Anti-Viral Drugs for Human Adenoviruses
Abstract
:Introduction
Adenovirus Biology and Structure
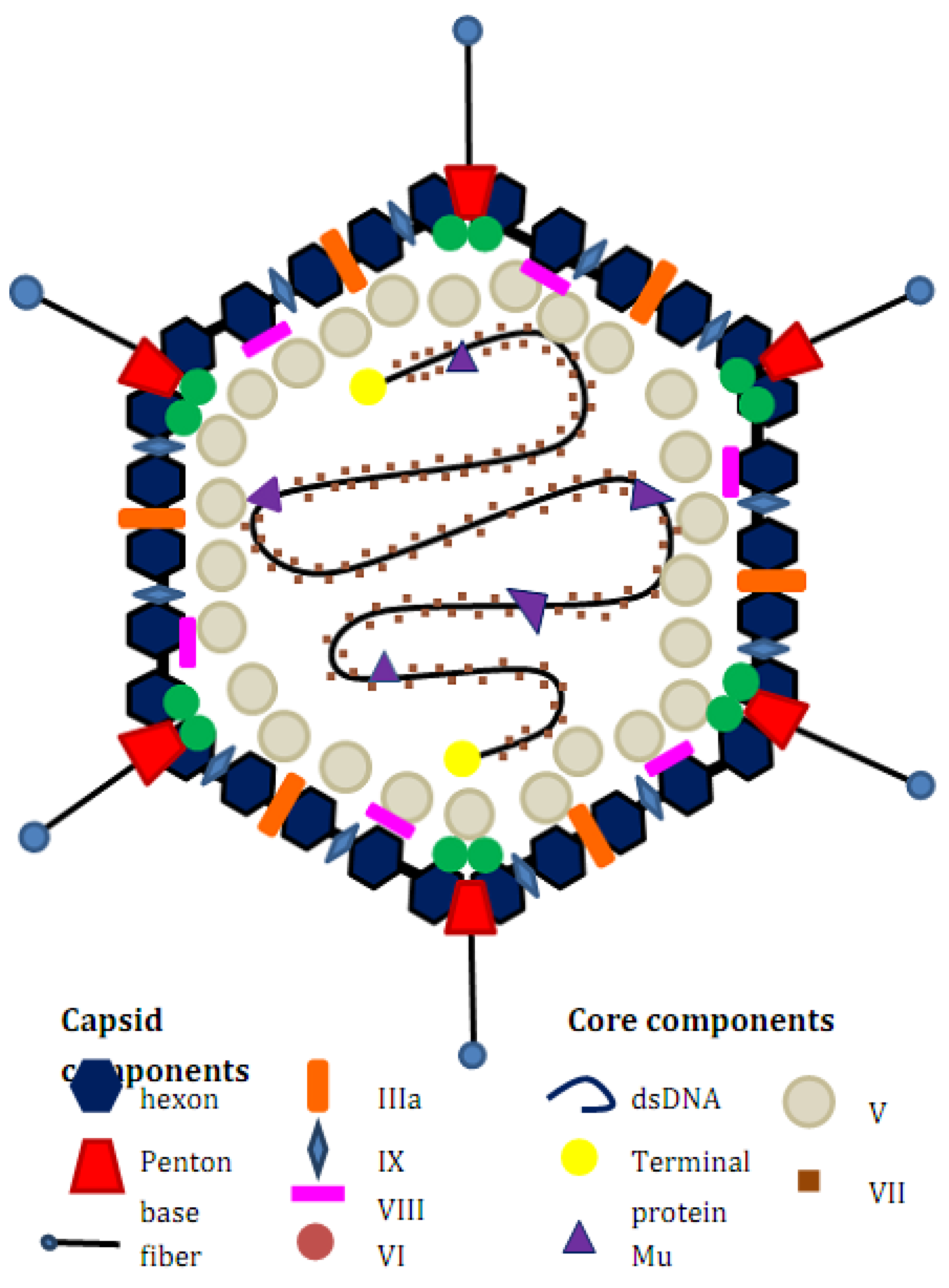

Rationale for Treatment
Current Anti-Viral Drugs
Cidofovir
Ribavirin
Efficacy as Established in Clinical Studies
Cidofovir
Ribavirin
Potential Anti-Viral Drugs
DHEA and epiandrosterone (EA) analogue
Transition metal complexes
Bispecific monoclonal antibodies
Camptothecin (CPT)
Water-soluble polymer complex of Arbidol
Stavudine
MicroRNA
Intravenous Immunoglobulin (IVIG) therapy and T- cell immunotherapy
Current Drug Screening Methods
New Methods for Drug Screening
RT-PCR method
Biosensor Method using Capacitance Sensor Arrays
Computation Method
Animal Model
Conclusions
Acknowledgements
References
- Kinchington, P.R; Romanowski, E.G.; Jerold, G.Y. Prospects for adenovirus antivirals. J. Antimicrob. Chemother. 2005, 55, 424–429. [Google Scholar]
- Muruve, D.A. The Innate Immune Response to Adenovirus Vectors. Hum. Gene Ther. 2004, 15, 1157–1166. [Google Scholar]
- Safrin, S.; Cherrington, J.; Jaffe, H.S. Clinical uses of cidofovir. Rev. Med. Virol. 1997, 7, 145–156. [Google Scholar]
- Hoffman, J.A.; Shah, A.J.; Ross, L.A.; Kapoor, N. Adenovirus infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2001, 7, 388–394. [Google Scholar]
- Carter, B.A.; Karpen, S.J.; Quiros-Tejeira, R.E.; Chang, I.F.; Clark, B.S.; Demmler, G.J.; Heslop, H.E.; Scott, J.D.; Seu, P.; Goss, J.A. Intravenous Cidofovir therapy for disseminated adenovirus in a pediatric liver transplant recipient. Transplantation 2002, 74, 1050–1052. [Google Scholar]
- Ljungman, P.; Ribaud, P.; Eyrich, M.; Matthes-Martin, S.; Einsele, H.; Bleakley, M.; Machaczka, M.; Bierings, M.; Bosi, A.; Gratecos, N.; Cordonnier, C. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003, 31, 481–486. [Google Scholar]
- Hromas, R.; Cornetta, K.; Srour, E.; Blanke, C.; Broun, E.R. Donor leukocyte infusion as therapy of life-threatening adenovirus infections after T-cell-depleted bone marrow transplantation. Blood 1994, 84, 1689–1690. [Google Scholar]
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gene. Virol. 2009, 90, 1–20. [Google Scholar]
- Wold, W.S.M.; Horwitz, M.S. Adenoviruses. In Fields Virology, 7th; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, Baltimore, MD, USA, 2007; pp. 1723–1740. [Google Scholar]
- Renard, G. Adenoviral keratoconjunctivitis. J. Fr. Ophtalmol. 2010, 33, 586–592. [Google Scholar]
- Flomenberg, P.; Babbitt, J.; Drobyski, W.R.; Ash, R.C.; Carrigan, D.R.; Sedmak, G.V.; McAuliffe, T.; Camitta, B.; Horowitz, M.M.; Bunin, N.; et al. Increasing incidence of adenovirus disease in bone marrow transplant recipients. J. Infect. Dis. 1994, 169, 775–781. [Google Scholar] [PubMed]
- Hale, G.A.; Heslop, H.E.; Krance, R.A.; Brenner, M.A.; Jayawardene, D.; Srivastava, D.K.; Patrick, C.C. Adenovirus infection after pediatric bone marrow transplantation. Bone Marrow Transplant. 1999, 23, 277–282. [Google Scholar]
- Myers, G.D.; Krance, R.A.; Weiss, H.; Kuehnle, I.; Demmler, G.; Heslop, H.E.; Bollard, C.M. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath). Bone Marrow Transplant. 2005, 36, 1001–1008. [Google Scholar]
- Suparno, C.; Milligan, D.W.; Moss, P.A.; Mautner, V. Adenovirus infections in stem cell transplant recipients:recent developments in understanding of pathogenesis, diagnosis and management. Leuk. Lymphoma 2004, 45, 873–885. [Google Scholar]
- Howard, D.S.; Phillips, G.L., II; Reece, D.E.; Munn, R.K.; Henslee-Downey, J.; Pittard, M.; Barker, M.; Pomeroy, C. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin.Infect.Dis. 1999, 29, 1494–1501. [Google Scholar]
- Sasseville, V.G.; Diters, R.W. Impact of Infections and Normal Flora in Nonhuman Primates on Drug Development. ILAR J. 2008, 49, 179–190. [Google Scholar]
- Kolehmainen, S.M. The Dangerous Promise of Gene Therapy. Abridged article from GeneWatch. Available online: http://www.actionbioscience.org/biotech/kolehmainen.html (accessed on 15 February 2000).
- Piedra, P.A.; Poveda, G.A.; Ramsey, B.; McCoy, K.; Hiatt, P.W. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics 1998, 101, 1013–1019. [Google Scholar] [PubMed]
- Atkinson, R.L. Viruses as an etiology of obesity. Mayo Clin. Proc. 2007, 82, 1192–1198. [Google Scholar]
- Atkinson, R.L.; Dhurandhar, N.V.; Allison, D.B.; Bowen, R.L.; Israel, B.A.; Albu, J.B.; Augustus, A.S. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int. J. Obes. (Land) 2005, 29, 281–286. [Google Scholar] [CrossRef]
- Hoffman, J.A.; Shah, A.J.; Ross, L.A.; Kapoor, N. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2001, 7, 388–394. [Google Scholar]
- Kinchington, P.R.; Araullo-Cruz, T.; Vergnes, J.P.; Yates, K.; Gordon, Y.J. Sequence changes in the human adenovirus type 5 DNA polymerase associated with resistance to the broad spectrum antiviral cidofovir. Antivir. Res. 2002, 56, 73–84. [Google Scholar]
- Graci, J.D.; Cameron, C.E. Mechanisms of action ofribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar]
- Morfin, F.; Dupuis-Girod, S.; Mundweiler, S.; Falcon, D.; Carrington, D.; Sedlacek, P.; Bierings, M.; Cetkovsky, P.; Kroes, A.C.; van Tol, M.J.; Thouvenot, D. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir.Ther. 2005, 10, 225–229. [Google Scholar]
- Hillenkamp, J.; Reinhard, T.; Ross, R.S.; Böhringer, D.; Cartsburg, O.; Roggendorf, M.; De Clercq, E.; Godehardt, E.; Sundmacher, R. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: A controlled clinical pilot study. Ophthalmology 2002, 109, 845–850. [Google Scholar]
- Hillenkamp, J.; Reinhard, T.; Ross, R.S.; Böhringer, D.; Cartsburg, O.; Roggendorf, M.; De Clercq, E.; Godehardt, E.; Sundmacher, R. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: A controlled clinical pilot study. Arch. Ophthal. 2001, 119, 1487–1491. [Google Scholar]
- Fowler, C.J.; Dunlap, J.; Troyer, D.; Stenzel, P.; Epner, E.; Maziarz, R.T. Life-threatening adenovirus infections in the setting of the immunocompromised allogeneic stem cell transplant patients. Adv.Hematol. 2010, 601548. [Google Scholar]
- Engelmann, G.; Heim, A.; Greil, J.; Schmitt, C.P.; Flechtenmacher, C.; Daum, E.; Küsters, U.; Schmidt, J.; Meyburg, J.; Schnitzler, P. Adenovirus infection and treatment with cidofovir in children after liver transplantation. Pediatric Transplant. 2009, 13, 421–428. [Google Scholar]
- Refaat, M.; McNamara, D.; Teuteberg, J.; Kormos, R.; McCurry, K.; Shullo, M.; Toyoda, Y.; Bermudez, C. Successful cidofovir treatment in an adult heart transplant recipient with severe adenovirus pneumonia. J Heart Lung Transplant. 2008, 27, 699–700. [Google Scholar]
- Saquib, R.; Melton, L.B.; Chandrakantan, A.; Rice, K.M.; Spak, C.W.; Saad, R.D.; Fenves, A.Z.; Barri, Y.M. Disseminated adenovirus infection in renal transplant recipients: The role of cidofovir and intravenous immunoglobulin. Transpl. Infect. Dis. 2010, 12, 77–83. [Google Scholar]
- Neofytos, D.; Ojha, A.; Mookerjee, B.; Wagner, J.; Filicko, J.; Ferber, A.; Dessain, S.; Grosso, D.; Brunner, J.; Flomenberg, N.; Flomenberg, P. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Bio Blood Marrow Transplant. 2007, 13, 74–81. [Google Scholar]
- Bhadri, V.A.; Lee-Horn, L.; Shaw, P.J. Safety and tolerability of cidofovir in high-risk pediatric patients. Transpl. Infect. Dis. 2009, 11, 373–379. [Google Scholar]
- Gavin, P.J.; Katz, B.Z. Intravenous ribavirin treatmentfor severe adenovirus disease in immunocompromised children. Pediatrics 2002, 110, e9. [Google Scholar]
- Arav-Boger, R.; Echavarria, M.; Forman, M.; Charache, P.; Persaud, D. Clearance of adenoviral hepatitis with ribavirintherapy in a pediatric liver transplant recipient. Pediatr. Infect. Dis. J. 2000, 19, 1097–1100. [Google Scholar]
- Cassano, W.F. Intravenous ribavirin therapy for adenoviruscystitis after allogeneic bone marrow transplantation. Bone Marrow Transplant 1991, 7, 247–248. [Google Scholar]
- Ljungman, P. Treatment of adenovirus infections inthe immunocompromised host. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 583–588. [Google Scholar]
- Toth, K.; Spencer, J.F.; Dhar, D.; Sagartz, J.E.; Buller, R.M.; Painter, G.R.; Wold, W.S. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc. Natl. Acad. Sci. USA 2008, 105, 7293–7297. [Google Scholar]
- Quenelle, D.C.; Collins, D.J.; Wan, W.B.; Beadle, J.R.; Hostetler, K.Y.; Kern, E.R. Oral treatmentof cowpox and vaccinia virus infections inmice with ether lipid esters of cidofovir. Antimicrob Agents Chemother 2004, 48, 404–412. [Google Scholar]
- Romanutti, C.; Bruttomesso, A.C.; Castilla, V.; Galagovsky, L.R.; Wachsman, M.B. Anti-Adenovirus Activity of Epiandrosterone and Dehydroepiandrosterone Derivatives. Chemotherapy 2010, 56, 158–165. [Google Scholar]
- Knight, D.A.; Hickey, T.E.; Bongard, J.E.; Thach, D.C.; Yngard, R.; Chang, E.L. Differential effects of Co(III), Ni(II), and Ru(III) amine complexes on Sindbis virus. J. Inorg. Biochem. 2010, 104, 592–598. [Google Scholar]
- Cao, Y.; Lam, L. Bispecific antibody conjugates in therapeutics. Advan. Drug Delivery Rev. 2003, 55, 171–197. [Google Scholar]
- Pantazis, P.; Han, Z.Y.; Chatterjee, D.; Wyche, J. Water-insoluble camptothecin analogues as potential antiviral drugs. J. Biomed. Sci. 1999, 6, 1–7. [Google Scholar]
- Eropkin, M.Y.; Solovskii, M.V.; Smirnova, M.Y.; Bryazzhikova,, T.S.; Gudkova, T.M.; Konovalova, N.I. Synthesis and biological activity of water-soluble polymer complexes of arbidol. Pharm. Chem. J. 2009, 43, 563–567. [Google Scholar]
- D'Cruz, O.J.; Uckun, F.M. Stampidine: A selective oculo-genital microbicide. J. Antimicrob. Chemother. 2005, 56, 10–19. [Google Scholar]
- Cawood, R.; Chen, H.H.; Carroll, F.; Bazan-Peregrino, M.; van Rooijen, N.; Leonard, W.S. Use of Tissue-Specific MicroRNA to Control Pathology of Wild-Type Adenovirus without Attenuation of Its Ability to Kill Cancer Cells. PLoS Pathog. 2009, 5, e1000440. [Google Scholar]
- Stiehm, R.E.; Ochs, H.D.; Winkelstein, J.A. Immunologic disorders in infants & children, 5th ed; Elsesier Health Sciences: Philadelphia, PA, USA, 2004; p. 337. [Google Scholar]
- Lenaerts, L.; De Clerc, E.; Naesens, L. Clinical Features and treatment of adenovirus infection. Rev. Med. Virol. 2008, 18, 357–374. [Google Scholar]
- García, J.M,; Español, T.; Gurbindo, M.D.; Casas, C.C. Update on the treatment of primary immunodeficiencies. Allergol. Immunopathol. (Madr) 2007, 35, 184–192. [Google Scholar]
- Saquib, R.; Melton, L.B.; Chandrakantan, A.; Rice, K.M.; Spak, C.W.; Saad, R.D.; Fenves, A.Z.; Barri, Y.M. Disseminated adenovirus infectionin renal transplant recipients: The role of cidofovir and intravenous immunoglobulin. Transpl. Infect. Dis. 2010, 12, 77–83. [Google Scholar]
- Gainotti, R.; Ricarte, C.; Ebekian, B.; Videla, C.; Carballal, G.; Damonte, E.B.; Echavarria, M. Real time PCR for rapid determination of susceptibility of adenovirus to antiviral drugs. J. Virol. Methods 2010, 164, 30–34. [Google Scholar]
- Beck, E.T.; Jurgens, L.A.; Kehl, S.C.; Bose, M.E.; Patitucci, T.; Lague, E.; Darga, P.; Wilkinson, K.; Witt, L.M.; Fan, J.; He, J.; Kumar, S.; Henrickson, K.J. Development of a Rapid Automated Influenza A, Influenza B, and Respiratory Syncytial Virus A/B Multiplex Real-Time RT-PCR Assay and Its Use during the 2009 H1N1 Swine-Origin Influenza Virus Epidemic in Milwaukee, Wisconsin. J. Mol. Diagn. 2010, 12, 74–81. [Google Scholar] [PubMed]
- Lee, R.; Kim, P.H.; Choi, J.W.; Oh-Joon, K.; Kim, K.; Kim, D.; Yun, C.-O.; Yoo, K.-H. Capacitance-based real time monitoring of receptor-mediated endocytosis. Biosens. Bioelectron. 2010, 25, 1325–1332. [Google Scholar]
- Klein, M.A.; Mayo, K.H.; Kratzke, R.A. p16(INK4a) Peptide mimetics identified via virtual screening. Bioorg. Medicinal Chem. Lett. 2010, 20, 403–405. [Google Scholar]
- Diaconu, I.; Cerullo, V.; Escutenaire, S.; Kanerva, A.; Bauerschmitz, G.J.; Hernandez-Alcoceba, R.; Pesonen, S.; Hemminki, A. Human adenovirus replication in immunocompetent Syrian hamsters can be attenuated with chlorpromazine or cidofovir. J. Gene Med. 2010, 12, 435–445. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Waye, M.M.Y.; Sing, C.W. Anti-Viral Drugs for Human Adenoviruses. Pharmaceuticals 2010, 3, 3343-3354. https://doi.org/10.3390/ph3103343
Waye MMY, Sing CW. Anti-Viral Drugs for Human Adenoviruses. Pharmaceuticals. 2010; 3(10):3343-3354. https://doi.org/10.3390/ph3103343
Chicago/Turabian StyleWaye, Mary Miu Yee, and Chor Wing Sing. 2010. "Anti-Viral Drugs for Human Adenoviruses" Pharmaceuticals 3, no. 10: 3343-3354. https://doi.org/10.3390/ph3103343




