1. Introduction
Cement has been prepared using alunite [
1], and a type of special cement containing calcium aluminate, anhydrite, and potassium sulfate has been prepared using anhydrite formed by the reaction between SO
3(g) that evolves from alunite and limestone via the following reaction:
Choi
et al. [
2] synthesized a calcium sulphoaluminate clinker consisting of 3CaO·3Al
2O
3·CaSO
4, CaO and CaSO
4 through the sintering for 2 hrs at a temperature of 1,200 °C of alunite, limestone and an anhydrite mixture at a weight ratio of 1:13:5. Their research results showed that alunite could be used in the preparation of a 3CaO·3Al
2O
3·CaSO
4 clinker provided that adequate mixing conditions are provided, despite that the expansion is relatively small compared to that of a clinker synthesized from reagents. However, the exact formation conditions of 3CaO·3Al
2O
3·CaSO
4 in the sintering state were not discussed. In addition, Han
et al. [
3,
4] synthesized a clinker containing calcium fluoroaluminate (C
11A
7·CaF
2) from domestic alunite and investigated its characteristics in an effort to develop a type of fast-hardening cement.
The authors [
5] carried out an investigation of the conditions under which 3CaO·3Al
2O
3·CaSO
4 is formed when mixtures of alunite and limestone are sintered. It was concluded that calcium langbeinite (2CaSO
4·K
2SO
4) forms from 700 °C and that calcium sulphoaluminate forms from 800 °C. Both are stable up to 1,300 °C, as shown in the equation below:
However, when alunite or limestone is incorporated in SiO2 so as to enable the formation of calcium sulphoaluminate, the molar ratios of CaO/alunite and CaO/SiO2 must be kept over 6.0 and 2.0, respectively. A clinker composed of calcium sulphoaluminate and calcium langbeinite was transformed into ettringite(3CaO·Al2O3·3CaSO4·32H2O) in water as calcium langbeinite is transformed into CaSO4·2H2O(s) and K2SO4 (aq) in water.
In the present study, the formation of 3CaO·3Al2O3·CaSO4 is identified in a K2SO4-CaO-Al2O3-CaSO4-SiO2 system using reagents of various types in a detailed investigation of the formation conditions of this species through the sintering of a mixture of alunite and limestone.
3. Results and Discussion
Alunite (K
2SO
4·Al
2(SO
4)
3·4Al(OH)) is transformed into KAl(SO
4)
2 and Al
2O
3 by dehydration at 500∼580 °C and KAl(SO
4)
2, K
2SO
4 and Al
2O
3 by desulphurization at 700∼780 °C via the reaction of K
2SO
4·Al
2(SO
4)
3·4Al(OH)
3 → K
2SO
4 + 2Al
2O
3 + 3SO
3(g) + 6H
2O(g), regardless of the partial pressure of CO
2(g). However, limestone decomposes from 650 °C in air and from 900 °C in a CO
2(g) saturated atmosphere [
6].
When the mixture of alunite and limestone is sintered in air and in a CO
2(g) saturated atmosphere, the rate of formation of anhydrite is relatively low, at 76.0% and 67.0%, respectively, at a CaCO
3/alunite stoichiometric molar ratio of 3. However, the rate of formation increases as the molar ratio of CaCO
3/alunite (particle size, 37∼44 μm) exceeds 6, showing rates of more than 99.0% and 95.0% in air and in a CO
2(g) saturated atmosphere, respectively [
7].
As shown in the results of aforementioned experiment, if alunite and limestone are mixed and sintered in air, most of the generated SO3 reacts with limestone to form anhydrite (CaSO4). Additionally, ignoring impurities such as Fe2O3, TiO2, and P2O5 included in the alunite, because alunite ore is composed of alunite, quartz(SiO2), and the aluminum silicate minerals of kaolinite, dickite, and pyrophyllite, the alunite and limestone mixture can be said to have five components, as does K2SO4-CaO-Al2O3-CaSO4-SiO2.
If 1 mol if pure alunite is heated to temperatures that exceed 800 °C, it is pyrolyzed as K2SO4·Al2(SO4)3·4Al(OH)3 → K2SO4 + 3Al2O3 + 3SO3 + 6H2O. Therefore, to formulate SO3(g) of 3 mol into anhydrite, 3 mol of CaCO3 is required, and to change 3 mol of Al2O3 into 3CaO·3Al2O3·CaSO4, 3 additional mol of CaCO3 and 1 mol of CaSO4 will be needed. Therefore, to change all of the Al2O3 in alunite into 3CaO·3Al2O3·CaSO4, and to generate SO3(g) by the thermal decomposition of alunite into anhydrite, theoretically, pure alunite and limestone should be mixed at a molar rate of 1:6. This mixture will be composed of five component systems as in K2SO4-CaO-Al2O3-CaSO4.
The present study aims to determine the mineral phases of a clinker generated in the K2SO4-CaO-Al2O3-CaSO4 and the K2SO4-CaO-Al2O3-CaSO4-SiO2 systems, using several types of reagents, including K2SO4, Al(OH)3, SiO2, CaSO4·2H2O, and CaCO3.
Figure 1 shows X-ray diffraction patterns of the materials generated, when a compound consisting of K
2SO
4-3CaO-3Al
2O
3-3CaSO
4 was sintered at 1,100 °C, 1,200 °C, and 1,300 °C, respectively, for 2 hours. As shown in this figure, 3CaO·3Al
2O
3·CaSO
4 and 2CaSO
4·K
2SO
4 is formed, but CaSO
4 does not react and remains at 1,100 °C. Meanwhile, at temperatures in excess of 1,200 °C 3CaO·3Al
2O
3·CaSO
4 and 2CaSO
4·K
2SO
4 were mainly generated. Accordingly, 1 mol of pure alunite reacts with 6 mol of limestone to form 1 mol of 3CaO·3Al
2O
3·CaSO
4 and 1 mol of 2CaSO
4·K
2SO
4 as follows; K
2SO
4·Al
2(SO
4)
3·4Al(OH)
3 + 6CaCO
3 → 2CaSO
4·K
2SO
4 + 3CaO·3Al
2O
3·CaSO
4 + 6H
2O(g) + 6CO
2(g).
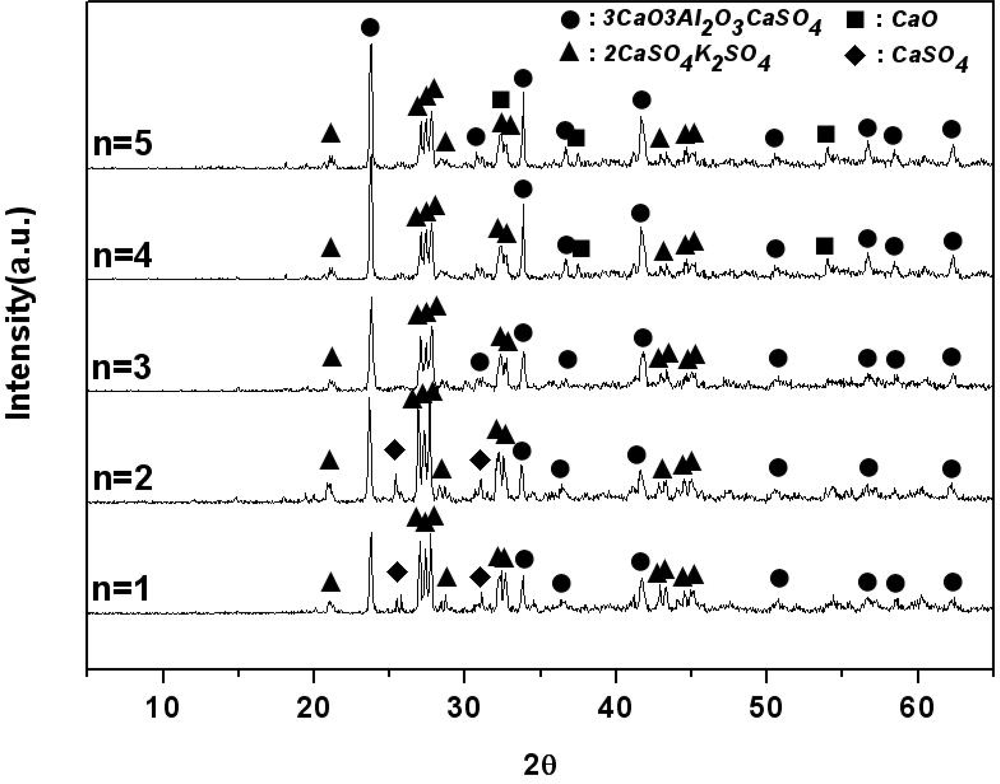
Figure 2 displays the materials generated from K
2SO
4-nCaO-3Al
2O
3-3CaSO
4 with various amounts of CaO sintered at 1,200 °C for two hours, as determined via an XRD analysis.
When it CaO is included in at an amount of 3 mol, 3CaO·3Al2O3·CaSO4 and 2CaSO4·K2SO4 are mainly generated. However, it was found that as the amount of CaO is increased from 3 mol to 5 mol, CaO does not participate in the formation reaction of 3CaO·3Al2O3·CaSO4. Thus, it can be said that 3CaO·3Al2O3·CaSO4 and 2CaSO4·K2SO4 are generated stably when CaO is 3 mol in the K2SO4-nCaO-3Al2O3-3CaSO4 system.
According to Fukuda [
8] 3CaO·3Al
2O
3·CaSO
4 is the only compound in the CaO-Al
2O
3-SO
3 system. This line of research was started in 1962 by Halstead,
et al. [
9] with Ca
2+ and SO
42− ions in a three-dimensional crystal structure sharing the angular point of a AlO
4 tetrahedron; an Al
3+ ion is coordinated with four O
2− ions, a Ca
2+ ion is surrounded asymmetrically by O
2− ions, and an isolated SO
42− ion is characterized by its ability to readily react with water. Kondo [
10] discovered that these ions become hardened in water. In general, they are produced by re-sintering after producing 3CaO·Al
2O
3 and regulating the mixture ratio of 3CaO·Al
2O
3, CaSO
4·2H
2O and Ca(OH)
2 and by sintering a mixture of CaCO
3, Al
2O
3, and CaSO
4·2H
2O.
2CaSO
4·K
2SO
4 is easily generated in the CaO-Al
2O
3-SiO
2-Fe
2O
3-MgO-CaSO
4-K
2SO
4 system and has a considerable influence on the condensation time and the hardening characteristics of cements. Known as a water-soluble alkali, as 2CaSO
4·K
2SO
4 can be easily separated from the liquid state of a clinker oxide and is said to become K
2SO
4·CaSO
4·H
2O(syngenite) and CaSO
4·2H
2O upon exposure to water [
11].
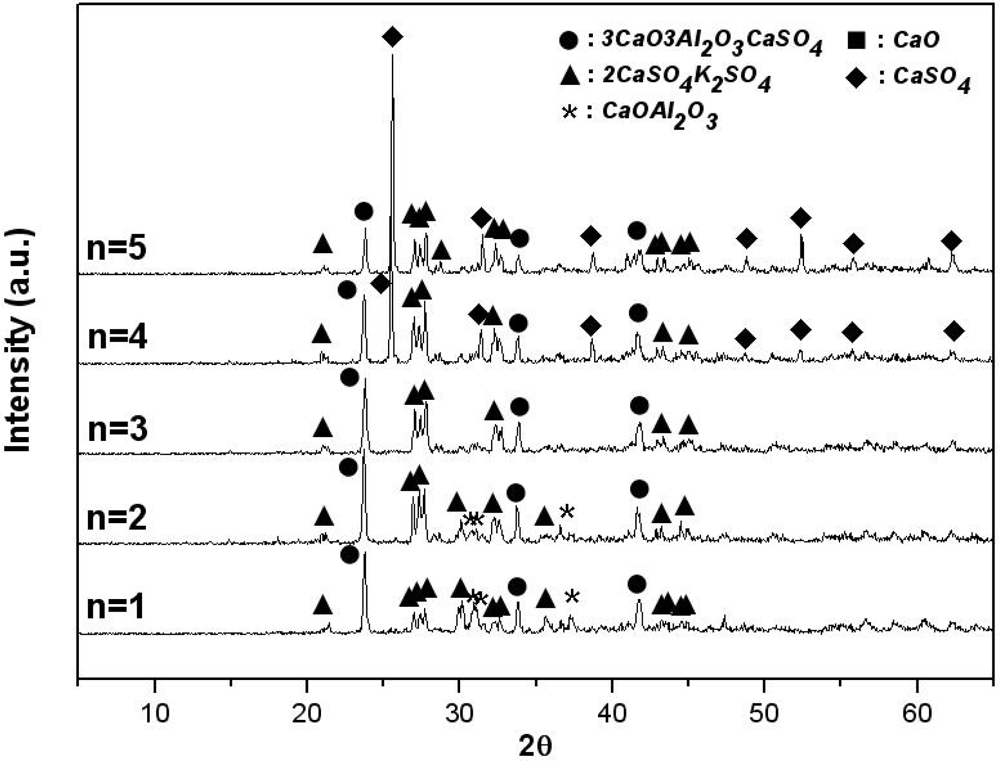
Figure 3 shows the materials generated from the K
2SO
4-3CaO-3Al
2O
3-nCaSO
4 system sintered at 1,200 °C for two hours with varying amounts of anhydrite, as determined via an XRD analysis.
As shown in this figure, when anhydrite is included in an amount of 3 mol, 3CaO·3Al2O3·CaSO4 and 2CaSO4·K2SO4 are generated. However, as the amount of anhydrite is decreased from 3 mol to 1 mol, it was observed that the K2SO4 that did not react with CaO·Al2O3 remained. As the amount of anhydrite is increased from 3 mol to 5 mol, only the diffraction strength of the CaSO4 that does not react increases. Thus, it can be said that an amount of CaSO4 in excess of the stoichiometric molar ratio is necessary to generate 3CaO·3Al2O3·CaSO4 stably.
As noted above, 3CaO·3Al2O3·CaSO4 and 2CaSO4·K2SO4 were mainly generated in the K2SO4-3CaO-3Al2O3-3CaSO4 system. However, because alunite from nature has impurities of SiO2 and aluminum silicate minerals such as kaolinite, dickite, and pyrophyllite, the alunite and limestone mixture is believed to be comprised of a K2SO4-CaO-Al2O3-CaSO4-SiO2 system to which SiO2 is added.
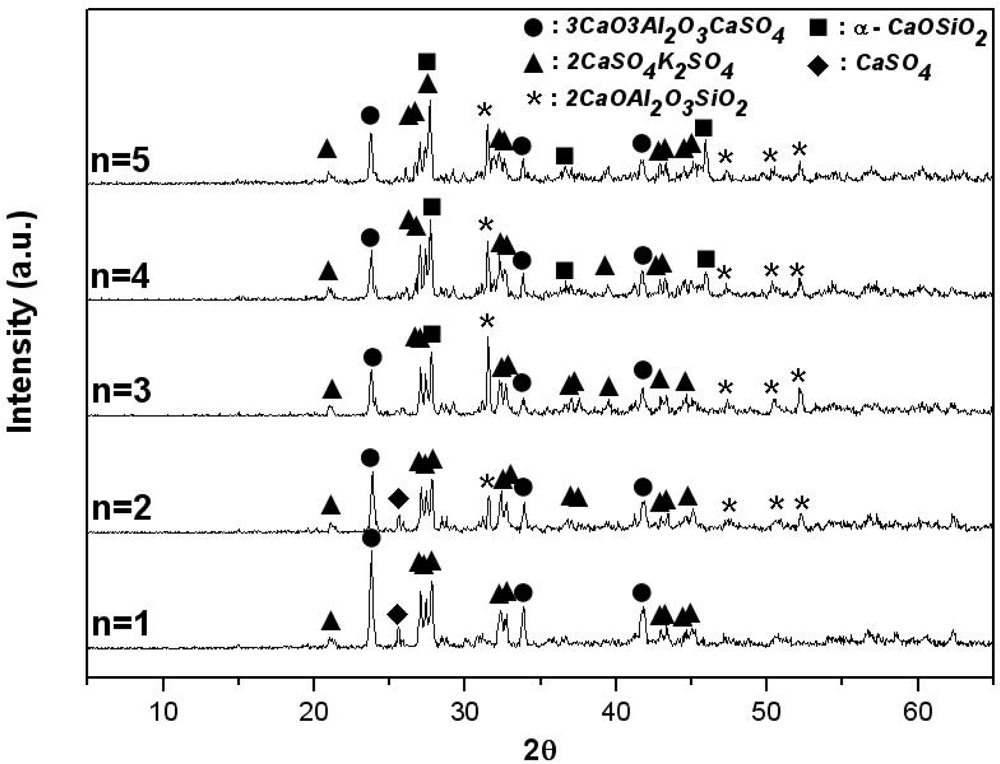
Figures 4 and
5 show, by XRD analysis, the materials generated from the K
2SO
4-3CaO-3Al
2O
3-3CaSO
4-nSiO
2 system and the K
2SO
4-(3+m)CaO-3Al
2O
3-3CaSO
4-nSiO
2 system, respectively, sintered at 1,200 °C for 2 hours, with various amounts of CaO (3 + m : mol number) and SiO
2(n: mol number). In
Figure 4, when there is no SiO
2 in the mixture, the result is identical to that obtained when 3 mol of CaO is used (
Figure 2). It is expected that 3CaO·3Al
2O
3·CaSO
4 and 2CaSO
4·K
2SO
4 will be generated. However, as the amount of SiO
2 is increased from 1 mol to 5 mol, the amount of 3CaO·3Al
2O
3·CaSO
4 generated is reduced, because gehlenite (2CaO·Al
2O
3·SiO
2) and wollastonite (α-CaO·SiO
2), which do not react with water, are generated. In addition, as shown in
Figure 5, when the mol rate of CaO/alunite is less than 6 (m less than 3), and that of CaO/SiO
2 is less than 2, the synthetic clinker contained 2CaO·Al
2O
3·SiO
2 and CaSO
4; these compounds do not participate in the formation reaction of 3CaO·3Al
2O
3·CaSO
4 because 3CaO·3Al
2O
3·CaSO
4 is the only compound in the CaO-Al
2O
3-SO
3 system as noted above[
8] and CaO and Al
2O
3 participate preferentially in the formation reaction of 2CaO·Al
2O
3·SiO
2. Accordingly, if all SO
3(g) generated by thermal decomposition of alunite reacts with CaCO
3 (or CaO, the thermal decomposition product of limestone) to form CaSO
4 in the alunite-limestone system, when the molar ratios of CaO/alunite exceed 6 and that of CaO/SiO
2 exceeds 2, 3CaO·3Al
2O
3·CaSO
4, 2CaSO
4·K
2SO
4 and β-2CaO·SiO
2 are generated stably.