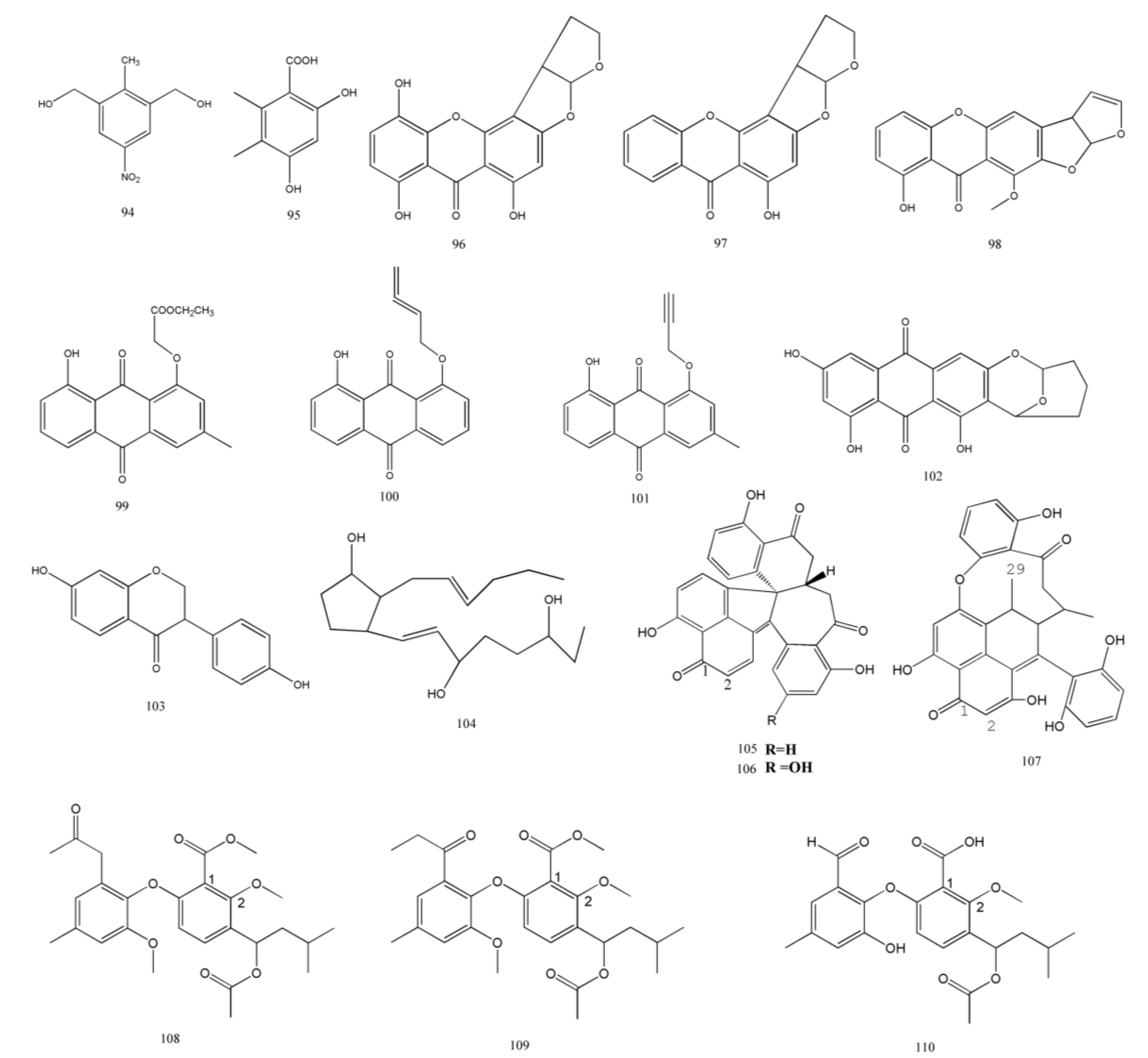
| Talaromyces sp. (SBE-14) | Tenelate A (108) and B (109) | [37] | |
| Tenellic acid C (110) | |||
| Sporothrix sp. (#4335) | Sporothrins A, B, and C (105–107) | Cytotoxic | [38] |
| Unidentified fungus (No. B77) | Anhydrofusarubin (17) | Anti-Gram-positive bacteria, Cytotoxic | [39] |
| Unidentified fungus (No. GX4-1B) | 1,10-dihydroxy-8-methyl-dibenz[b,e]oxepin-6,11-dione (54) | [40] | |
| 6-hydroxy-4-hydroxymethyl-8-methoxy-3-methylisocoumarin (55) | |||
| 3-hydroxymethyl-6,8-dimethoxycoumarin (56) | |||
| 1,10-dihydroxy-dibenz[b ,e]oxepin-6,11-dione (57) | |||
| Unidentified fungus (No. ZH19) | 1-hydroxy-4,7-dimethoxy-6-(3-oxobutyl)-9 H-xanthen-9-one (84) | Cytotoxic | [41] |
| 1,7-dihydroxy-2-methoxy-3-(3-methylbut-2-enyl)-9 H-xanthen-9-one (85) | Cytotoxic |
2.1. Anthracenediones
2.2. Secalonic Acid Family
2.3. Xyloketals
2.4. Sesquiterpenoids
2.5. Chromones


2.6. Lactones

2.7. Coumarins and Isocoumarin Derivatives
2.8. Xanthones and Peroxides

2.9. Other
3. Conclusions
Acknowledgments
References
- Vinothkumar, S.; Parameswaran, P.S. Recent advances in marine drug research. Biotechnol. Adv. 2013, in press. [Google Scholar]
- Montaser, R.; Luesch, H. Marine natural products: a new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Wang, J.F.; Hao, Y.Y.; Wang, Y. Recent advances in the discovery and development of marine microbial natural products. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef]
- De Rosa, S. Marine natural products—Analysis, structure elucidation, bioactivity and potential use as drugs. In Mediterranean Marine Organisms as Source of New Potential Drugs, Proceedings of the Phytochemical Society of Europe, Fund calouste gulbenkian, Lisbon, Portugal, April 2000; Rauter, A.P., Palma, F.B., Justino, J., Araujo, M.E., PinaDosSantos, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 441–461. [Google Scholar]
- Newman, D.J.; Cragg, G.M. The Discovery of Anticancer Drugs from Natural Sources; Humana Press Inc: Totowa, NJ, USA, 2005; pp. 129–168. [Google Scholar]
- Singh, R.; Sharma, M.; Joshi, P.; Rawat, D.S. Clinical status of anti-cancer agents derived from marine sources. Anticancer Agents Med. Chem. 2008, 8, 603–617. [Google Scholar]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antiviral Res. 2010, 86, 231–240. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef]
- Fraenkel, G.S. The raison d′etre of secondary plant substances; these odd chemicals arose as a means of protecting plants from insects and now guide insects to food. Science 1959, 129, 1466–1470. [Google Scholar]
- Secondary metabolite. Available online: http://en.wikipedia.org/wiki/Secondary_metabolites (accessed on 15 August 2013).
- Xu, J. Biomolecules produced by mangrove-associated microbes. Curr. Med. Chem. 2011, 18, 5224–5266. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Xie, G.; Zhu, X.; Li, Q.; Gu, M.; He, Z.; Wu, J.; Li, J.; Lin, Y.; Li, M.; She, Z.; et al. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br. J. Pharmacol. 2010, 159, 689–697. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Gao, Y.; Lin, H.; Du, L; Yang, S.; Long, S.; She, Z.; Cai, X.; Zhou, S.; et al. The anthracenedione compound bostrycin induces mitochondria-mediated apoptosis in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 297–308. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Chen, L.M.; Mi, Y.J.; Zheng, L.S.; Wang, F.; She, Z.G.; Lin, Y.C.; To, K.K.; et al. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Mar. Drugs 2010, 8, 1469–1481. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Huang, Y.; Chen, H.; Li, Y.; Zhong, L.; Chen, Y.; Chen, S.; Wang, J.; Kang, J.; et al. Anti-mycobacterial activity of marine fungus-derived 4-deoxybostrycin and nigrosporin. Molecules 2013, 18, 1728–1740. [Google Scholar] [CrossRef]
- Huang, C.H.; Pan, J.H.; Chen, B.; Yu, M.; Huang, H.B.; Zhu, X.; Lu, Y.J.; She, Z.G.; Lin, Y.C. Three bianthraquinone derivatives from the mangrove endophytic fungus Alternaria sp. ZJ9-6B from the South China Sea. Mar. Drugs 2011, 9, 832–843. [Google Scholar] [CrossRef]
- Zhai, A.; Zhang, Y.; Zhu, X.; Liang, J.; Wang, X.; Lin, Y.; Chen, R. Secalonic acid A reduced colchicine cytotoxicity through suppression of JNK, p38 MAPKs and calcium influx. Neurochem. Int. 2011, 58, 85–91. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Yan, Y.Y.; Dai, C.L.; Xia, X.K.; She, Z.G.; Lin, Y.C.; Fu, L.W. Secalonic acid D induced leukemia cell apoptosis and cell cycle arrest of G(1) with involvement of GSK-3beta/beta-catenin/c-Myc pathway. Cell Cycle 2009, 8, 2444–2450. [Google Scholar] [CrossRef]
- Hu, Y.P.; Tao, L.Y.; Wang, F.; Zhang, J.Y.; Liang, Y.J.; Fu, L.W. Secalonic acid D reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem. Pharmacol. 2013, 85, 1619–1625. [Google Scholar] [CrossRef]
- Liao, G.; Zhou, J.; Wang, H.; Mao, Z.; Xiao, W.; Wang, H.; She, Z.; Zhu, Y. The cell toxicity effect of secalonic acid D on GH3 cells and the related mechanisms. Oncol. Rep. 2010, 23, 387–395. [Google Scholar]
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.L. A novel marine compound xyloketal B protects against oxidized LDL-induced cell injury in vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Y.; Wang, J.; Pang, J.; Huang, C.; Wu, X.; She, Z.; Vrijmoed, L.L.; Jones, E.B.; Lin, Y. Benzofuran derivatives from the mangrove endophytic Fungus Xylaria sp. (#2508). J. Nat. Prod. 2008, 71, 1251–1253. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Huang, H.; Ma, L.; Wang, J.; Gu, Y.; Liu, L.; Lin, Y. Four eremophilane sesquiterpenes from the mangrove endophytic fungus Xylaria sp. BL321. Mar. Drugs 2012, 10, 340–348. [Google Scholar] [CrossRef]
- Wei, M.Y.; Wang, C.Y.; Liu, Q.A.; Shao, C.L.; She, Z.G.; Lin, Y.C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef]
- Gao, H.; Guo, W.; Wang, Q.; Zhang, L.; Zhu, M.; Zhu, T.; Gu, Q.; Wang, W.; Li, D. Aspulvinones from a mangrove rhizosphere soil-derived fungus Aspergillus terreus Gwq-48 with anti-influenza A viral (H1N1) activity. Bioorg. Med. Chem. Lett. 2013, 23, 1776–1778. [Google Scholar] [CrossRef]
- Zang, L.Y.; Wei, W.; Guo, Y.; Wang, T.; Jiao, R.H.; Ng, S.W.; Tan, R.X.; Ge, H.M. Sesquiterpenoids from the mangrove-derived endophytic fungus Diaporthe sp. J. Nat. Prod. 2012, 75, 1744–1749. [Google Scholar] [CrossRef]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.; Edrada, R.; Lin, W.; Wu, J.; Proksch, P. Chromones from the endophytic fungus Pestalotiopsis sp. isolated from the chinese mangrove plant Rhizophora mucronata. J. Nat. Prod. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Xu, J.; Kjer, J.; Sendker, J.; Wray, V.; Guan, H.; Edrada, R.; Muller, W.E.; Bayer, M.; Lin, W.; Wu, J.; et al. Cytosporones, coumarins, and an alkaloid from the endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Bioorg. Med. Chem. 2009, 17, 7362–7367. [Google Scholar] [CrossRef]
- Tao, Y.; Zeng, X.; Mou, C.; Li, J.; Cai, X.; She, Z.; Zhou, S.; Lin, Y. 1H and 13C NMR assignments of three nitrogen containing compounds from the mangrove endophytic fungus (ZZF08). Magn. Reson. Chem. 2008, 46, 501–505. [Google Scholar] [CrossRef]
- Huang, Z.; Cai, X.; Shao, C.; She, Z.; Xia, X.; Chen, Y.; Yang, J.; Zhou, S.; Lin, Y. Chemistry and weak antimicrobial activities of phomopsins produced by mangrove endophytic fungus Phomopsis sp. ZSU-H76. Phytochemistry 2008, 69, 1604–1608. [Google Scholar] [CrossRef]
- Yang, J.X.; Qiu, S.X.; She, Z.G.; Lin, Y.C. A new xanthone derivative from the marine fungus Phomopsis sp. (No. SK7RN3G1). Chem. Nat. Compd. 2013, 49, 31–33. [Google Scholar] [CrossRef]
- Han, Z.; Mei, W.L.; Zhao, Y.X.; Deng, Y.Y.; Dai, H.F. A new cytotoxic isocoumarin from endophytic fungus Penicillium sp. 091402 of the mangrove plant Bruguiera sexangula. Chem. Nat. Compd. 2009, 45, 805–807. [Google Scholar] [CrossRef]
- Shao, C.; Wang, C.; Wei, M.; Gu, Y.; Xia, X.; She, Z.; Lin, Y. Structure elucidation of two new xanthone derivatives from the marine fungus Penicillium sp. (ZZF 32#) from the South China Sea. Magn. Reson. Chem. 2008, 46, 1066–1069. [Google Scholar]
- Li, H.; Huang, H.; Shao, C.; Huang, H.; Jiang, J.; Zhu, X.; Liu, Y.; Liu, L.; Lu, Y.; Li, M.; et al. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J. Nat. Prod. 2011, 74, 1230–1235. [Google Scholar] [CrossRef]
- Liu, F.; Li, Q.; Yang, H.; Cai, X.L.; Xia, X.K.; Chen, S.P.; Li, M.F.; She, Z.G.; Lin, Y.C. Structure elucidation of three diphenyl ether derivatives from the mangrove endophytic fungus SBE-14 from the South China Sea. Magn. Reson. Chem. 2009, 47, 453–455. [Google Scholar] [CrossRef]
- Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W.L.; Vrijmoed, L.L.; Jones, E.B.; Lin, Y. Three metabolites from the mangrove endophytic fungus Sporothrix sp. (#4335) from the South China Sea. J. Org. Chem. 2009, 74, 1093–1098. [Google Scholar] [CrossRef]
- Shao, C.; Wang, C.; Zheng, C.; She, Z.; Gu, Y.; Lin, Y. A new anthraquinone derivative from the marine endophytic fungus Fusarium sp. (No. b77). Nat. Prod. Res. 2010, 24, 81–85. [Google Scholar] [CrossRef]
- Huang, H.; Li, Q.; Feng, X.; Chen, B.; Wang, J.; Liu, L.; She, Z.; Lin, Y. Structural elucidation and NMR assignments of four aromatic lactones from a mangrove endophytic fungus (No. GX4-1B). Magn. Reson. Chem. 2010, 48, 496–499. [Google Scholar]
- Huang, Z.; Yang, R.; Yin, X.; She, Z.; Lin, Y. Structure elucidation and NMR assignments for two xanthone derivatives from a mangrove endophytic fungus (No. ZH19). Magn. Reson. Chem. 2010, 48, 80–82. [Google Scholar]
- Zhu, X.; He, Z.; Wu, J.; Yuan, J.; Wen, W.; Hu, Y.; Jiang, Y.; Lin, C.; Zhang, Q.; Lin, M.; et al. A marine anthraquinone SZ-685C overrides adriamycin-resistance in breast cancer cells through suppressing Akt signaling. Mar. Drugs 2012, 10, 694–711. [Google Scholar] [CrossRef]
- Chen, C.H.; Xiao, W.W.; Jiang, X.B.; Wang, J.W.; Mao, Z.G.; Lei, N.; Fan, X.; Song, B.B.; Liao, C.X.; Wang, H.J.; et al. A novel marine drug, SZ-685C, induces apoptosis of MMQ pituitary tumor cells by downregulating miR-200c. Curr. Med. Chem. 2013, 20, 2145–2154. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Liu, Q.; Wang, M.; Wang, C.; Yang, H. SZ-685C exhibits potent anticancer activity in both radiosensitive and radioresistant NPC cells through the miR-205-PTEN-Akt pathway. Oncol. Rep. 2013, 29, 2341–2347. [Google Scholar]
- Shao, C.L.; Wang, C.Y.; Deng, D.S.; She, Z.G.; Gu, Y.C.; Lin, Y.S. Crystal Structure of a Marine Natural Compound, Anhydrofusarubin. Chinese J. Struct. Chem. 2008, 27, 824–828. [Google Scholar]
- Zhai, A.; Zhu, X.; Wang, X.; Chen, R.; Wang, H. Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP)-induced cell death via the mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2013, 713, 58–67. [Google Scholar] [CrossRef]
- Steyn, P.S. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 1970, 26, 51–57. [Google Scholar] [CrossRef]
- Dhulipala, V.C.; Welshons, W.V.; Reddy, C.S. Cell cycle proteins in normal and chemically induced abnormal secondary palate development: A review. Hum. Exp. Toxicol. 2006, 25, 675–682. [Google Scholar] [CrossRef]
- Dhulipala, V.C.; Maddali, K.K.; Ray, B.K.; Welshons, W.V.; Reddy, C.S. Role of p21 and cyclin E in normal and secalonic acid D-inhibited proliferation of human embryonic palatal mesenchymal cells. Hum. Exp. Toxicol. 2011, 30, 1222–1232. [Google Scholar] [CrossRef]
- Hong, R. Secalonic acid D as a novel DNA topoisomerase I inhibitor from marine lichen-derived fungus Gliocladium sp. T31. Pharm. Biol. 2011, 49, 796–769. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.Y.; Lin, Y.C.; Liu, J.; Huang, R.; Wang, G.L.; Pei, Z.; et al. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res. 2009, 1302, 240–247. [Google Scholar] [CrossRef]
- Tao, L.Y.; Zhang, J.Y.; Liang, Y.J.; Chen, L.M.; Zhen, L.S.; Wang, F.; Mi, Y.J.; She, Z.G.; To, K.K.; Lin, Y.C.; et al. Anticancer effect and structure-activity analysis of marine products isolated from metabolites of mangrove fungi in the South China Sea. Mar. Drugs 2010, 8, 1094–1105. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.; Mao, Z.-G.; Song, B.-B.; Chen, C.-H.; Xiao, W.-W.; Hu, B.; Wang, J.-W.; Jiang, X.-B.; Zhu, Y.-H.; Wang, H.-J. Advances in the Study of the Structures and Bioactivities of Metabolites Isolated from Mangrove-Derived Fungi in the South China Sea. Mar. Drugs 2013, 11, 3601-3616. https://doi.org/10.3390/md11103601
Wang X, Mao Z-G, Song B-B, Chen C-H, Xiao W-W, Hu B, Wang J-W, Jiang X-B, Zhu Y-H, Wang H-J. Advances in the Study of the Structures and Bioactivities of Metabolites Isolated from Mangrove-Derived Fungi in the South China Sea. Marine Drugs. 2013; 11(10):3601-3616. https://doi.org/10.3390/md11103601
Chicago/Turabian StyleWang, Xin, Zhi-Gang Mao, Bing-Bing Song, Chun-Hua Chen, Wei-Wei Xiao, Bin Hu, Ji-Wen Wang, Xiao-Bing Jiang, Yong-Hong Zhu, and Hai-Jun Wang. 2013. "Advances in the Study of the Structures and Bioactivities of Metabolites Isolated from Mangrove-Derived Fungi in the South China Sea" Marine Drugs 11, no. 10: 3601-3616. https://doi.org/10.3390/md11103601




