1. Introduction
Driver fatigue is a vaguely defined term in a physiology sense, but its effect on traffic accidents is well documented. According to a Gallop poll in 2003, driver fatigue was the second most important cause of death (behind driving under the influence of alcohol) in automobile accidents. Nearly a third of survivors that responded to the survey remembered falling asleep while driving. People often have “micro-sleeps” without being aware of them. Numerous statistics and studies have shown that long hours driving resulted in physical tiredness and slowdown in mental judgment. In another report published by Shinar [
1], a significant portion of highway accidents was attributed to driver fatigue. The National Transportation Safety Board in the U.S. investigated 286 accidents involving commercial vehicles and discovered that 38% of accidents were caused by the drivers’ drowsiness or distraction. It is believed that a better understanding in driving-associated fatigue on the physiological level may lead to greater awareness of driving risks in general and new development of fatigue preventive devices in particular [
1].
Drivers often have a hard time maintaining concentration on a road with repetitious and unexciting scenery. The limited hip and leg space in vehicles also constrain the driver’s lower body from active movement. As a result, the “pumping” action by leg muscle contraction, which assists the venous blood to flow back to the heart, is largely lost [
2–
3]. It has been our belief that immobilization of the lower body is one of the root causes of driving fatigue. Immobilization not only hinders systemic blood circulation, it also causes body conditions to deviate from homeostasis. We further believe that an effective preventive means or device for driving fatigue and associated traffic accidents must address the problems of blood circulation and homeostasis.
It is well known that when local or systemic circulation is obstructed, the autonomic nervous system (ANS) in the body activates swiftly to compensate. Through its sympathetic and parasympathetic branches, ANS helps the cardiovascular system to maintain proper blood supply under these compromised circumstances [
4]. If the activation and execution of ANS is effective, deviations of physiological parameters from the homeostatic states can be avoided. On the other hand, any significant change in vital physiological parameters from baseline may point to an “exhausting” body that is unable to respond to new physiological needs. Eventually, brain hypoxia might occur and sleepiness would soon follow if blood supply to the brain is below basic requirements.
HRV has been used in various studies for the assessment of physiological states. Originated from the sub-conscious cyclic variation in heartbeat period induced by ANS activities, HRV is commonly analyzed in both time and frequency domains to give rise to parameters that are linked to total ANS activity [HRV or SDNN (standard deviation of NN intervals); TP (Total Power)], sympathetic activity [LF(AU) and LF(NU)], parasympathetic (or vagal) activity [HF(AU) and HF(NU)], and sympatho-vagal balance (LF/HF) indexes. Hjortskov
et al. [
5] and Garde
et al. [
6] monitored HRV parameter changes in volunteers before and after a computer task during which various degrees of mental stresses were introduced. The authors reported that the stressors led to changes in HRV [increase in LF(AU), HF(AU), and LF/HF compared to those under resting conditions], and a sustained increase in blood pressures (SYS and DIA). Garde
et al. [
6] reported an increase in heart rate, blood pressure, and LF(NU) and a decrease in TP(AU) and HF(NU) in response to a physically demanding reference computer task. Wahlstrom
et al. [
7] also introduced time and verbal stresses during a mouse-driven computer task to investigate the physiological and psychological changes based upon heart rate, blood pressures (SYS and DIA), and HRV. Increases in both the physiological (HR, BP, LF/HF) and psychological reactions were observed compared to control conditions. These reports suggest that physical and mental stresses may cause the activation of sympathetic nervous system as indicated by increased BP, HR, LF, and LF/HF.
Several authors have investigated the effect of simulated flight on physiological parameters [
8–
10]. Similar to the indoor driving employed in this study, simulated flight training also involves various degrees of mental stress in a tight-sitting posture. Their general finding is that the complexity of a pilot’s task in operating a flight often caused an increase in HR and BP (SYS and DIA), and a decrease in HRV. Lee
et al. [
9] clearly showed that when the pilots conducted tasks that required high concentration, such as during take-off and landing, their heart rates increased significantly. Among various tasks performed by pilots (take off, climb and cruise, descent and approach, and landing), HRV was seen lowest during approach as it was the most critical period of piloting. It is noted that there are two conditions in simulated flight training sessions that are potentially different from indoor simulated driving studies: they tend to be shorter in duration and more extensive in mental stress.
In the area of indoor simulated driving, Yang
et al. [
11] utilized ECG to monitor the driver’s HRV changes. They discovered four HRV parameters that were significantly changed after driving, namely increased HRV (or SDNN), increased LF(AU), decreased HF(NU), and increased LF/HF. Yang
et al. [
11] also reported that as the degree of fatigue increased (indicated by increasing driving hour), SDNN (equivalent to HRV in this study), LF(NU) and LF/HF all increased while HF(NU) decreased progressively. They believed that the increase in LF/HF was an indication of increase in degree of driving fatigue, as the balance of ANS shifted towards the sympathetic branch. Li
et al. [
12] also based their indoor driving and HRV study on ECG data and found three HRV parameters with significant changes after simulated driving, including increased LF(NU), decreased HF(NU), and increased LF/HF. After three hours of continuous driving, the drivers showed an increase in mental response time, a drop in judgment accuracy, and a lower heart rate. Subjective written questionnaire also showed elevated symptoms of driving fatigue. They proposed using HRV as a quantitative index for driving fatigue. Li
et al. [
13–
14] further studied the effect of acupuncture on driving fatigue. Their findings suggested that driving fatigue induced symptoms could be attenuated by acupuncture.
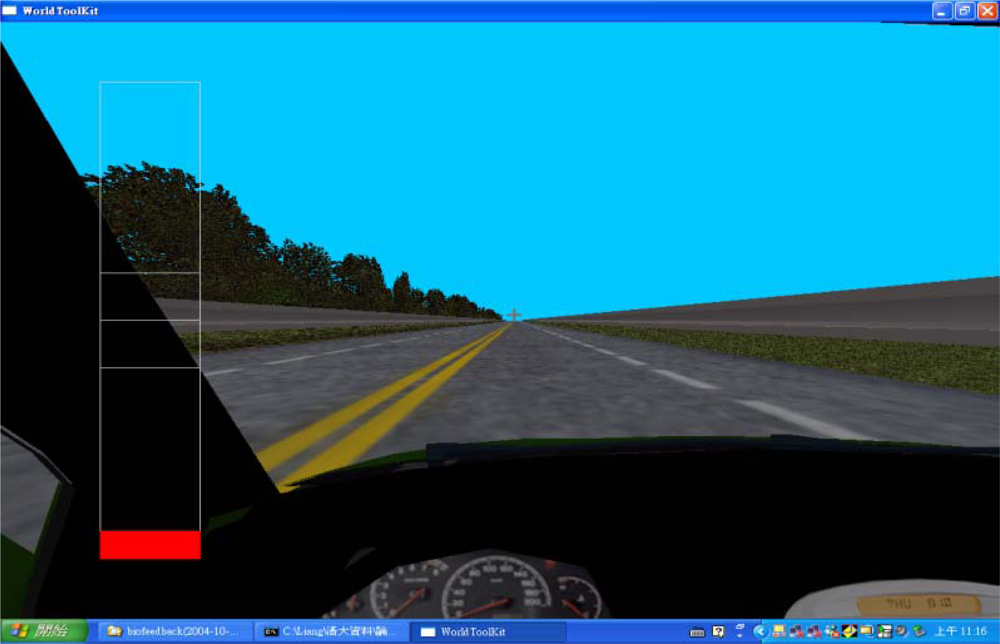
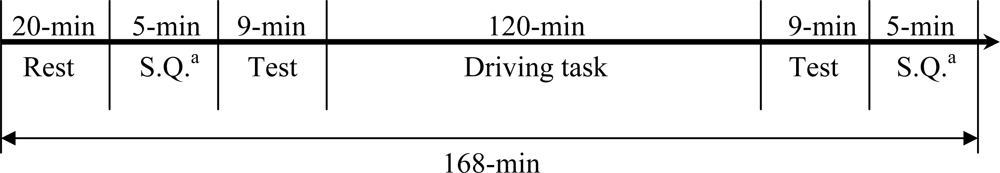
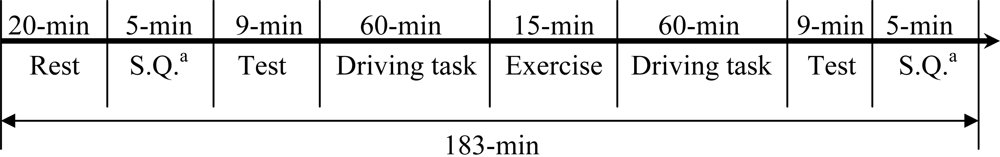
In this study, we monitored multiple physiological parameters, including palm temperatures (left and right), HR, BP (SYS/DIA), and HRV parameters before and after an indoor driving task lasting for 120 minutes. Volunteers were divided into two groups. One group conducted the driving task continuously while the other group had a 15-min exercise break taken at mid-term (60-min from start) of driving. By comparing the physiological changes between the two groups, the effect of exercise break on driving fatigue could be investigated. In addition, a written questionnaire was filled by each participant before and after the driving task to gauge the subjective feeling of driving fatigue. It is our expectation that the results of this series of studies (including Liang
et al. [
15] that was published earlier) could provide useful information for the quantitative definition of driving fatigue based upon physiological parameter changes. Identification of key parameters for monitoring driving fatigue is the next goal for these studies. This is a prerequisite step in the development of driving fatigue preventive devices.
4. Discussion
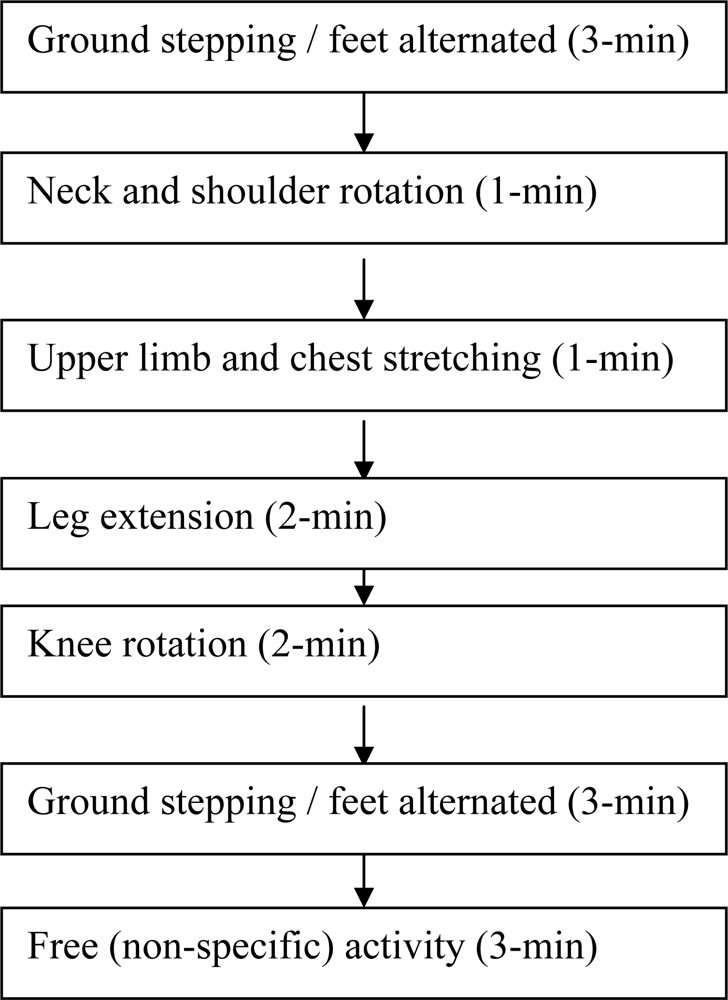
Multiple vital physiological parameters, in addition to a written questionnaire, were monitored in the study to evaluate driving-induced fatigue. Furthermore, an attempt to reduce driving fatigue symptoms was made by the introduction of a 15-min exercise break in group B. Physiological parameter changes and their implications and interactions are discussed below.
Systolic pressure (SYS) was lowered from 116.7 mmHg before driving to 110.2 mmHg after driving for group A (without exercise). In contrast, SYS was almost unchanged (116.7 mmHg before driving and 116.4 mmHg after driving) for group B with the 15-min exercise break. Independent t-test confirmed that SYS was significantly different between the two driving groups after driving (see Section 3.2.2 in Results). Diastolic pressure was little changed in either driving group (
Tables 4 and
5). Overall, homeostasis of blood pressure was better maintained in group B with the assistance of the 15-min exercise which was specifically designed to boost lower body and systemic circulation (see
Figure 2). The results are in agreement with written questionnaire where group A volunteers felt more exhausted than group B (see Section 3.3.3 in Results). From these results, deviation of systolic pressure from homeostasis could be a candidate for quantitative index of driver fatigue. On the other hand, diastolic pressure change is not a sensitive parameter for monitoring fatigue. Our results differ from those reported by Fumio
et al. [
28] where SYS was found to increase in taxi drivers measured during city road traffic. SYS increase was reported by other authors in various studies where subjects were asked to perform a stress-laden task [
5–
7,
10]. The authors of this study believe that several experimental settings are different between this and those cited studies. Our indoor driving is based upon highway scenery with the objective of simulating multiple-hour driving. The traffic conditions are neither complex nor action-demanding. It normally would not produce high degree of nervousness or anxiety in drivers. On the other hand, poor circulation caused by long-hour lower body immobilization is a characteristic of the experimental setting. In contrast, city road driving tends to be short in duration but requires frequent and spontaneous actions by drivers. Immobilization is not a major concern as drivers often get off the seat to assist passengers. Computer tasks and indoor flight simulations both influence more in mental stress and less in systemic circulation. Consequently, SYS was seen increased in those studies due to increased mental load. One would argue that if those studies were to extend the time of study (into several hours), mental stress would lead to fatigue eventually. One would further expect that SYS would be lowered under these circumstances.
Heart rate (HR) was reduced significantly after driving in both group A (from 70.4 before driving to 65.6 after driving) and group B (from 71.3 before driving to 64.2 after driving). The finding is in agreement with other indoor driving studies [
14,
18,
25] where HR was seen decreased as well. Indoor simulated driving tends to be less stressful compared to actual road driving, due in part to the volunteer’s recognition that the simulation is not real. A stressful condition might have caused an increase in HR. In addition, body movement is limited during simulated driving with the waist, hip, and legs almost stationary. As a combined result, HR is lowered below that under a restful condition. The authors believe that HR decrease is a reflection of poor systematic circulation and decreased cardiac output. Thus, change in HR could be a candidate for quantitative index of driver fatigue.
HRV, an index for total autonomic nervous system (ANS) activity, increased significantly after driving in both group A (from 47.7 ms before driving to 58.6 ms after driving) and group B (from 48.3 ms before driving to 58.7 ms after driving). One can conduct a further analysis by using the mathematic relationship that HRV square is equal to TP (or total power in the frequency domain analysis) which is the summation of HF(AU), LF(AU), and VLF(AU) (all with a unit of ms
2). From this relationship, the increase in HRV for group A (without exercise) was contributed 78% from very low frequency VLF(AU) (from 1233.6 ms
2 before driving to 2135.6 ms
2 after driving), 21% from the parasympathetic activity index HF(AU) (from 506.3 before driving to 757.2 after driving), and 1% from the sympathetic activity index LF(AU) (from 738.3 before driving to 825.5 after driving). By the same analysis for group B (with exercise), HRV increase consisted of 41% contribution from VLF(AU) (from 1550.3 before driving to 1974.0 after driving), 37% from the sympathetic activity index LF(AU) (from 499.8 before driving to 883.5 after driving), and 22% from the parasympathetic activity index HF(AU) (from 638.8 before driving to 875.1 after driving). VLF(AU) was the largest contributor to HRV increase for both group A and group B. Unfortunately its physiological meaning is not defined in the 1996 Standard. For group A, HF(AU) was a significant contributor while LF(AU) was negligible. In contrast, LF(AU)’s contribution (37%) was larger than that of HF(AU) (22%) for group B. The authors attributed the stark difference to the effect of exercise break. It has been observed clinically that during daytime the sympathetic modulation is the dominant mode while during sleep or nighttime the parasympathetic modulation dominates [
26–
27]. In other words, the sympathetic nervous system is activated when the body activity (mental or physical) is increased. The opposite is true for activation of the parasympathetic nervous system. Furthermore, when the body needs rest due to exhaustion, activation of the parasympathetic branch is called for. In the first publication of this series of studies [
15], the same authors compared physiological changes induced by indoor driving conducted in the morning versus in the afternoon. While both driving groups saw a decrease in HR and palm temperatures, and increase in HRV and VLF(AU), three striking differences were observed between the two: (1) LF(AU) and LF(NU) increased while HF(NU) decreased for the morning group; In contrast, LF(NU) decreased while HF(NU) increased for the afternoon group (2) systolic pressure (SYS) was maintained in the morning group but dropped in the afternoon group, and (3) morning group volunteers felt less exhausted than those in the afternoon group as indicated by written questionnaire (all p < 0.05). From the two studies conducted by us, it can be seen that for the driving group that showed higher fatigue scores (the afternoon group in our first study and group A without exercise break in this study), the net effect of ANS was to activate the parasympathetic nervous system, as indicated by an increase in HF(AU) or HF(NU), or a decrease in LF(AU) or LF(NU). Thus, increase in the parasympathetic activity index HF is a good candidate for quantifying driver fatigue. Since LF(NU), HF(NU), and LF/HF are inter-related with well defined mathematic relationship (see Section 2.5), all three are equally good for quantifying driver fatigue. More specifically, the authors propose that an increase in HF(NU), a decrease in LF(NU), or a decrease in LF/HF could be an indication for driver fatigue. The authors prefer using normalized indices of LF and HF (or LF/HF in this sense) instead of absolute counterparts on the ground that they each represent the net effect of ANS activities, rather than the activity of a specific branch. The authors do not believe that change in HRV is a good candidate for driver fatigue correlation. HRV may increase or decrease upon a new physiological need as ANS adjusts the relative contribution of each branch. Thus, the total change in ANS activity does not necessarily represent body fatigue.
Along the same lines, as HR and SYS decreased due to long-hour driving observed in our studies, ANS promptly activated to adjust body conditions for new physiological needs. In the morning versus afternoon study, activation of the sympathetic branch successfully kept SYS from being lowered for the morning group. Volunteers in this group were in their fresh state. Sympathetic stimulation helped to increase cardiac output and contract blood vessels. As a result, SYS was maintained. For the afternoon group in that study, exhaustion must have kicked in to the extent that the body activated the parasympathetic branch to prime for rest. As a result, SYS was lowered. Group A in this study behaved similarly to the afternoon group in the earlier study, as expected. The mid-term exercise break introduced in this study for group B, however, effectively attenuated body exhaustion, as confirmed by questionnaire data analyses (
Tables 7–
10). With sufficient energy and strength, the body again activated the sympathetic branch (instead of the parasympathetic branch) to meet driving needs. As a result, SYS was maintained for group B. In view of the data shown in
Table 5 in this study, physiological behaviors in group B of this study are a lot similar to those recorded for the morning group of our earlier study. In other words, drivers in the afternoon acted like those in the morning with the help of a mid-term exercise break. Our findings here explain well in physiological terms why driving while being tired is highly risky.
The interpretation of our overall findings in this study can be summarized here. As driving fatigue develops, cardio-vascular system is unable to fulfill the basic physiological needs. Hands and feet become cold (T
LP and T
RP lowered as observed), heart rate slows, and eventually blood pressures go down. Poor circulation causes muscle pain and numbness. Hypoxia in brain induces drowsiness and loss of concentration. Under these circumstances, our body re-assesses the new physiological needs and activates ANS instantly (seen with changes in HRV parameters in the study). If re-assessment finds the body under exhaustion which requires an immediate rest, the parasympathetic nerve branch in ANS is called upon (seen with group A in this study). On the other hand, if re-assessment determines that a boost to cardiovascular system can meet the new physiological needs, the sympathetic nerve branch is activated (group B in this study) (also see [
4]). The success of ANS action depends on several factors, including mental alertness, heart strength, and peripheral circulation resistance [
5–
6,
9–
10]. Immobilization of the lower body, which increases blood flow resistance significantly, could compromise the sympathetic nerve’s function in boosting systemic circulation [
29]. Since the monitoring of physiological parameters was done at the beginning and the ending of driving in the study, it is unclear whether or not the sympathetic nerve branch was activated first in the early face of driving for group A. Noted that at the end of driving for this group, the parasympathetic activity was enhanced. Under these conditions, if the driver continues to drive, the risk of traffic accidents would be extremely high. For group B, the sympathetic nerve was enhanced at the end of driving, which helped to maintain systolic pressure and other vital parameters. But even the ANS action in this group is regarded as a partial success since palm temperatures and heart rate were still below baseline.
The results of this study indicate that driving fatigue can be tracked by vital physiological parameters, including extremity temperatures, heart rate, and blood pressures. Significant deviation of any vital parameter from baseline during or after driving should be viewed as a symptom of fatigue. We further propose that among the HRV parameters recorded, the normalized parasympathetic index HF(NU) (possibly in a positive correlation to fatigue within certain range), the normalized sympathetic index LF(NU) (possibly in a negative correlation to fatigue within certain range), and the sympatho-vagal balance index LF/HF (possibly in a negative correlation to fatigue within certain range) are three most promising parameters that could be further developed to quantify driver fatigue.