Minimally-Myelosuppressive Asparaginase-Containing Induction Regimen for Treatment of a Jehovah’s Witness with mutant IDH1/NPM1/NRAS Acute Myeloid Leukemia
Abstract
:1. Introduction
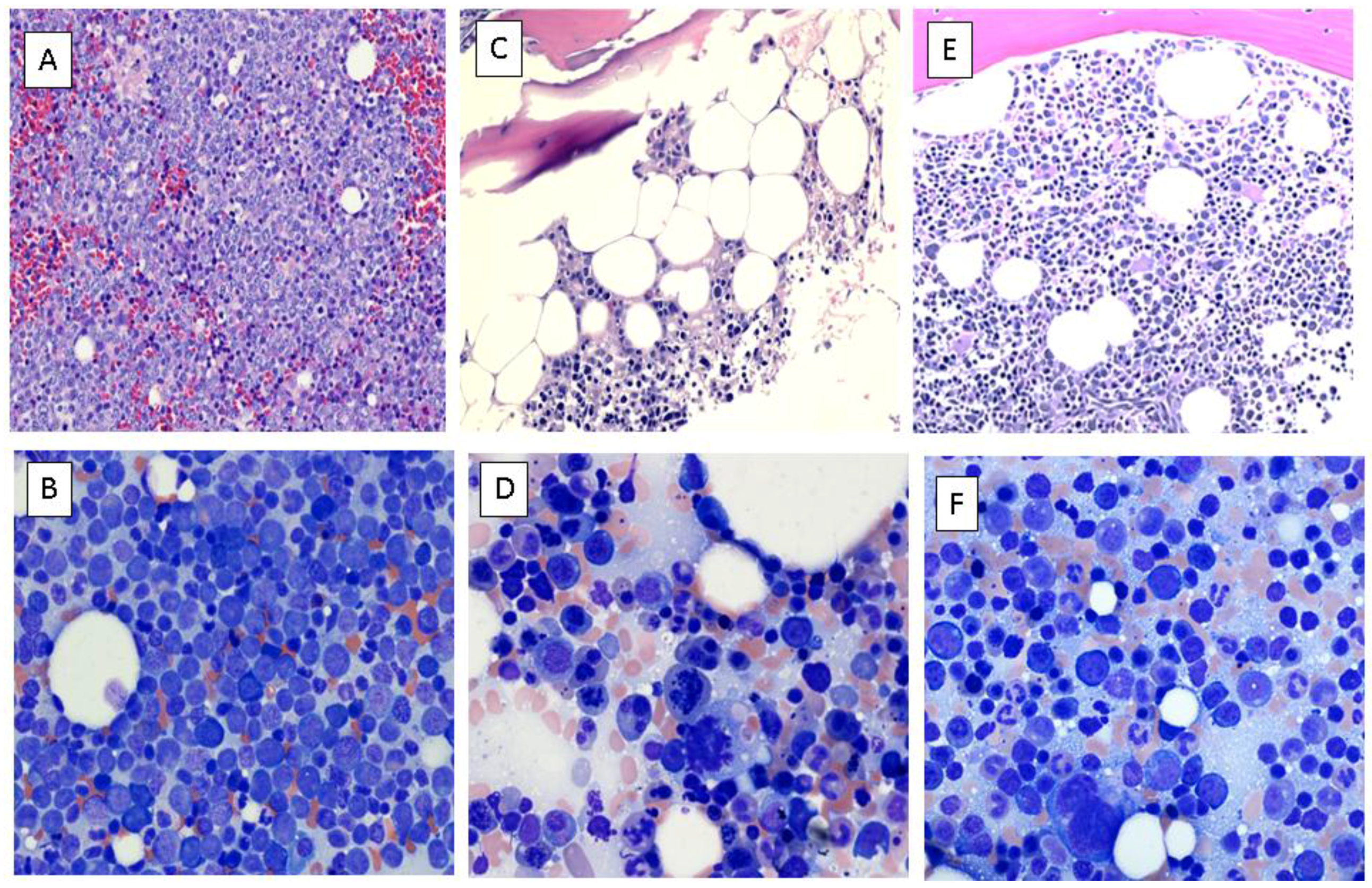
Case Report
2. Discussion
3. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kerridge, I.; Lowe, M.; Seldon, M.; Enno, A.; Deveridge, S. Clinical and ethical issues in the treatment of a jehovah’s witness with acute myeloblastic leukemia. Arch. Intern. Med. 1997, 157, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Cullis, J.O.; Duncombe, A.S.; Dudley, J.M.; Lumley, H.S.; Apperley, J.F.; Smith, A.G. Acute leukaemia in jehovah’s witnesses. Br. J. Haematol. 1998, 100, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Keck, G.; Ford, P.A. Acute myeloid leukemia in jehovah witnesses. Leuk. Lymphoma 2008, 49, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Karp, J.E. The state of the union on treatment of acute myeloid leukemia. Leuk. Lymphoma 2014, 55, 2423–2425. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Karp, J.E. The clinically relevant pharmacogenomic changes in acute myelogenous leukemia. Pharmacogenomics 2012, 13, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, B.; Duong, V.H.; Gourdin, T.S.; Tidwell, M.L.; Chen, C.; Ning, Y.; Emadi, A.; Sausville, E.A.; Baer, M.R. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk. Lymphoma 2014, 55, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- F.D.A. Label for asparaginase. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/101063s5169lbl.pdf (accessed on 3 March 2016).
- F.D.A. Label for erwinaze. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125359lbl.pdf (accessed on 3 March 2016).
- F.D.A. Label for oncaspar. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103411s5180lbl.pdf (accessed on 3 March 2016).
- Emadi, A.; Zokaee, H.; Sausville, E.A. Asparaginase in the treatment of non-all hematologic malignancies. Cancer Chemother. Pharmacol. 2014, 73, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Iguchi, M.; Tachibana, T.; Takemura, S.; Taguchi, J.; Tanaka, M.; Maruta, A.; Ishigatsubo, Y. Remission induction treatment for 6 patients of jehova’s witnesses with de novo acute leukemia. Blood 2006, 108, 217B–218B. [Google Scholar]
- Avramis, V.I. Asparaginases: A successful class of drugs against leukemias and lymphomas. J. Pediatr. Hematol. Oncol. 2011, 33, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Willems, L.; Jacque, N.; Jacquel, A.; Neveux, N.; Maciel, T.T.; Lambert, M.; Schmitt, A.; Poulain, L.; Green, A.S.; Uzunov, M.; et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood 2013, 122, 3521–3532. [Google Scholar] [CrossRef] [PubMed]
- Samudio, I.; Konopleva, M. Asparaginase unveils glutamine-addicted aml. Blood 2013, 122, 3398–3400. [Google Scholar] [CrossRef] [PubMed]
- Jacque, N.; Ronchetti, A.M.; Larrue, C.; Meunier, G.; Birsen, R.; Willems, L.; Saland, E.; Decroocq, J.; Thiago, T.T.; Lambert, M.; et al. Targeting glutaminolysis has antileukemic activity in acute myeloid leukemia and synergizes with bcl-2 inhibition. Blood 2015, 126, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A. Exploiting aml vulnerability: Glutamine dependency. Blood 2015, 126, 1269–1270. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. New Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated idh1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.C.; Hou, H.A.; Chen, C.Y.; Tang, J.L.; Yao, M.; Tsay, W.; Ko, B.S.; Wu, S.J.; Huang, S.Y.; Hsu, S.C.; et al. Distinct clinical and biologic characteristics in adult acute myeloid leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood 2010, 115, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The common feature of leukemia-associated idh1 and idh2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar]
- Abbas, S.; Lugthart, S.; Kavelaars, F.G.; Schelen, A.; Koenders, J.E.; Zeilemaker, A.; van Putten, W.J.; Rijneveld, A.W.; Lowenberg, B.; Valk, P.J. Acquired mutations in the genes encoding idh1 and idh2 both are recurrent aberrations in acute myeloid leukemia: Prevalence and prognostic value. Blood 2010, 116, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Maharry, K.; Wu, Y.Z.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Holland, K.B.; Whitman, S.P.; Becker, H.; Schwind, S.; et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A cancer and leukemia group b study. J. Clin. Oncol. 2010, 28, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Paschka, P.; Schlenk, R.F.; Gaidzik, V.I.; Habdank, M.; Kronke, J.; Bullinger, L.; Spath, D.; Kayser, S.; Zucknick, M.; Gotze, K.; et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with npm1 mutation without flt3 internal tandem duplication. J. Clin. Oncol. 2010, 28, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zeng, Y.; Zhang, D.F.; Zou, S.H.; Cheng, Y.F.; Yao, Y.G. IDH1 and IDH2 mutations are frequent in chinese patients with acute myeloid leukemia but rare in other types of hematological disorders. Biochem. Biophys. Res. Commun. 2010, 402, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Gonen, M.; Figueroa, M.E.; Fernandez, H.; Sun, Z.; Racevskis, J.; Van Vlierberghe, P.; Dolgalev, I.; Thomas, S.; Aminova, O.; et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. New Engl. J. Med. 2012, 366, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Iwanaga, E.; Tokunaga, K.; Nanri, T.; Shimomura, T.; Suzushima, H.; Mitsuya, H.; Asou, N. IDH1 and IDH2 mutations confer an adverse effect in patients with acute myeloid leukemia lacking the npm1 mutation. Eur. J. Haematol. 2014, 92, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, J.; Xu, Z.; Peng, B.; Huang, Q.; Arnold, E.; Ding, J. Structures of human cytosolic nadp-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J. Biol. Chem. 2004, 279, 33946–33957. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jun, S.A.; Tsukamoto, T.; Fathi, A.T.; Minden, M.D.; Dang, C.V. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with idh mutations. Exp. Hematol. 2014, 42, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T.; Wander, S.A.; Faramand, R.; Emadi, A. Biochemical, epigenetic, and metabolic approaches to target idh mutations in acute myeloid leukemia. Semin. Hematol. 2015, 52, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.J.; Caljouw, M.A.; Hop, W.C.; van Rhenen, D.J.; Schipperus, M.R. Feasibility of a restrictive red-cell transfusion policy for patients treated with intensive chemotherapy for acute myeloid leukaemia. Transfus. Med. 2004, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Schlaifer, D.; Cooper, M.R.; Attal, M.; Sartor, A.O.; Trepel, J.B.; Laurent, G.; Myers, C.E. Myeloperoxidase: An enzyme involved in intrinsic vincristine resistance in human myeloblastic leukemia. Blood 1993, 81, 482–489. [Google Scholar] [PubMed]
- McGrath, T.; Center, M.S. Mechanisms of multidrug resistance in hl60 cells: Evidence that a surface membrane protein distinct from p-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988, 48, 3959–3963. [Google Scholar] [CrossRef]
- Ozgen, U.; Savasan, S.; Stout, M.; Buck, S.; Ravindranath, Y. Further elucidation of mechanism of resistance to vincristine in myeloid cells: Role of hypochlorous acid in degradation of vincristine by myeloperoxidase. Leukemia 2000, 14, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Panetta, J.C.; Cai, X.; Yang, W.; Pei, D.; Cheng, C.; Kornegay, N.; Pui, C.H.; Relling, M.V. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J. Clin. Oncol. 2008, 26, 1932–1939. [Google Scholar] [CrossRef] [PubMed]
- Kawedia, J.D.; Liu, C.; Pei, D.; Cheng, C.; Fernandez, C.A.; Howard, S.C.; Campana, D.; Panetta, J.C.; Bowman, W.P.; Evans, W.E.; et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood 2012, 119, 1658–1664. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emadi, A.; Bade, N.A.; Stevenson, B.; Singh, Z. Minimally-Myelosuppressive Asparaginase-Containing Induction Regimen for Treatment of a Jehovah’s Witness with mutant IDH1/NPM1/NRAS Acute Myeloid Leukemia. Pharmaceuticals 2016, 9, 12. https://doi.org/10.3390/ph9010012
Emadi A, Bade NA, Stevenson B, Singh Z. Minimally-Myelosuppressive Asparaginase-Containing Induction Regimen for Treatment of a Jehovah’s Witness with mutant IDH1/NPM1/NRAS Acute Myeloid Leukemia. Pharmaceuticals. 2016; 9(1):12. https://doi.org/10.3390/ph9010012
Chicago/Turabian StyleEmadi, Ashkan, Najeebah A. Bade, Brandi Stevenson, and Zeba Singh. 2016. "Minimally-Myelosuppressive Asparaginase-Containing Induction Regimen for Treatment of a Jehovah’s Witness with mutant IDH1/NPM1/NRAS Acute Myeloid Leukemia" Pharmaceuticals 9, no. 1: 12. https://doi.org/10.3390/ph9010012




