The Tissue Selective Estrogen Complex: A Promising New Menopausal Therapy
Abstract
:1. Introduction
2. Hormone Therapy
2.1. Types of HT
2.2. Efficacy of HT on Menopausal Symptoms
2.3. Effects of HT on Bone
2.4. Effects of HT on Sleep and Quality of Life
2.5. Safety and Tolerability of HT
2.5.1. Estrogen Therapy
| HR (95% CI) | Age group at randomization | ||
|---|---|---|---|
| 50 to 59 years | 60 to 69 years | 70 to 79 years | |
| CHD [42] | |||
| CEE alone | 0.63 (0.36–1.09) | 0.94 (0.71–1.24) | 1.13 (0.82–1.54) |
| CEE/MPA | 1.29 (0.79–2.12) | 1.03 (0.74–1.43) | 1.48 (1.04–2.11) |
| Stroke [42] | |||
| CEE alone | 0.89 (0.47–1.69) | 1.62 (1.15–2.27) | 1.21 (0.84–1.75) |
| CEE/MPA | 1.41 (0.75–2.65) | 1.37 (0.95–1.97) | 1.21 (0.82–1.78) |
| VTE [45,48] | |||
| CEE alone | 1.37 (0.70–2.68) | 2.82 (1.59–5.01) | 3.77 (2.07–6.89) |
| CEE/MPA | 2.27 (1.19–4.33) | 4.28 (2.38–7.72) | 7.46 (4.32–14.38) |
2.5.2. Estrogen-Progestin Therapy
3. Other Therapies for Menopausal Symptoms or Postmenopausal Osteoporosis
3.1. The Tissue Selective Estrogen Complex
Preclinical Evidence for Potential SERM/CE Combinations
3.2. Clinical Studies of BZA/CE
| SMART-1 [78,79,80,81,86,87,88] | SMART-2 [82,83] | SMART-3 [84,85] |
|---|---|---|
| Enrolled non-hysterectomized postmenopausal women | ||
| Aged 40–75 years with acceptable endometrial biopsy results at screening (N = 3,397) | Aged 40–65 years with acceptable endometrial biopsy results and ≥7 moderate-to-severe hot flushes/d at screening (N = 318) | Aged 40–65 years with acceptable endometrial biopsy results and ≥1 moderate-to-severe VVA symptom at screening (N = 652) |
| Substudies | ||
| N/A | N/A |
| Study duration | ||
| 2 years | 12 weeks | 12 weeks |
|
|
|
| Primary endpoints | ||
|
|
|
| Secondary endpoints | ||
|
|
|
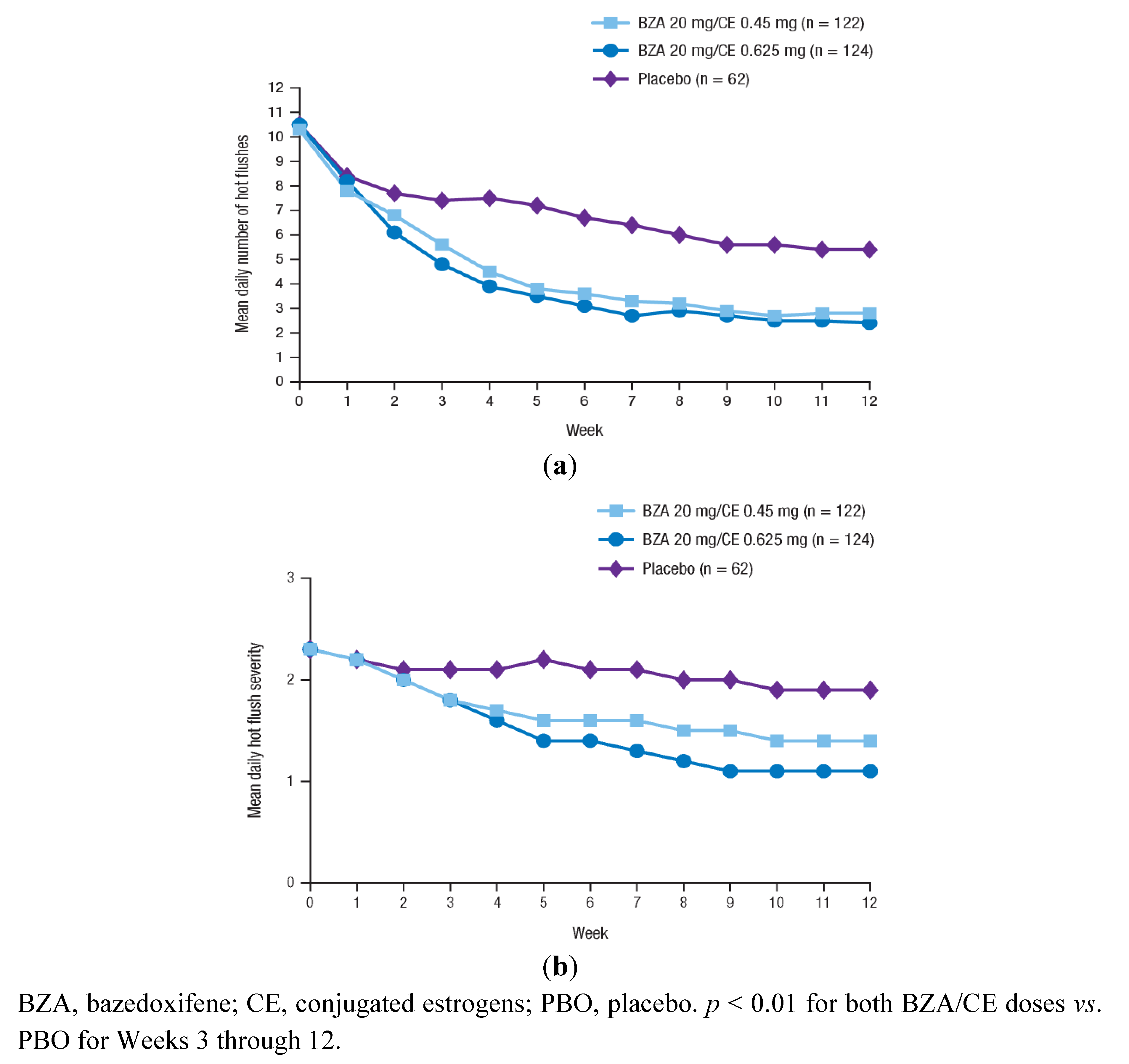
3.2.1. Efficacy of BZA/CE on VMS
| SMART-1 | SMART-2 | SMART-3 |
|---|---|---|
| Efficacy on vasomotor symptoms | ||
|
| N/A |
| Efficacy on vulvar/vaginal atrophy | ||
| N/A |
|
| Effects on bone | ||
| N/A | N/A |
| Effects on sleep and quality of life | ||
| N/A |
| |
| Satisfaction with treatment | ||
| N/A |
|
|
3.2.2. Efficacy of BZA/CE on VVA
3.2.3. Effects of BZA/CE on Bone
3.2.4. Effects of BZA/CE on Sleep, Quality of Life, and Satisfaction with Treatment
3.2.5. Safety and Tolerability of BZA/CE
3.2.5.1. Cardiovascular
3.2.5.2. Endometrium
3.2.5.3. Breast
| SMART-1 | SMART-2 | SMART-3 |
|---|---|---|
| Overall safety | ||
| ||
| Endometrial safety | ||
| ||
| Tolerability | ||
|
|
|
3.2.5.4. Uterine Bleeding
4. TSEC Summary: Menopause Symptom Relief without a Progestin
5. Conclusions
Conflict of Interest
Acknowledgements
References
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef]
- Burger, H.G. The endocrinology of the menopause. Maturitas 1996, 23, 129–136. [Google Scholar] [CrossRef]
- Levine, J.P. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend. Med. 2011, 8, 57–68. [Google Scholar] [CrossRef]
- Lewis, V. Undertreatment of menopausal symptoms and novel options for comprehensive management. Curr. Med. Res. Opin. 2009, 25, 2689–2698. [Google Scholar]
- Dennerstein, L.; Dudley, E.C.; Hopper, J.L.; Guthrie, J.R.; Burger, H.G. A prospective population-based study of menopausal symptoms. Obstet. Gynecol. 2000, 96, 351–358. [Google Scholar]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar]
- National Osteoporosis Foundation. Fast facts on osteoporosis. Available online: http://www.nof.org/node/40/ (accessed on 17 November 2011).
- Utian, W.H. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health. Qual. Life Outcomes 2005, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause 2012, 19, 257–271.
- North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 Position statement of The North American Menopause Society. Menopause 2010, 17, 25–54.
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar]
- Smith, D.C.; Prentice, R.; Thompson, D.J.; Herrmann, W.L. Association of exogenous estrogen and endometrial carcinoma. N. Engl. J. Med. 1975, 293, 1164–1167. [Google Scholar] [CrossRef]
- Weiderpass, E.; Adami, H.O.; Baron, J.A.; Magnusson, C.; Bergstrom, R.; Lindgren, A.; Correia, N.; Persson, I. Risk of endometrial cancer following estrogen replacement with and without progestins. J. Natl. Cancer Inst. 1999, 91, 1131–1137. [Google Scholar]
- Ziel, H.K.; Finkle, W.D. Increased risk of endometrial carcinoma among users of conjugated estrogens. N. Engl. J. Med. 1975, 293, 1167–1170. [Google Scholar]
- North American Menopause Society. Role of progestogen in hormone therapy for postmenopausal women: Position statement of The North American Menopause Society. Menopause 2003, 10, 113–132.
- Utian, W.H.; Shoupe, D.; Bachmann, G.; Pinkerton, J.V.; Pickar, J.H. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertil. Steril. 2001, 75, 1065–1079. [Google Scholar]
- MacLennan, A.H.; Broadbent, J.L.; Lester, S.; Moore, V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst. Rev. 2004, CD002978. [Google Scholar]
- Greendale, G.A.; Reboussin, B.A.; Hogan, P.; Barnabei, V.M.; Shumaker, S.; Johnson, S.; Barrett-Connor, E. Symptom relief and side effects of postmenopausal hormones: Results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet. Gynecol. 1998, 92, 982–988. [Google Scholar]
- Pornel, B.; Spielmann, D. A study of the control of climacteric symptoms in postmenopausal women following sequential regimens of 1 mg 17b-estradiol and trimegestone compared with a regimen containing 1 mg estradiol valerate and norethisterone over a 2-year period. Gynecol. Endocrinol. 2005, 21, 74–81. [Google Scholar] [CrossRef]
- Gambacciani, M.; Spielmann, D.; Genazzani, A.R. Efficacy on climacteric symptoms of a continuous combined regimen of 1 mg 17b-estradiol and trimegestone versus two regimens combining 1 or 2 mg 17b-estradiol and norethisterone acetate. Gynecol. Endocrinol. 2005, 21, 65–73. [Google Scholar] [CrossRef]
- Schurmann, R.; Holler, T.; Benda, N. Estradiol and drospirenone for climacteric symptoms in postmenopausal women: A double-blind, randomized, placebo-controlled study of the safety and efficacy of three dose regimens. Climacteric 2004, 7, 189–196. [Google Scholar]
- Rowan, J.P.; Simon, J.A.; Speroff, L.; Ellman, H. Effects of low-dose norethindrone acetate plus ethinyl estradiol (0.5 mg/2.5 mg) in women with postmenopausal symptoms: Updated analysis of three randomized, controlled trials. Clin. Ther. 2006, 28, 921–932. [Google Scholar]
- Cardozo, L.; Bachmann, G.; McClish, D.; Fonda, D.; Birgerson, L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: Second report of the Hormones and Urogenital Therapy Committee. Obstet. Gynecol. 1998, 92, 722–727. [Google Scholar] [CrossRef]
- North American Menopause Society. The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 Position statement of The North American Menopause Society. Menopause 2007, 14, 357–369.
- Dorr, M.B.; Nelson, A.L.; Mayer, P.R.; Ranganath, R.P.; Norris, P.M.; Helzner, E.C.; Preston, R.A. Plasma estrogen concentrations after oral and vaginal estrogen administration in women with atrophic vaginitis. Fertil. Steril. 2010, 94, 2365–2368. [Google Scholar]
- Bachmann, G.; Bouchard, C.; Hoppe, D.; Ranganath, R.; Altomare, C.; Vieweg, A.; Graepel, J.; Helzner, E. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009, 16, 719–727. [Google Scholar]
- Jackson, R.D.; Wactawski-Wende, J.; LaCroix, A.Z.; Pettinger, M.; Yood, R.A.; Watts, N.B.; Robbins, J.A.; Lewis, C.E.; Beresford, S.A.; Ko, M.G.; et al. Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: Results from the women’s health initiative randomized trial. J. Bone Miner. Res. 2006, 21, 817–828. [Google Scholar]
- Cauley, J.A.; Robbins, J.; Chen, Z.; Cummings, S.R.; Jackson, R.D.; LaCroix, A.Z.; LeBoff, M.; Lewis, C.E.; McGowan, J.; Neuner, J.; et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA 2003, 290, 1729–1738. [Google Scholar]
- Lindsay, R.; Gallagher, J.C.; Kleerekoper, M.; Pickar, J.H. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA 2002, 287, 2668–2676. [Google Scholar] [CrossRef]
- Torgerson, D.J.; Bell-Syer, S.E. Hormone replacement therapy and prevention of nonvertebral fractures: A meta-analysis of randomized trials. JAMA 2001, 285, 2891–2897. [Google Scholar]
- Wells, G.; Tugwell, P.; Shea, B.; Guyatt, G.; Peterson, J.; Zytaruk, N.; Robinson, V.; Henry, D.; O’Connell, D.; Cranney, A. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr. Rev. 2002, 23, 529–539. [Google Scholar]
- Hays, J.; Ockene, J.K.; Brunner, R.L.; Kotchen, J.M.; Manson, J.E.; Patterson, R.E.; Aragaki, A.K.; Shumaker, S.A.; Brzyski, R.G.; LaCroix, A.Z.; et al. Effects of estrogen plus progestin on health-related quality of life. N. Engl. J. Med. 2003, 348, 1839–1854. [Google Scholar]
- Brunner, R.L.; Gass, M.; Aragaki, A.; Hays, J.; Granek, I.; Woods, N.; Mason, E.; Brzyski, R.; Ockene, J.; Assaf, A.; et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: Results from the Women’s Health Initiative Randomized Clinical Trial. Arch. Intern. Med. 2005, 165, 1976–1986. [Google Scholar]
- Polo-Kantola, P.; Erkkola, R.; Helenius, H.; Irjala, K.; Polo, O. When does estrogen replacement therapy improve sleep quality? Am. J. Obstet. Gynecol. 1998, 178, 1002–1009. [Google Scholar] [CrossRef]
- Barnabei, V.M.; Grady, D.; Stovall, D.W.; Cauley, J.A.; Lin, F.; Stuenkel, C.A.; Stefanick, M.L.; Pickar, J.H. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet. Gynecol. 2002, 100, 1209–1218. [Google Scholar]
- Gelfand, M.M.; Moreau, M.; Ayotte, N.J.; Hilditch, J.R.; Wong, B.A.; Lau, C.Y. Clinical assessment and quality of life of postmenopausal women treated with a new intermittent progestogen combination hormone replacement therapy: A placebo-controlled study. Menopause 2003, 10, 29–36. [Google Scholar]
- Hlatky, M.A.; Boothroyd, D.; Vittinghoff, E.; Sharp, P.; Whooley, M.A. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: Results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial. JAMA 2002, 287, 591–597. [Google Scholar]
- Haines, C.J.; Yim, S.F.; Chung, T.K.; Lam, C.W.; Lau, E.W.; Ng, M.H.; Chin, R.; Lee, D.T. A prospective, randomized, placebo-controlled study of the dose effect of oral oestradiol on menopausal symptoms, psychological well being, and quality of life in postmenopausal Chinese women. Maturitas 2003, 44, 207–214. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Hsia, J.; Criqui, M.H.; Herrington, D.M.; Manson, J.E.; Wu, L.; Heckbert, S.R.; Allison, M.; McDermott, M.M.; Robinson, J.; Masaki, K. Conjugated equine estrogens and peripheral arterial disease risk: The Women’s Health Initiative. Am. Heart J. 2006, 152, 170–176. [Google Scholar]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar]
- Manson, J.E.; Allison, M.A.; Rossouw, J.E.; Carr, J.J.; Langer, R.D.; Hsia, J.; Kuller, L.H.; Cochrane, B.B.; Hunt, J.R.; Ludlam, S.E.; et al. Estrogen therapy and coronary-artery calcification. N. Engl. J. Med. 2007, 356, 2591–2602. [Google Scholar]
- Hendrix, S.L.; Wassertheil-Smoller, S.; Johnson, K.C.; Howard, B.V.; Kooperberg, C.; Rossouw, J.E.; Trevisan, M.; Aragaki, A.; Baird, A.E.; Bray, P.F.; et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation 2006, 113, 2425–2434. [Google Scholar]
- Curb, J.D.; Prentice, R.L.; Bray, P.F.; Langer, R.D.; van Horn, L.; Barnabei, V.M.; Bloch, M.J.; Cyr, M.G.; Gass, M.; Lepine, L.; et al. Venous thrombosis and conjugated equine estrogen in women without a uterus. Arch. Intern. Med. 2006, 166, 772–780. [Google Scholar]
- Stefanick, M.L.; Anderson, G.L.; Margolis, K.L.; Hendrix, S.L.; Rodabough, R.J.; Paskett, E.D.; Lane, D.S.; Hubbell, F.A.; Assaf, A.R.; Sarto, G.E.; et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA 2006, 295, 1647–1657. [Google Scholar] [CrossRef]
- LaCroix, A.Z.; Chlebowski, R.T.; Manson, J.E.; Aragaki, A.K.; Johnson, K.C.; Martin, L.; Margolis, K.L.; Stefanick, M.L.; Brzyski, R.; Curb, J.D.; et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA 2011, 305, 1305–1314. [Google Scholar]
- Cushman, M.; Kuller, L.H.; Prentice, R.; Rodabough, R.J.; Psaty, B.M.; Stafford, R.S.; Sidney, S.; Rosendaal, F.R. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004, 292, 1573–1580. [Google Scholar]
- Wassertheil-Smoller, S.; Hendrix, S.L.; Limacher, M.; Heiss, G.; Kooperberg, C.; Baird, A.; Kotchen, T.; Curb, J.D.; Black, H.; Rossouw, J.E.; et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. JAMA 2003, 289, 2673–2684. [Google Scholar] [CrossRef]
- Topal, N.B.; Ayhan, S.; Topal, U.; Bilgin, T. Effects of hormone replacement therapy regimens on mammographic breast density: The role of progestins. J. Obstet. Gynaecol. Res. 2006, 32, 305–308. [Google Scholar] [CrossRef]
- Kaewrudee, S.; Anuwutnavin, S.; Kanpittaya, J.; Soontrapa, S.; Sakondhavat, C. Effect of estrogen-progestin and estrogen on mammographic density. J. Reprod. Med. 2007, 52, 513–520. [Google Scholar]
- Santen, R. Menopausal hormone therapies: Their effect on mammographic density and breast cancer risk. Gynecol. Endocrinol. 2005, 21, 12–16. [Google Scholar] [CrossRef]
- Chen, F.P.; Cheung, Y.C.; Soong, Y.K. Factors that influence changes in mammographic density with postmenopausal hormone therapy. Taiwan J. Obstet. Gynecol. 2010, 49, 413–418. [Google Scholar]
- Boyd, N.F.; Rommens, J.M.; Vogt, K.; Lee, V.; Hopper, J.L.; Yaffe, M.J.; Paterson, A.D. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005, 6, 798–808. [Google Scholar]
- McCormack, V.A.; dos Santos Silva, I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1159–1169. [Google Scholar] [CrossRef]
- Pinsky, R.W.; Helvie, M.A. Mammographic breast density: Effect on imaging and breast cancer risk. J. Natl. Compr. Canc. Netw. 2010, 8, 1157–1164. [Google Scholar]
- Anderson, G.L.; Chlebowski, R.T.; Rossouw, J.E.; Rodabough, R.J.; McTiernan, A.; Margolis, K.L.; Aggerwal, A.; David, C.J.; Hendrix, S.L.; Allan, H.F.; et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas 2006, 55, 103–115. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Gass, M.; Lane, D.S.; Aragaki, A.K.; Kuller, L.H.; Manson, J.E.; Stefanick, M.L.; Ockene, J.; Sarto, G.E.; et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010, 304, 1684–1692. [Google Scholar] [CrossRef]
- Manonai, J.; Theppisai, U.; Suchartwatnachai, C.; Jetsawangsri, T.; Chittacharoen, A. Compliance with hormone replacement therapy in Thai women. Maturitas 2003, 44, 201–205. [Google Scholar] [CrossRef]
- Barnabei, V.M.; Cochrane, B.B.; Aragaki, A.K.; Nygaard, I.; Williams, R.S.; McGovern, P.G.; Young, R.L.; Wells, E.C.; O’Sullivan, M.J.; Chen, B.; et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet. Gynecol. 2005, 105, 1063–1073. [Google Scholar]
- Crandall, C.J.; Aragaki, A.K.; Cauley, J.A.; McTiernan, A.; Manson, J.E.; Anderson, G.; Chlebowski, R.T. Breast tenderness and breast cancer risk in the estrogen plus progestin and estrogen-alone women’s health initiative clinical trials. Breast Cancer Res. Treat. 2012, 132, 275–285. [Google Scholar] [CrossRef]
- Textbook of Medical Physiology; Guyton, A.; Hall, J. (Eds.) W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 1017–1032.
- Fournier, A.; Berrino, F.; Clavel-Chapelon, F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res. Treat. 2008, 107, 103–111. [Google Scholar]
- Nelson, H.D.; Vesco, K.K.; Haney, E.; Fu, R.; Nedrow, A.; Miller, J.; Nicolaidis, C.; Walker, M.; Humphrey, L. Nonhormonal therapies for menopausal hot flashes: Systematic review and meta-analysis. JAMA 2006, 295, 2057–2071. [Google Scholar]
- North American Menopause Society. The role of soy isoflavones in menopausal health: Report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause 2011, 18, 732–753.
- Howes, L.G.; Howes, J.B.; Knight, D.C. Isoflavone therapy for menopausal flushes: A systematic review and meta-analysis. Maturitas 2006, 55, 203–211. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Komm, B.S. A new approach to menopausal therapy: The tissue selective estrogen complex. Reprod. Sci. 2008, 15, 984–992. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Connor, C.E.; Wijayaratne, A.; Chang, C.Y.; Norris, J.D. Definition of the molecular and cellular mechanisms underlying the tissue-selective agonist/antagonist activities of selective estrogen receptor modulators. Recent Prog. Horm. Res. 2002, 57, 295–316. [Google Scholar] [CrossRef]
- Kharode, Y.; Bodine, P.V.; Miller, C.P.; Lyttle, C.R.; Komm, B.S. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology 2008, 149, 6084–6091. [Google Scholar] [CrossRef]
- Komm, B.S.; Vlasseros, F.; Samadfam, R.; Chouinard, L.; Smith, S.Y. Skeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized rats. Bone 2011, 49, 376–386. [Google Scholar]
- Peano, B.J.; Crabtree, J.S.; Komm, B.S.; Winneker, R.C.; Harris, H.A. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 2009, 150, 1897–1903. [Google Scholar]
- Stovall, D.W.; Utian, W.H.; Gass, M.L.; Qu, Y.; Muram, D.; Wong, M.; Plouffe, L., Jr. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause 2007, 14, 510–517. [Google Scholar]
- Davis, S.R.; O’Neill, S.M.; Eden, J.; Baber, R.; Ekangaki, A.; Stocks, J.M.; Thiebaud, D. Transition from estrogen therapy to raloxifene in postmenopausal women: Effects on treatment satisfaction and the endometrium-a pilot study. Menopause 2004, 11, 167–175. [Google Scholar] [CrossRef]
- Carranza-Lira, S.; Gooch, A.L.; Saldivar, N.; Osterwalder, M.S. Climacteric symptom control after the addition of low-dose esterified conjugated estrogens to raloxifene standard doses. Int. J. Fertil. Womens Med. 2007, 52, 93–96. [Google Scholar]
- Pinkerton, J.V.; Shifren, J.L.; La, V.J.; Rosen, A.; Roesinger, M.; Siddhanti, S. Influence of raloxifene on the efficacy of an estradiol-releasing ring for treating vaginal atrophy in postmenopausal women. Menopause 2003, 10, 45–52. [Google Scholar]
- Valiati, B.; Capp, E.; Edelweiss, M.I.; de Freitas, F.M.; Wender, M.C. Effect of raloxifene and low-dose percutaneous 17beta-estradiol on menopause symptoms and endometrium—A randomized controlled trial. Maturitas 2009, 62, 81–84. [Google Scholar] [CrossRef]
- Archer, D.F.; Lewis, V.; Carr, B.R.; Olivier, S.; Pickar, J.H. Bazedoxifene/conjugated estrogens (BZA/CE): Incidence of uterine bleeding in postmenopausal women. Fertil. Steril. 2009, 92, 1039–1044. [Google Scholar]
- Lindsay, R.; Gallagher, J.C.; Kagan, R.; Pickar, J.H.; Constantine, G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil. Steril. 2009, 92, 1045–1052. [Google Scholar]
- Lobo, R.A.; Pinkerton, J.V.; Gass, M.L.; Dorin, M.H.; Ronkin, S.; Pickar, J.H.; Constantine, G. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil. Steril. 2009, 92, 1025–1038. [Google Scholar]
- Pickar, J.H.; Yeh, I.-T.; Bachmann, G.; Speroff, L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil. Steril. 2009, 92, 1018–1024. [Google Scholar]
- Pinkerton, J.V.; Utian, W.H.; Constantine, G.D.; Olivier, S.; Pickar, J.H. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: A randomized, controlled trial. Menopause 2009, 16, 1116–1124. [Google Scholar]
- Utian, W.; Yu, H.; Bobula, J.; Mirkin, S.; Olivier, S.; Pickar, J.H. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas 2009, 63, 329–335. [Google Scholar]
- Bachmann, G.; Bobula, J.; Mirkin, S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010, 13, 132–140. [Google Scholar] [CrossRef]
- Kagan, R.; Williams, R.S.; Pan, K.; Mirkin, S.; Pickar, J.H. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010, 17, 281–289. [Google Scholar]
- Fenton, A.; Chines, A.; Mirkin, S. Endometrial safety and bleeding profile of bazedoxifene paired with conjugated estrogens: Results from 2 years of therapy. In Presentation at the 4th Triennial Scientific Meeting of the Asia Pacific Menopause Federation, Sydney, Australia, 26–29 September 2010.
- Fenton, A.; Chines, A.; Mirkin, S. Effect of bazedoxifene paired with conjugated estrogens on vasomotor symptoms over 2 years of therapy. In Presented at the 4th Triennial Scientific Meeting of the Asia Pacific Menopause Federation, Sydney, Australia, 26–29 September 2010.
- Harvey, J.A.; Pinkerton, J.V.; Baracat, E.C.; Shi, H.; Mirkin, S.; Chines, A.A. Evaluation of changes in mammographic breast density associated with bazedoxifene/conjugated estrogens in postmenopausal women. Endocr. Rev. 2011, 32, P1–P79, (Abstract). [Google Scholar]
- Yu, H.; Racketa, J.; Mirkin, S.; Chines, A. Hot flush symptom-free days with bazedoxifene/conjugated estrogens in a randomized, controlled trial of postmenopausal women. Menopause 2010, 17, 1238, Abstract P-56. [Google Scholar]
- Silverman, S.L.; Christiansen, C.; Genant, H.K.; Vukicevic, S.; Zanchetta, J.R.; de Villiers, T.J.; Constantine, G.D.; Chines, A.A. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: Results from a 3-year, randomized, placebo- and active-controlled clinical trial. J. Bone Miner. Res. 2008, 23, 1923–1934. [Google Scholar] [CrossRef]
- Haines, C.J.; Pan, K.; Mirkin, S.; Chines, A.A. Safety and tolerability of bazedoxifene and conjugated estrogens: Pooled analysis from the Selective estrogens, Menopause And Response to Therapy (SMART)-1, SMART-2, and SMART-3 trials. In Poster presented at the 4th Triennial Scientific Meeting of the Asia Pacific Menopause Federation, Sydney, Australia, 26–29 September 2010.
- Christiansen, C.; Chesnut, C.H., III; Adachi, J.D.; Brown, J.P.; Fernandes, C.E.; Kung, A.W.; Palacios, S.; Levine, A.B.; Chines, A.A.; Constantine, G.D. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet. Disord. 2010, 11, 130. [Google Scholar]
- Pinkerton, J.V.; Archer, D.F.; Utian, W.H.; Menegoci, J.C.; Levine, A.B.; Chines, A.A.; Constantine, G.D. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause 2009, 16, 1102–1108. [Google Scholar] [CrossRef]
- Archer, D.F.; Pinkerton, J.V.; Utian, W.H.; Menegoci, J.C.; de Villiers, T.J.; Yuen, C.K.; Levine, A.B.; Chines, A.A.; Constantine, G.D. Bazedoxifene, a selective estrogen receptor modulator: Effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 2009, 16, 1109–1115. [Google Scholar]
- Harvey, J.A.; Holm, M.K.; Ranganath, R.; Guse, P.A.; Trott, E.A.; Helzner, E. The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause 2009, 16, 1193–1196. [Google Scholar] [CrossRef]
- Pinkerton, J.A.; Constantine, G.D.; Komm, B.S.; Yu, H.; Pickar, J.H. Breast effects of bazedoxifene/conjugated estrogens in a randomized, controlled trial of postmenopausal women. Menopause 2008, 15, 1221, Abstract P-46. [Google Scholar]
- Archer, D.F.; Dorin, M.; Lewis, V.; Schneider, D.L.; Pickar, J.H. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertil. Steril. 2001, 75, 1080–1087. [Google Scholar]
- Archer, D.F.; Pickar, J.H.; Bottiglioni, F. Bleeding patterns in postmenopausal women taking continuous combined or sequential regimens of conjugated estrogens with medroxyprogesterone acetate. Menopause Study Group. Obstet. Gynecol. 1994, 83, 686–692. [Google Scholar]
- Crandall, C.J.; Karlamangla, A.; Huang, M.H.; Ursin, G.; Guan, M.; Greendale, G.A. Association of new-onset breast discomfort with an increase in mammographic density during hormone therapy. Arch. Intern. Med. 2006, 166, 1578–1584. [Google Scholar] [CrossRef]
- Wattanakumtornkul, S.; Chichareon, S.; Geater, A.; Suwan, K. Compliance with hormone replacement therapy at Songklanagarind Hospital. J. Obstet. Gynaecol. Res. 2003, 29, 380–387. [Google Scholar] [CrossRef]
- Komm, B.S.; Lyttle, C.R. Developing a SERM: Stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann. NY Acad. Sci. 2001, 949, 317–326. [Google Scholar]
- Komm, B.S.; Kharode, Y.P.; Bodine, P.V.; Harris, H.A.; Miller, C.P.; Lyttle, C.R. Bazedoxifene acetate: A selective estrogen receptor modulator with improved selectivity. Endocrinology 2005, 146, 3999–4008. [Google Scholar]
- Gallagher, J.C.; Lindsay, R.; Pan, K.; Mirkin, S.; Chines, A. Effects of bazedoxifene/conjugated estrogens on bone mineral density and bone turnover markers in postmenopausal women: A double-blind, randomized, placebo- and active-controlled phase 3 study. J. Bone Miner. Res. 2011, 26, S46, Abstract 1133. [Google Scholar]
- Pinkerton, J.V.; Pan, K.; Abraham, L.; Racketa, J.; Chines, A.A.; Mirkin, S. Effects of bazedoxifene/conjugated estrogens on sleep parameters and health-related quality of life in postmenopausal women. Menopause 2011, 18, 1346, Abstract S-13. [Google Scholar]
- Archer, D.F.; Lobo, R.A.; Pan, K.; Chines, A.A.; Mirkin, S. Safety and tolerability of bazedoxifene/conjugated estrogens in postmenopausal women: Findings from a 1-year, randomized, placebo- and active-controlled, phase 3 trial. Menopause 2011, 18, 1355–1356, Abstract P-25. [Google Scholar]
- Harvey, J.A.; Pinkerton, J.V.; Pan, K.; Thompson, J.R.; Ryan, K.A.; Mirkin, S.; Chines, A.A. The effects of bazedoxifene/conjugated estrogens on breast density in postmenopausal women. Menopause 2011, 18, 1342, Abstract S-1. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Komm, B.S.; Mirkin, S. The Tissue Selective Estrogen Complex: A Promising New Menopausal Therapy. Pharmaceuticals 2012, 5, 899-924. https://doi.org/10.3390/ph5090899
Komm BS, Mirkin S. The Tissue Selective Estrogen Complex: A Promising New Menopausal Therapy. Pharmaceuticals. 2012; 5(9):899-924. https://doi.org/10.3390/ph5090899
Chicago/Turabian StyleKomm, Barry S., and Sebastian Mirkin. 2012. "The Tissue Selective Estrogen Complex: A Promising New Menopausal Therapy" Pharmaceuticals 5, no. 9: 899-924. https://doi.org/10.3390/ph5090899




