Temporal Trends in the Secondary Metabolite Production of the Sponge Aplysina aerophoba
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Profile of Aplysina aerophoba
2.2. Natural Product Variations
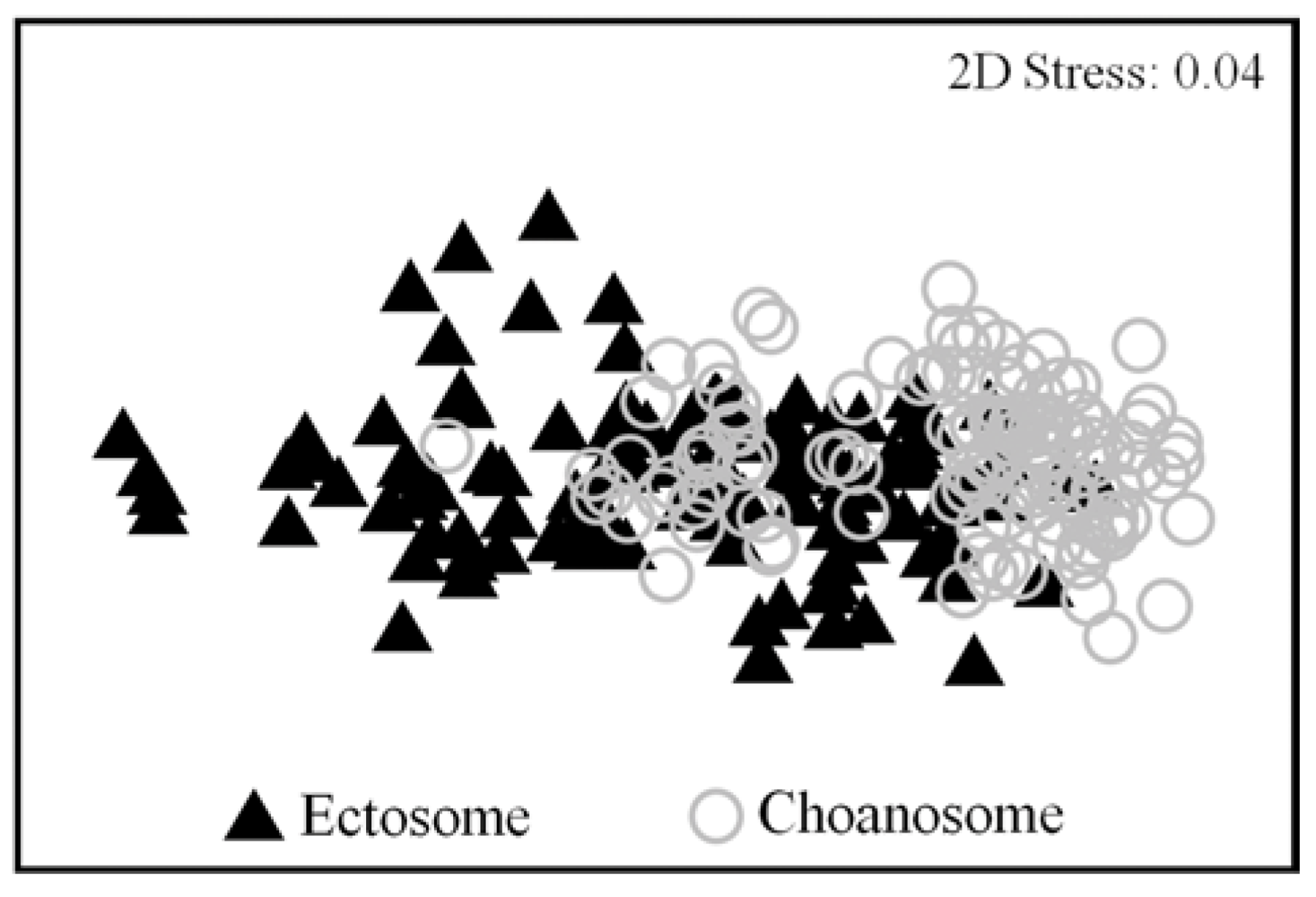
| Ectosome | Choanosome | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Year | Season | Month | Error | Year | Season | Month | Error |
| Aero2 | 0.869 | 0.190 | 0.027/14.85% | 78.13% | 0.059 | 0.491 | 0.030/13.67% | 76.29% |
| Aply1 | 0.368 | 0.001/25.82% | 0.539 | 74.18% | 0.463 | 0.438 | 0.025/15.80% | 83.80% |
| Iso3 | 0.260 | 0.003/24.44% | 0.322 | 69.30% | 0.551 | 0.201 | 0.031/14.03% | 79.60% |
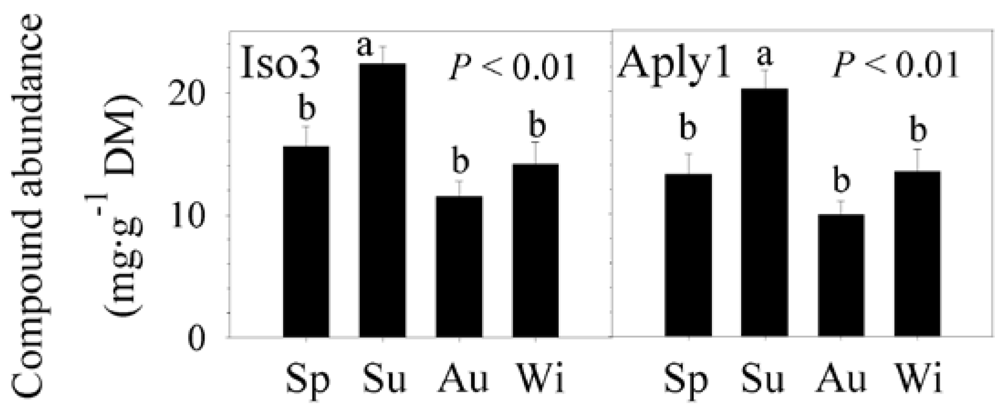
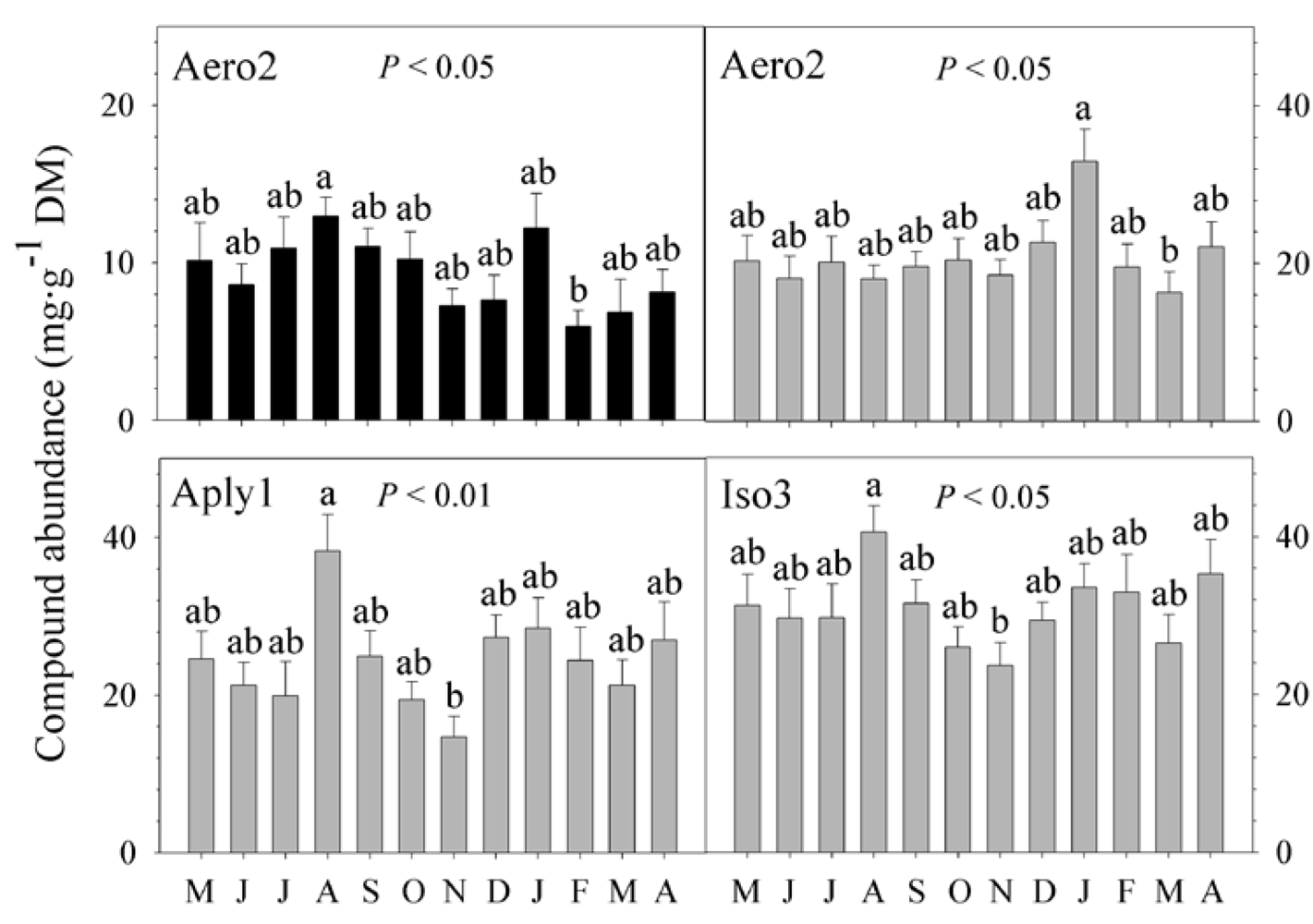
2.3. Natural Product Trends

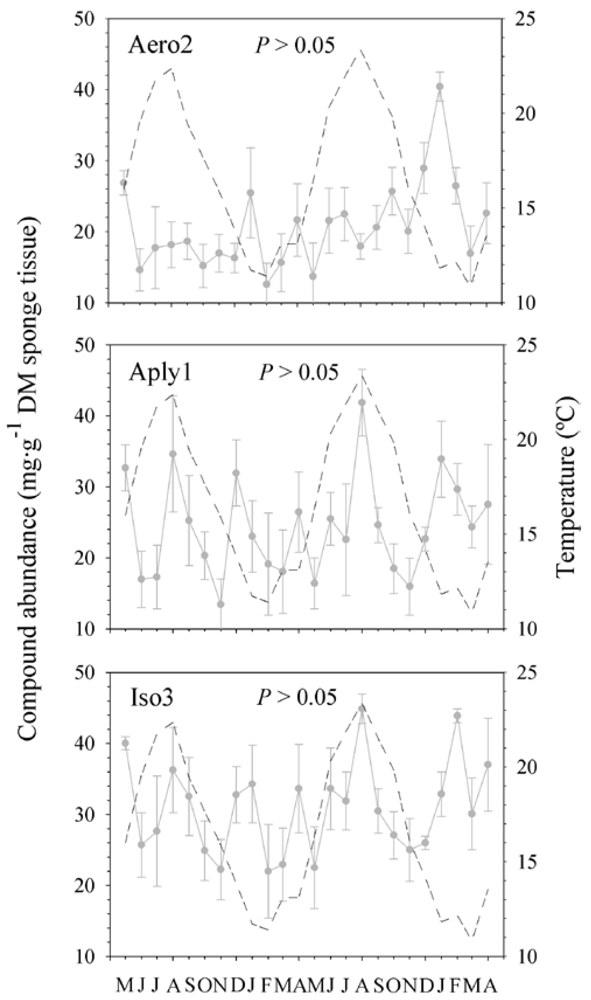
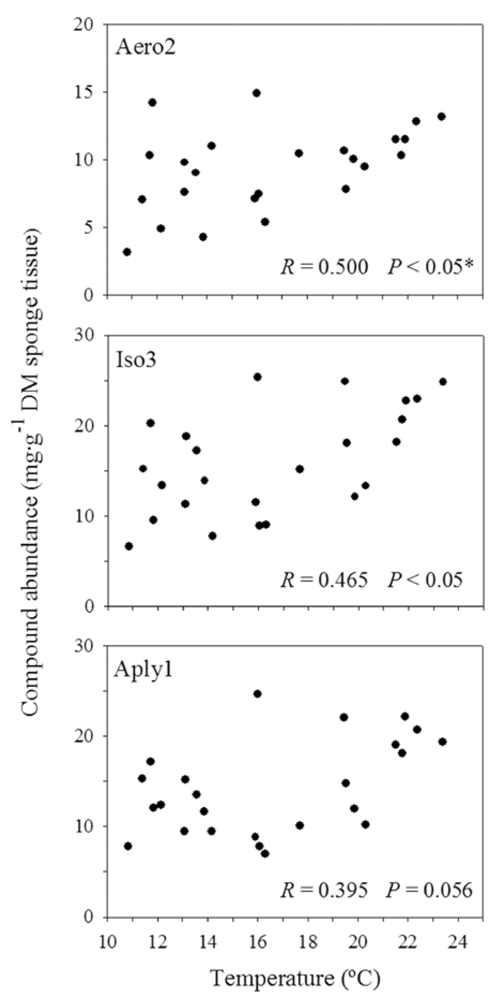
2.4. Sea Water Temperature and Concentration of BAs
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Egan, S.; Thomas, T.; Kjelleberg, S. Unlocking the diversity and biotechnological potential of marine surface associated microbial communities. Curr. Opin. Microbiol. 2008, 11, 219–225. [Google Scholar]
- Paul, V.J.; Puglisi, M.P.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2006, 23, 153–180. [Google Scholar]
- Sipkema, D.; Franssen, M.C.R.; Osinga, R.; Tramper, J.; Wijffels, R.H. Marine sponges as pharmacy. Mar. Biotechnol. 2005, 7, 142–162. [Google Scholar]
- Burns, E.; Ifrach, I.; Carmeli, S.; Pawlik, J.R.; Ilan, M. Comparison of anti-predatory defenses of Red Sea and Caribbean sponges. I. Chemical defense. Mar. Ecol. Prog. Ser. 2003, 252, 105–114. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Chanas, B.; Toonen, R.J.; Fenical, W. Defenses of Caribbean sponges against predatory reef fish. I. Chemical deterrency. Mar. Ecol. Prog. Ser. 1995, 127, 183–194. [Google Scholar] [CrossRef]
- Ruzicka, R.; Gleason, D.F. Sponge community structure and anti-predator defenses on temperate reefs of the South Atlantic Bight. J. Exp. Mar. Biol. Ecol. 2009, 380, 36–46. [Google Scholar]
- Waddell, B.; Pawlik, J.R. Defenses of Caribbean sponges against invertebrate predators. I. Assays with hermit crabs. Mar. Ecol. Prog. Ser. 2000, 195, 125–132. [Google Scholar] [CrossRef]
- Waddell, B.; Pawlik, J.R. Defenses of Caribbean sponges against invertebrate predators. II. Assays with sea stars. Mar. Ecol. Prog. Ser. 2000, 195, 133–144. [Google Scholar] [CrossRef]
- Engel, S.; Pawlik, J.R. Allelopathic activities of sponge extracts. Mar. Ecol. Prog. Ser. 2000, 207, 273–281. [Google Scholar]
- Luter, H.M.; Duckworth, A.R. Influence of size and spatial competition on the bioactivity of coral reef sponges. Biochem. Syst. Ecol. 2010, 38, 146–153. [Google Scholar]
- Becerro, M.A.; López, N.I.; Turon, X.; Uriz, M.J. Antimicrobial activity and surface bacterial film in marine sponges. J. Exp. Mar. Biol. Ecol. 1994, 179, 195–205. [Google Scholar]
- Uriz, M.J.; Rosell, D.; Martin, D. The sponge population of the cabrera archipelago (balearic islands)—Characteristics, distribution, and abundance of the most representative species. Mar. Ecol. 1992, 13, 101–117. [Google Scholar] [CrossRef]
- Kelman, D.; Kashman, Y.; Rosenberg, E.; Ilan, M.; Ifrach, I.; Loya, Y. Antimicrobial activity of the reef sponge Amphimedon viridis from the Red Sea: Evidence for selective toxicity. Aquat. Microb. Ecol. 2001, 24, 9–16. [Google Scholar]
- Newbold, R.W.; Jensen, P.R.; Fenical, W.; Pawlik, J.R. Antimicrobial activity of Caribbean sponge extracts. Aquat. Microb. Ecol. 1999, 19, 279–284. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar]
- Munro, M.H.G.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.H.; Lill, R.E.; Li, S.X.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar]
- Erwin, P.M.; Lopez-Legentil, S.; Schuhmann, P.W. The pharmaceutical value of marine biodiversity for anti-cancer drug discovery. Ecol. Econ. 2010, 70, 445–451. [Google Scholar]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2000, 17, 7–55. [Google Scholar]
- Corriero, G.; Liaci, L.S.; Ruggiero, D.; Pansini, M. The sponge community of a semi-submerged Mediterranean cave. Mar. Ecol. 2000, 21, 85–96. [Google Scholar]
- Reiswig, H.M. Population dynamics of three Jamaican demospongiae. Bull. Mar. Sci. 1973, 23, 191–226. [Google Scholar]
- Voultsiadou, E. Demosponge distribution in the eastern Mediterranean: A NW-SE gradient. Helgol. Mar. Res. 2005, 59, 237–251. [Google Scholar]
- Wilkinson, C.R.; Cheshire, A.C. Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs 1989, 8, 127–134. [Google Scholar]
- Zenkevitch, L.A.; Birstein, J.A. On the problem of the antiquity of the deep-sea fauna. Deep-Sea Res. 1960, 7, 10–23. [Google Scholar] [CrossRef]
- Becerro, M.A. Quantitative trends in sponge ecology research. Mar. Ecol. Evol. Persp. 2008, 29, 167–177. [Google Scholar]
- McKey, D. Adaptive patterns in alkaloid physiology. Am. Nat. 1974, 108, 305–320. [Google Scholar]
- McKey, D. The Distribution of Secondary Compounds within Plants. In Herbivores: Their Interaction with Secondary Plant Metabolites; Rosenthal, G.A., Janzen, G.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 56–134. [Google Scholar]
- Rhoades, D.F. Evolution of Plant Chemical Defense Against Herbivores. In Herbivores: Their Interaction with Secondary Plant Metabolites; Rosenthal, G.A., Janzen, G.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 3–54. [Google Scholar]
- Skogsmyr, I.; Fagerstrom, T. The cost of antiherbivory defense—An evaluation of some ecological and physiological factors. Oikos 1992, 64, 451–457. [Google Scholar]
- Zangerl, A.R.; Rutledge, C.E. The probability of attack and patterns of constitutive and induced defense: A test of optimal defense theory. Am. Nat. 1996, 147, 599–608. [Google Scholar]
- Becerro, M.A.; Turon, X.; Uriz, M.J. Natural variation of toxicity in encrusting sponge Crambe crambe (Schmidt) in relation to size and environment. J. Chem. Ecol. 1995, 21, 1931–1946. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Henkel, T.P.; McMurray, S.E.; Lopez-Legentil, S.; Loh, T.L.; Rohde, S. Patterns of sponge recruitment and growth on a shipwreck corroborate chemical defense resource trade-off. Mar. Ecol. Prog. Ser. 2008, 368, 137–143. [Google Scholar]
- Page, M.; West, L.; Northcote, P.; Battershill, C.; Kelly, M. Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. J. Chem. Ecol. 2005, 31, 1161–1174. [Google Scholar] [CrossRef]
- Ferretti, C.; Vacca, S.; de Ciucis, C.; Marengo, B.; Duckworth, A.R.; Manconi, R.; Pronzato, R.; Domenicotti, C. Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Mar. Ecol. Evol. Persp. 2009, 30, 327–336. [Google Scholar] [CrossRef]
- Thompson, J.E.; Murphy, P.T.; Bergquist, P.R.; Evans, E.A. Environmentally induced variation in diterpene composition of the marine sponge Rhopaloeides odorabile. Biochem. Syst. Ecol. 1987, 15, 595–606. [Google Scholar] [CrossRef]
- Abdo, D.A.; Motti, C.A.; Battershill, C.N.; Harvey, E.S. Temperature and spatiotemporal variability of salicylihalamide A in the sponge Haliclona sp. J. Chem. Ecol. 2007, 33, 1635–1645. [Google Scholar] [CrossRef]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Seasonal patterns of toxicity in benthic invertebrates: The encrusting sponge Crambe crambe (Poecilosclerida). Oikos 1996, 75, 33–40. [Google Scholar] [CrossRef]
- Duckworth, A.R.; Battershill, C.N. Population dynamics and chemical ecology of New Zealand demospongiae Latrunculia sp. nov. and Polymastia croceus (Poecilosclerida: Latrunculiidae: Polymastiidae). N. Z. J. Mar. Freshwat. Res. 2001, 35, 935–949. [Google Scholar] [CrossRef]
- Becerro, M.A.; Uriz, M.J.; Turon, X. Chemically-mediated interactions in benthic organisms: The chemical ecology of Crambe crambe (Porifera, Poecilosclerida). Hydrobiologia 1997, 355, 77–89. [Google Scholar] [CrossRef]
- Ciminiello, P.; Fattorusso, E.; Forino, M.; Magno, S.; Pansini, M. Chemistry of Verongida sponges. 8. Bromocompounds from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. Tetrahedron 1997, 53, 6565–6572. [Google Scholar]
- Ebel, R.; Brenzinger, M.; Kunze, A.; Gross, H.J.; Proksch, P. Wound activation of protoxins in marine sponge Aplysina aerophoba. J. Chem. Ecol. 1997, 23, 1451–1462. [Google Scholar] [CrossRef]
- Thoms, C.; Ebel, R.; Proksch, P. Activated chemical defense in Aplysina sponges revisited. J. Chem. Ecol. 2006, 32, 97–123. [Google Scholar] [CrossRef]
- Weiss, B.; Ebel, R.; Elbrächter, M.; Kirchner, M.; Proksch, P. Defense metabolites from the marine sponge Verongia aerophoba. Biochem. Syst. Ecol. 1996, 24, 1–12. [Google Scholar] [CrossRef]
- Kreuter, M.H.; Robitzki, A.; Chang, S.; Steffen, R.; Michaelis, M.; Kljajic, Z.; Bachmann, M.; Schröder, H.C.; Müller, W.E.G. Production of the cytostatic agent aeroplysinin by the sponge Verongia aerophoba in in vitro culture. Comp. Biochem. Phys. C 1992, 101, 183–187. [Google Scholar]
- Sacristán-Soriano, O.; Banaigs, B.; Casamayor, E.O.; Becerro, M.A. Exploring the links between natural products and bacterial assemblages in the sponge Aplysina aerophoba. Appl. Environ. Microbiol. 2011, 77, 862–870. [Google Scholar]
- Turon, X.; Becerro, M.A.; Uriz, M.J. Distribution of brominated compounds within the sponge Aplysina aerophoba: Coupling of X-ray microanalysis with cryofixation techniques. Cell Tissue Res. 2000, 301, 311–322. [Google Scholar] [CrossRef]
- Sacristán-Soriano, O.; Banaigs, B.; Becerro, M.A. Relevant spatial scales of chemical variation in Aplysina aerophoba. Mar. Drugs 2011, 9, 2499–2513. [Google Scholar] [CrossRef] [Green Version]
- Schupp, P.; Eder, C.; Paul, V.; Proksch, P. Distribution of secondary metabolites in the sponge Oceanapia sp. and its ecological implications. Mar. Biol. 1999, 135, 573–580. [Google Scholar] [CrossRef]
- Becerro, M.A.; Paul, V.J.; Starmer, J. Intracolonial variation in chemical defenses of the sponge Cacospongia sp. and its consequences on generalist fish predators and the specialist nudibranch predator Glossodoris pallida. Mar. Ecol. Prog. Ser. 1998, 168, 187–196. [Google Scholar] [CrossRef]
- Puyana, M.; Fenical, W.; Pawlik, J.R. Are there activated chemical defenses in sponges of the genus Aplysina from the Caribbean? Mar. Ecol. Prog. Ser. 2003, 246, 127–135. [Google Scholar] [CrossRef]
- Swearingen, D.C.; Pawlik, J.R. Variability in the chemical defense of the sponge Chondrilla nucula against predatory reef fishes. Mar. Biol. 1998, 131, 619–627. [Google Scholar] [CrossRef]
- Becerro, M.A.; Turon, X.; Uriz, M.J. Multiple functions for secondary metabolites in encrusting marine invertebrates. J. Chem. Ecol. 1997, 23, 1527–1547. [Google Scholar]
- Teeyapant, R.; Woerdenbag, H.J.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba. Z. Naturforsch. C 1993, 48, 939–945. [Google Scholar]
- Becerro, M.A.; Turon, X.; Uriz, M.J.; Templado, J. Can a sponge feeder be a herbivore? Tylodina perversa (Gastropoda) feeding on Aplysina aerophoba (Demospongiae). Biol. J. Linn. Soc. 2003, 78, 429–438. [Google Scholar] [CrossRef]
- Becerro, M.A.; Bonito, V.; Paul, V.J. Effects of monsoon-driven wave action on coral reefs of Guam and implications for coral recruitment. Coral Reefs 2006, 25, 193–199. [Google Scholar]
- Thoms, C.; Wolff, M.; Padmakumar, K.; Ebel, R.; Proksch, P. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z. Naturforsch. C 2004, 59, 113–122. [Google Scholar]
- Teeyapant, R.; Proksch, P. Biotransformation of brominated compounds in the marine sponge Verongia aerophoba—Evidence for an induced chemical defense. Naturwissenschaften 1993, 80, 369–370. [Google Scholar] [CrossRef]
- Leong, W.; Pawlik, J.R. Evidence of a resource trade-off between growth and chemical defenses among Caribbean coral reef sponges. Mar. Ecol. Prog. Ser. 2010, 406, 71–78. [Google Scholar]
- Gallissian, M.F.; Vacelet, J. Ultrastructure de quelques stades de l'ovogenèse de spongiaires du genre Verongia (Dictyoceratida). Ann. Sci. Nat. Zool. 1976, 18, 381–404. [Google Scholar]
- Tsurumi, M.; Reiswig, H.M. exual versus asexual reproduction in an oviparous rope-form sponge, Aplysina cauliformis (Porifera; Verongida). Invertebr. Reprod. Dev. 1997, 32, 1–9. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cook, S.D.C. Order Verongida Bergquist, 1978. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J.N.A., van Soest, R.M.W., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; p. 1093. [Google Scholar]
- Scalera-Liaci, L.; Sciscioli, M.; Matarrese, A.; Giove, C. Osservazioni sui cicli sessuali di alcune Keratosa (Poriferi) e loro interesse negli studi filogenetici. Atti Soc. Pelorit. Sci. FisicheMat. E Nat. 1971, 17, 33–52. [Google Scholar]
- Smale, D.A.; Wernberg, T. Satellite-derived SST data as a proxy for water temperature in nearshore benthic ecology. Mar. Ecol. Prog. Ser. 2009, 387, 27–37. [Google Scholar]
- Maldonado, M.; Sanchez-Tocino, L.; Navarro, C. Recurrent disease outbreaks in corneous demosponges of the genus Ircinia: Epidemic incidence and defense mechanisms. Mar. Biol. 2010, 157, 1577–1590. [Google Scholar] [CrossRef]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2004, 21, 519–538. [Google Scholar]
- Unson, M.D.; Faulkner, D.J. Cyanobacterial symbiont biosynthesis of chlorinated metabolites from Dysidea herbacea (Porifera). Experientia 1993, 49, 349–353. [Google Scholar] [CrossRef]
- Unson, M.D.; Holland, N.D.; Faulkner, D.J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of a crystalline metabolite in the sponge tissue. Mar. Biol. 1994, 119, 1–11. [Google Scholar]
- Siegl, A.; Hentschel, U. PKS and NRPS gene clusters from microbial symbiont cells of marine sponges by whole genome amplification. Environ. Microbiol. Rep. 2010, 2, 507–513. [Google Scholar]
- Cimino, G.; Derosa, S.; Destefano, S.; Self, R.; Sodano, G. The bromo-compounds of the true sponge Verongia aerophoba. Tetrahedron Lett. 1983, 24, 3029–3032. [Google Scholar]
- NASA Goddard Space Flight Center. Available online: http://oceancolor.gsfc.nasa.gov/ (accessed on 1 March 2011).
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- SPSS, Systat 9: Statistics I, 9.0, SPSS Inc.: Chicago, IL, USA, 1999.
- SPSS, Systat 9: Statistics II, 9.0, SPSS Inc.: Chicago, IL, USA, 1999.
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sacristán-Soriano, O.; Banaigs, B.; Becerro, M.A. Temporal Trends in the Secondary Metabolite Production of the Sponge Aplysina aerophoba. Mar. Drugs 2012, 10, 677-693. https://doi.org/10.3390/md10040677
Sacristán-Soriano O, Banaigs B, Becerro MA. Temporal Trends in the Secondary Metabolite Production of the Sponge Aplysina aerophoba. Marine Drugs. 2012; 10(4):677-693. https://doi.org/10.3390/md10040677
Chicago/Turabian StyleSacristán-Soriano, Oriol, Bernard Banaigs, and Mikel A. Becerro. 2012. "Temporal Trends in the Secondary Metabolite Production of the Sponge Aplysina aerophoba" Marine Drugs 10, no. 4: 677-693. https://doi.org/10.3390/md10040677




