Preparation and Characterization of Ferrofluid Stabilized with Biocompatible Chitosan and Dextran Sulfate Hybrid Biopolymer as a Potential Magnetic Resonance Imaging (MRI) T2 Contrast Agent
Abstract
:1. Introduction
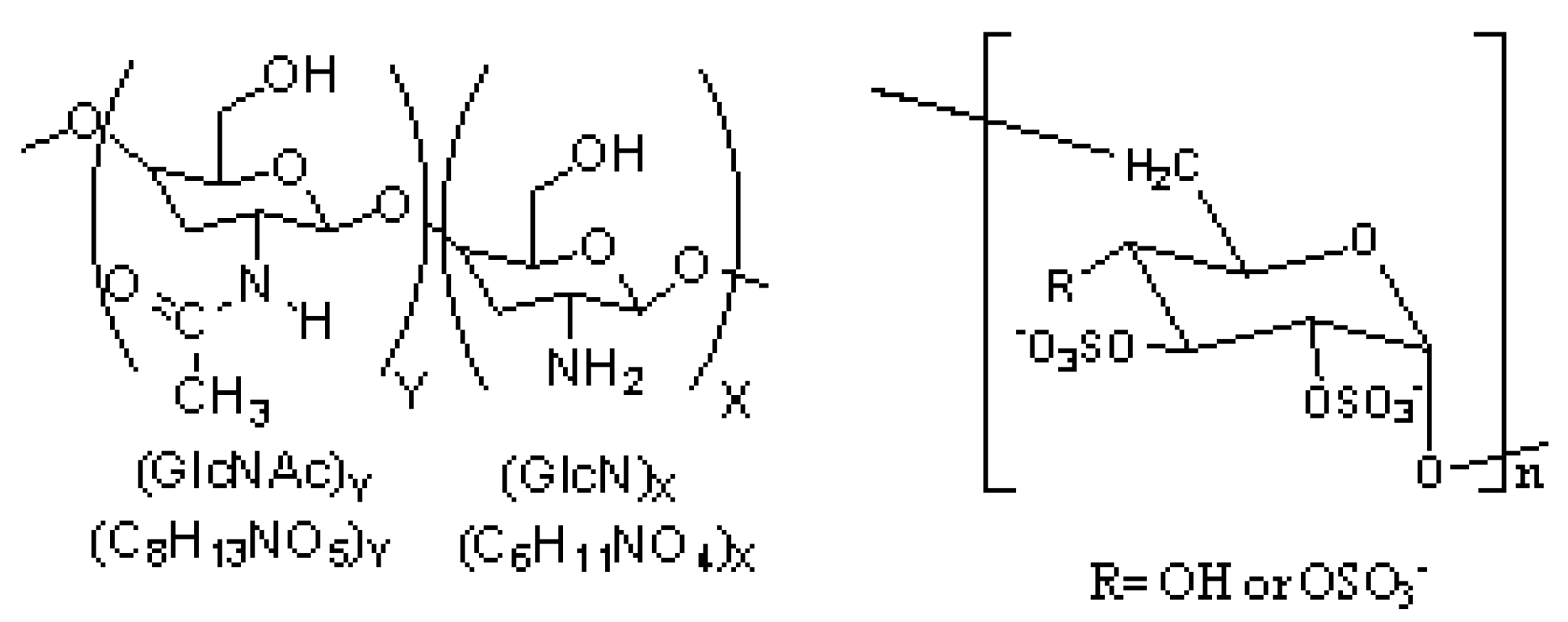
2. Results and Discussion
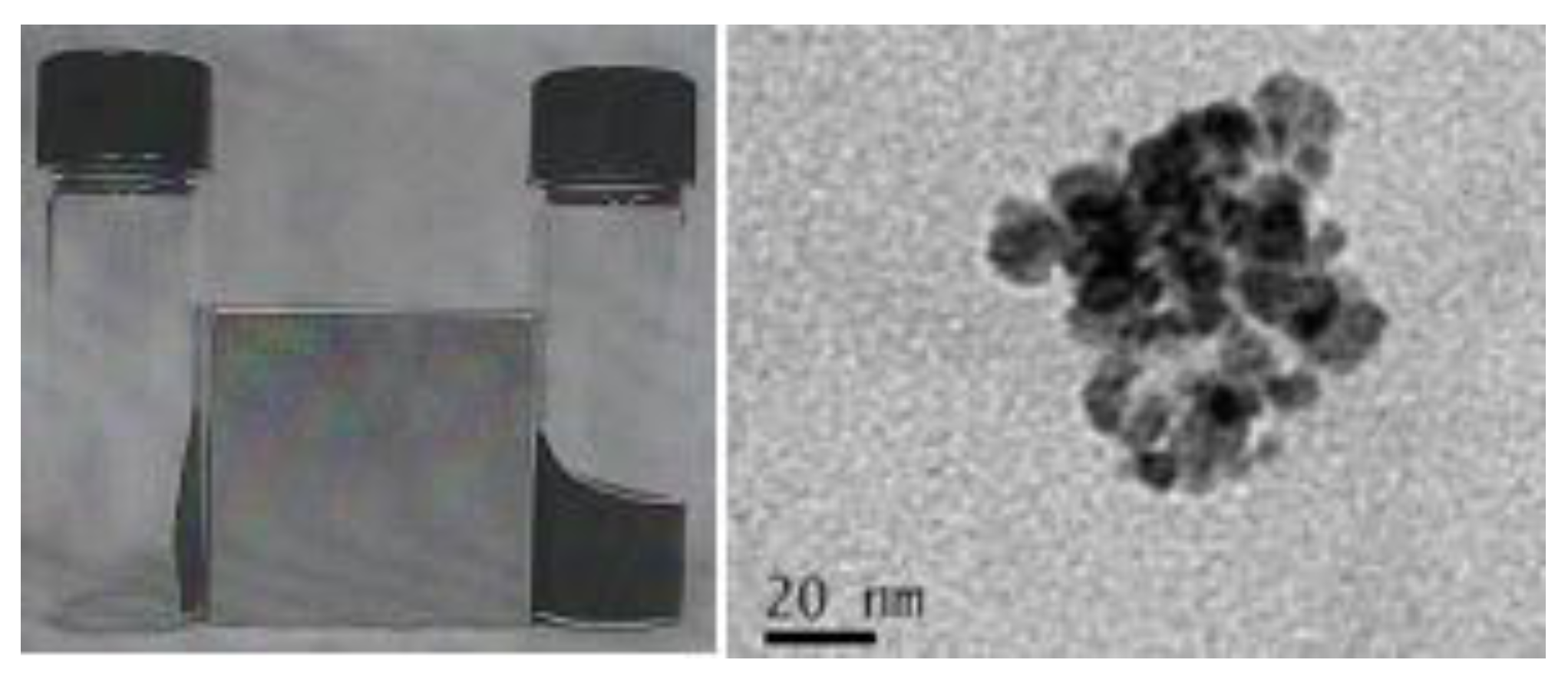
2.1. Synthesis of the Hybrid Ferrofluid (Hybrid SPIONs)
2.2. Physicochemical Features of the Hybrid Ferrofluid
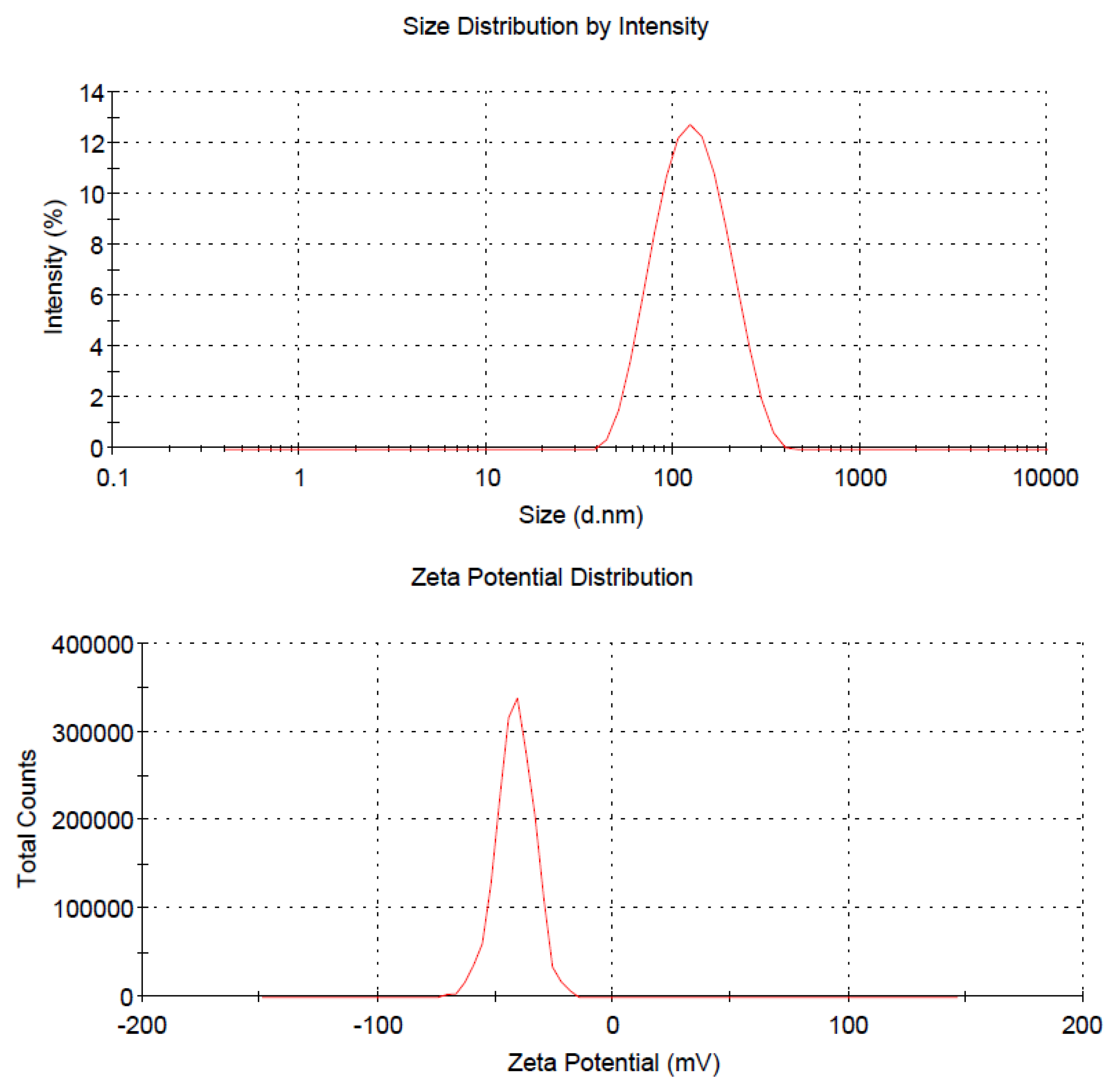
| Dilution | z-average diameter (nm) | polydispersity index | zeta potential (mV) | |||
|---|---|---|---|---|---|---|
| Ferrofluid | PECs | Ferrofluid | PECs | Ferrofluid | PECs | |
| Stock a | 194 | 133.3 | 0.173 | 0.150 | −22.7 | −36.8 |
| 2× | 152.9 | 134.4 | 0.163 | 0.134 | −33.4 | −38.3 |
| 5× | 133.7 | 140.4 | 0.179 | 0.120 | −39.4 | −38.7 |
| 10× | 124.9 | 147.3 | 0.176 | 0.123 | −40.2 | −32.7 |
| 200× | 114.3 | 150.3 | 0.174 | 0.181 | −41.5 | −32.2 |
2.3. Relaxometry of the Hybrid Ferrofluid

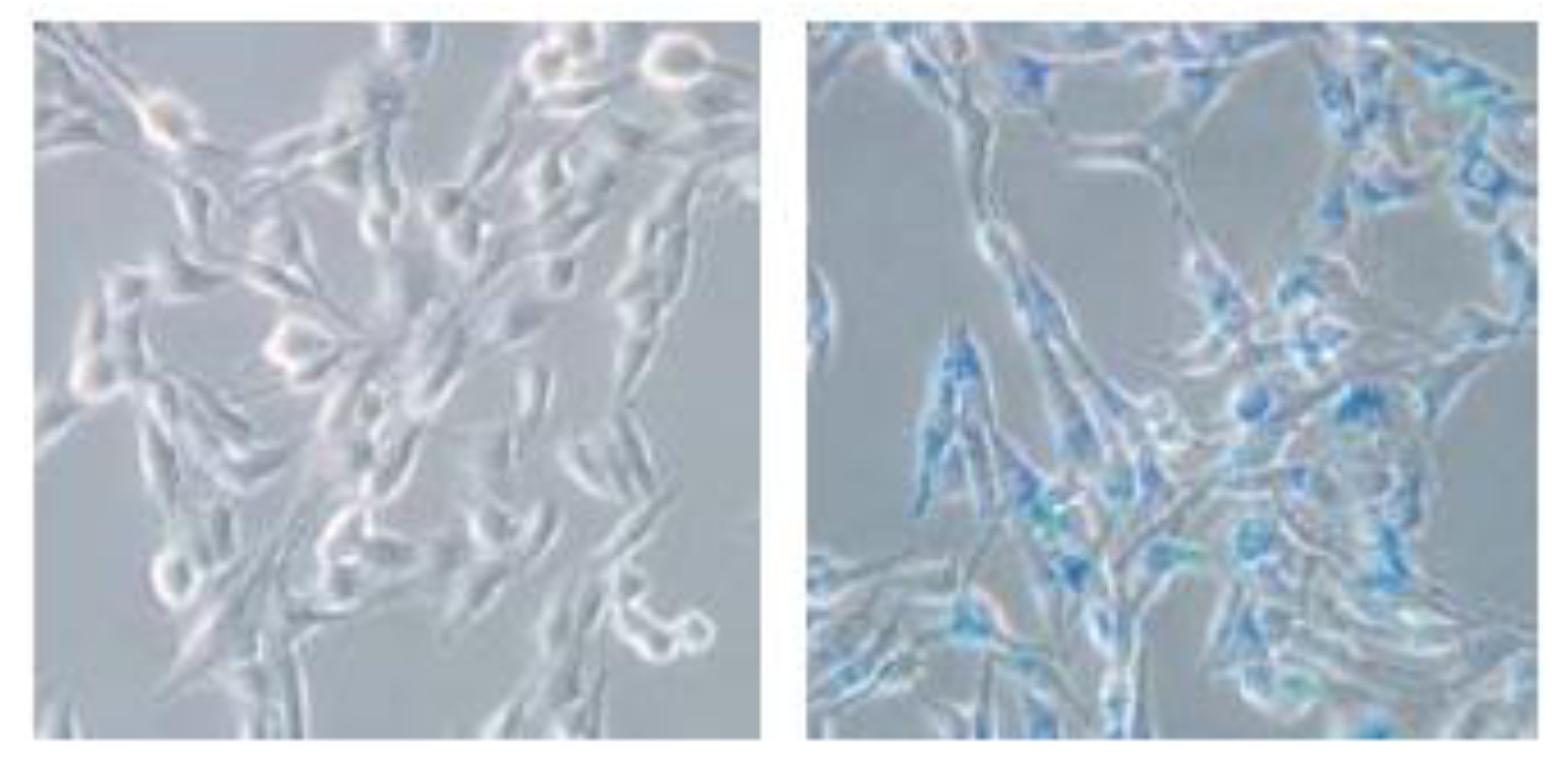
2.4. Biocompatibility and Cellular Uptake Analysis
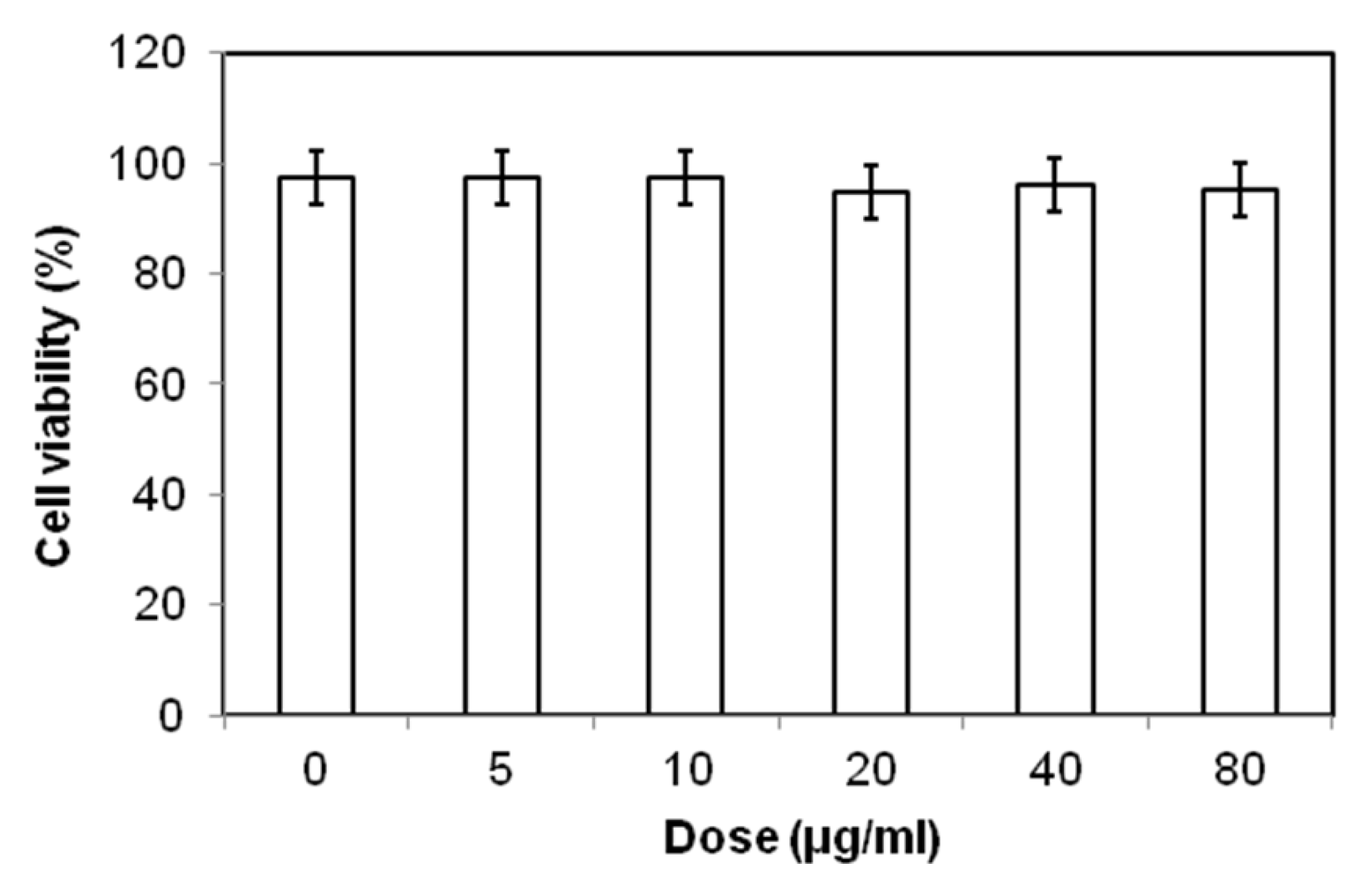
3. Experimental Section
3.1. Materials
3.2. Preparation of the Hybrid SPION Ferrofluid
3.3. Characterization of the Hybrid SPION Ferrofluid
3.4. Cell Survival
3.5. Cellular Uptake
4. Conclusions
Acknowledgments
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Latham, A.H.; Williams, M.E. Controlling transport and chemical functionality of magnetic nanoparticles. Acc. Chem. Res. 2008, 41, 411–420. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, S.; Simchi, A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar]
- Villaraza, A.J.; Bumb, A.; Brechbiel, M.W. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: The interplay between size, function, and pharmacokinetics. Chem. Rev. 2010, 110, 2921–2959. [Google Scholar] [CrossRef]
- Wang, Y.-X.J.; Quercy-Jouvet, T.; Wang, H.-H.; Li, A.-W.; Chak, C.-P.; Xuan, S.; Shi, L.; Wang, D.-F.; Lee, S.-F.; Leung, P.-C.; et al. Efficacy and durability in direct labeling of mesenchymal stem cells using ultrasmall superparamagnetic iron oxide nanoparticles with organosilica, dextran, and PEG coatings. Materials 2011, 4, 703–715. [Google Scholar] [CrossRef]
- Somsook, E.; Hinsin, D.; Buakhrong, P.; Teanchai, R.; Mophan, N.; Pohmakotr, M.; Shiowatana, J. Interactions between iron(III) and sucrose, dextran, or starch in complexes. Carbohydr. Polym. 2005, 61, 281–287. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Bahadur, K.C.R.; Aryal, S.; Khil, M.S.; Kim, H.Y. N-Acylated chitosan stabilized iron oxide nanoparticles as a novel nano-matrix and ceramic modification. Carbohydr. Polym. 2007, 69, 467–477. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Maharramov, A.; Ramazanov, M.A. Synthesis and characterization of superparamagnetic nanoparticles coated with carboxymethyl starch (CMS) for magnetic resonance imaging technique. Carbohydr. Polym. 2009, 78, 292–295. [Google Scholar] [CrossRef]
- Tsai, Z.-T.; Wang, J.-F.; Kuo, H.-Y.; Shen, C.-R.; Wang, J.-J.; Yen, T.-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating with chitosan: A potential MRI contrast agent useful for cell tracking. J. Magn. Magn. Mater. 2010, 322, 208–213. [Google Scholar] [CrossRef]
- Qin, J.; Laurent, S.; Jo, Y.S.; Roch, A.; Mikhaylova, M.; Bhujwalla, Z.M.; Muller, R.N.; Muhammed, M. A High-performance magnetic resonance imaging T2 contrast agent. Adv. Mater. 2007, 19, 1874–1878. [Google Scholar]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef]
- Chen, J.-K.; Yeh, C.-H.; Wang, L.-C.; Liou, T.-H.; Shen, C.-R.; Liu, C.-L. Chitosan, the marine functional food, is a potent adsorbent of humic acid. Mar. Drugs 2011, 9, 2488–2498. [Google Scholar] [CrossRef]
- Maganti, N.; Venkat Surya, P.K.C.; Thein-Han, W.W.; Pesacreta, T.C.; Misra, R.D.K. Structure–Process–Property relationship of biomimetic chitosan-based nanocomposite scaffolds for tissue engineering: Biological, physico-chemical, and mechanical functions. Adv. Eng. Mater. 2011, 13, B108–B122. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.K.; Kitiyanant, Y. Chitosan—Gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP—buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Misra, R.D.K. Biomimetic chitosan—nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 1182–1197. [Google Scholar] [CrossRef]
- Yuan, Q.; Hein, S.; Misra, R.D.K. New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: Synthesis, characterization and in vitro drug delivery response. Acta Biomater. 2010, 6, 2732–2739. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R.D.K. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B-chitosan-dextran sulfate nanoparticles. Int. J. Pharm. 2007, 329, 142–149. [Google Scholar] [CrossRef]
- Drogoz, A.; David, L.; Rochas, C.; Domard, A.; Delair, T. Polyelectrolyte complexes from polysaccharides: Formation and stoichiometry monitoring. Langmuir 2007, 23, 10950–10958. [Google Scholar] [CrossRef]
- Schatz, C.; Domard, A.; Viton, C.; Pichot, C.; Delair, T. Versatile and efficient formation of colloids of biopolymer-based polyelectrolyte complexes. Biomacromolecules 2004, 5, 1882–1892. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Ferreira, D.; Neufeld, R. Oral bioavailability of insulin contained in polysaccharide nanoparticles. Biomacromolecules 2007, 8, 3054–3060. [Google Scholar] [CrossRef]
- Huang, M.; Vitharana, S.N.; Peek, L.J.; Coop, T.; Berkland, C. Polyelectrolyte complexes stabilize and controllably release vascular endothelial growth factor. Biomacromolecules 2007, 8, 1607–1614. [Google Scholar] [CrossRef]
- Lauten, E.H.; VerBerkmoes, J.; Choi, J.; Jin, R.; Edwards, D.A.; Loscalzo, J.; Zhang, Y.Y. Nanoglycan complex formulation extends VEGF retention time in the lung. Biomacromolecules 2010, 11, 1863–1872. [Google Scholar] [CrossRef]
- Tan, M.L.; Friedhuber, A.M.; Dunstan, D.E.; Choong, P.F.; Dass, C.R. The performance of doxorubicin encapsulated in chitosan-dextran sulphate microparticles in an osteosarcoma model. Biomaterials 2010, 31, 541–551. [Google Scholar] [CrossRef]
- Anitha, A.; Deepagan, V.G.; Divya Rani, V.V.; Menon, D.; Nair, S.V.; Jayakumar, R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate—chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1158–1164. [Google Scholar] [CrossRef]
- Drogoz, A.; Munier, S.; Verrier, B.; David, L.; Domard, A.; Delair, T. Towards biocompatible vaccine delivery systems: Interactions of colloidal PECs based on polysaccharides with HIV-1 p24 antigen. Biomacromolecules 2008, 9, 583–591. [Google Scholar]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef]
- Shen, C.-R.; Juang, J.-H.; Tsai, Z.-T.; Wu, S.-T.; Tsai, F.-Y.; Wang, J.-J.; Liu, C.-L.; Yen, T.-C. Preparation, characterization and application of superparamagnetic iron oxide encapsulated with N-[(2-hydroxy-3-trimethylammonium) propyl] chitosan chloride. Carbohydr. Polym. 2011, 84, 781–787. [Google Scholar] [CrossRef]
- Shen, C.-R.; Wu, S.-T.; Tsai, Z.-T.; Wang, J.-J.; Yen, T.-C.; Tsai, J.-S.; Shih, M.-F.; Liu, C.-L. Characterization of quaternized chitosan-stabilized iron oxide nanoparticles as a novel potential magnetic resonance imaging contrast agent for cell tracking. Polym. Int. 2011, 60, 945–950. [Google Scholar] [CrossRef]
- Juang, J.H.; Wang, J.J.; Shen, C.R.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging of transplanted mouse islets labeled with chitosan-coated superparamagnetic iron oxide nanoparticles. Transplant. Proc. 2010, 42, 2104–2108. [Google Scholar] [CrossRef]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Lin, M.Y.; Wu, S.T.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging study of mouse islet allotransplantation. Transplant. Proc. 2010, 42, 4217–4220. [Google Scholar] [CrossRef]
- Lim, L.Y.; Khor, E.; Koo, O. Gamma irradiation of chitosan. J. Biomed. Mater. Res. 1998, 43, 282–290. [Google Scholar]
- Bakandritsos, A.; Psarras, G.C.; Boukos, N. Some physicochemical aspects of nanoparticulate magnetic iron oxide colloids in neat water and in the presence of poly(vinyl alcohol). Langmuir 2008, 24, 11489–11496. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tsai, Z.-T.; Tsai, F.-Y.; Yang, W.-C.; Wang, J.-F.; Liu, C.-L.; Shen, C.-R.; Yen, T.-C. Preparation and Characterization of Ferrofluid Stabilized with Biocompatible Chitosan and Dextran Sulfate Hybrid Biopolymer as a Potential Magnetic Resonance Imaging (MRI) T2 Contrast Agent. Mar. Drugs 2012, 10, 2403-2414. https://doi.org/10.3390/md10112403
Tsai Z-T, Tsai F-Y, Yang W-C, Wang J-F, Liu C-L, Shen C-R, Yen T-C. Preparation and Characterization of Ferrofluid Stabilized with Biocompatible Chitosan and Dextran Sulfate Hybrid Biopolymer as a Potential Magnetic Resonance Imaging (MRI) T2 Contrast Agent. Marine Drugs. 2012; 10(11):2403-2414. https://doi.org/10.3390/md10112403
Chicago/Turabian StyleTsai, Zei-Tsan, Fu-Yuan Tsai, Wei-Cheng Yang, Jen-Fei Wang, Chao-Lin Liu, Chia-Rui Shen, and Tzu-Chen Yen. 2012. "Preparation and Characterization of Ferrofluid Stabilized with Biocompatible Chitosan and Dextran Sulfate Hybrid Biopolymer as a Potential Magnetic Resonance Imaging (MRI) T2 Contrast Agent" Marine Drugs 10, no. 11: 2403-2414. https://doi.org/10.3390/md10112403




