Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook
Abstract
:1. Introduction
- (i) The analytical meaning of the pH value, (ii) traditional and novel pH measuring techniques, and (iii) the variety of pH sensitive materials and (iv) preparation methods described in the literature.
- Platinum metal oxides are presented as materials which are able to replicate similar proton-exchange processes occuring at common glass membranes. This justifies the title “ion-exchanging surfaces”, because the bulk of platinum metal oxides is mainly electronically conducting. Less expensive than commercial glass electrodes, disposable metal oxide probes are useful, e.g., for applications in aqueous and biological media.
- Additionally, pH dependent redox processes occur at platinum metal oxides. With respect to a future direct pH indicator, the redox pseudocapacitance of hydrous RuO2 is considered as a model system, which requires the passage of protons through grain boundaries and cracks on the porous electrode surface for the establishment of the equilibrium potential.
- Finally, this article points out the role of (i) the support material, and (ii) the reference electrode. The pH sensitive material is usually coated on a support material to create a durable electrode.
2. pH Monitoring Using Glassy Materials and Similar Proton Probes
2.1. Glass Electrodes
- a)
- 2,2-Bis(hydroxyethyl)amino-tris(hydroxymethyl)methane, 20.924 g/L, in HCl, 0.05 mol/L; pH 6.58
- b)
- Trishydroxymethylamine, 18.17 g/L, in HCl, 0.1 mol/L; pH 7.90
- c)
- Ethanolamine, 3.054 g/L, in HCl, 0.03 mol/L; pH 9.39
- d)
- Piperidine, 10,644 g/L, in HCl, 0.1 mol/L; pH 11.34
- e,f,g)
- Tetramethylammoniumhydroxide, 25% (54,69 g/L, 105.73 g/L or 328,14 g/L, respectively), in HCl, 0.1 mol/L, exhibits pH 12.61, pH 13,3, and pH 14.0 respectively. NaCl is added to this solution.
2.2. Absolute Measurement of pH in Dilute Aqueous Solution: IUPAC Recommendation
- The cell (–) Pt|H2|HCl(m)|AgCl|Ag (+) is filled with hydrochloric acid (e.g. mHCl = 0.01 mol kg–1, γ±HCl = 0.904 at 25 °C), and the potential difference according to the spontaneous cell reaction, ½H2 + AgCl → Ag(s) + H+ + Cl– is measured. From this E0 is calculated (p0 = 101 325 Pa):
- The Harned cell is then filled with a test solution, and the acidity function p(aHγCl) = –lg(aHγH) is measured for at least three molalities of added potassium chloride (I < 0.1 mol kg–1). p(aHγCl)0 is found by linear extrapolation towards infinite dilution. In practice, the cells of step 1 and step 2 are operated simultaneously in a thermostat bath at 25 °C, so that ΔE2–ΔE1 is independent of the standard potential difference and the assumption that the standard hydrogen potential equals E0(H+|H2) = 0 at all temperatures:
- The pH value in Equation (6b) is calculated according to the Bates-Guggenheim convention that the immeasurable activity coefficient of the chloride ion γCl is estimated by help of the Debye-Hückel theory. For KCl (z+ = z– = 1), the ionic strength I, and the molar concentration c (in mol/L), or molality m (in mol kg–1), respectively, are identical:
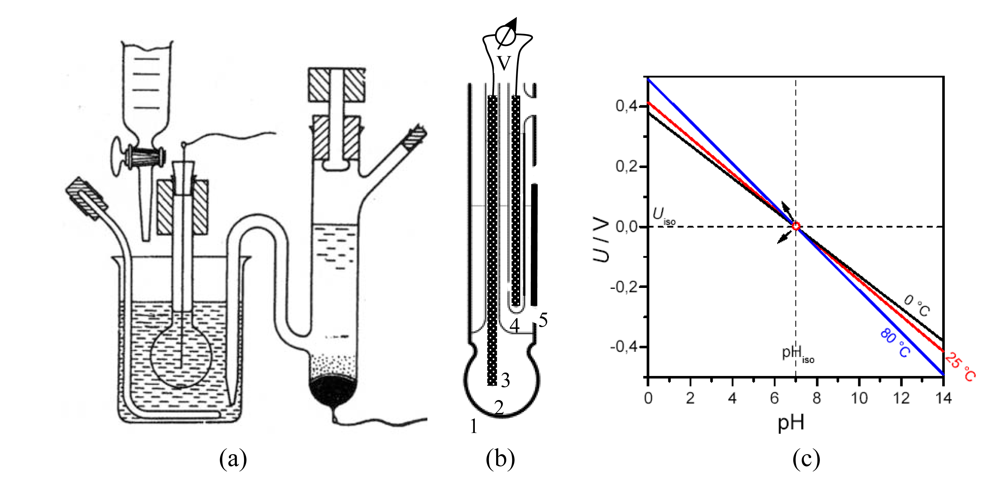
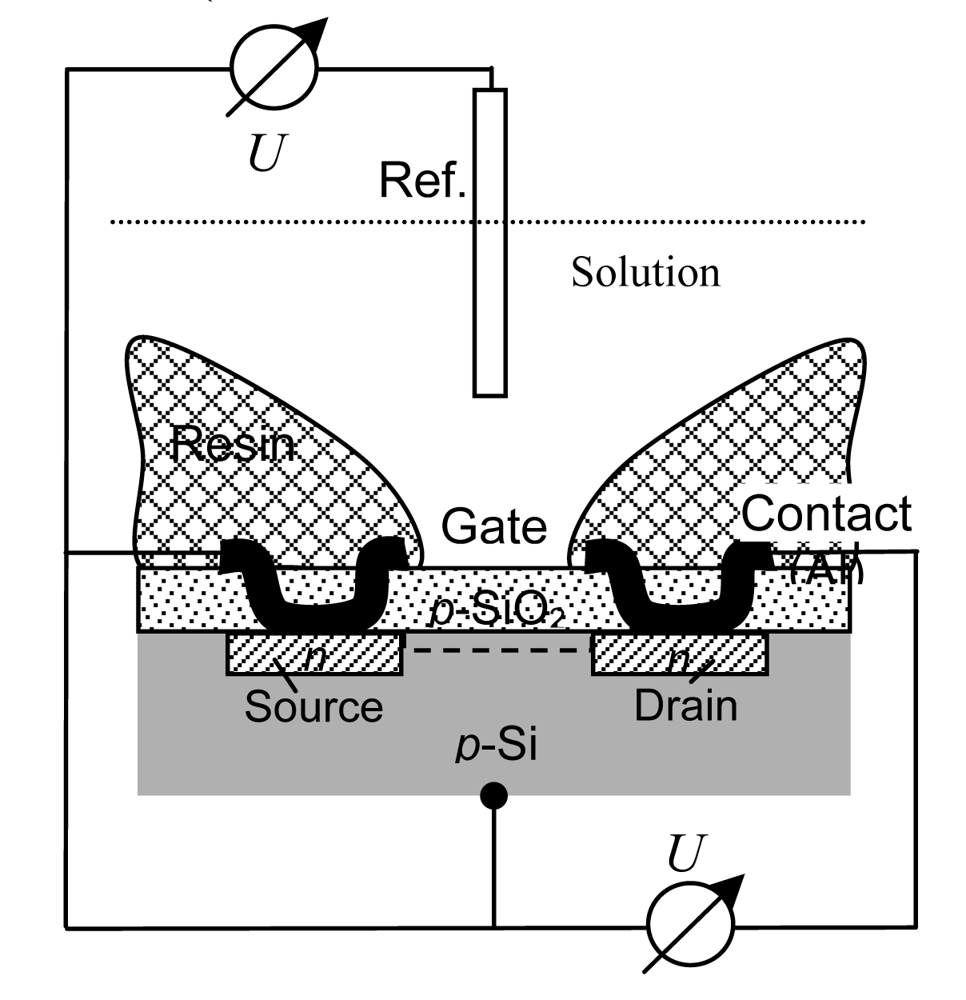
2.3. Solid State pH Electrodes
- Drift effect: The slow nonrandom change of output voltage with time (by some mV h–1) in a solution with constant composition and temperature. Measurement circuit, reference electrode, and device body are greatly affected.
- Hysteresis or memory effect: When the ISFET is measured many times in the same pH buffer solution, different output voltages at the buffer solution–insulator interface occur. This apparent delay of the pH response creates a loop cycle with different hysteresis widths in different pH buffer solutions (Figure 3-b).
- Optical effect: The output voltage of the ISFET changes (by some ten mV) when the light is switched on and off. For surface potential mapping, on the dry backside of the silicon substrate, a light-emitting diode may be applied to generate a photocurrent, the size being a measure of the surface potential at that particular region.
2.4. Enamel Electrode
2.5. Gel Membrane Electrodes
2.6. Electrochemically Active Monolayers
2.7. Colorimetric Sensors and Optodes
3. Metal Electrodes for pH Determination
3.1. Hydrogen Electrodes and Storage Electrodes
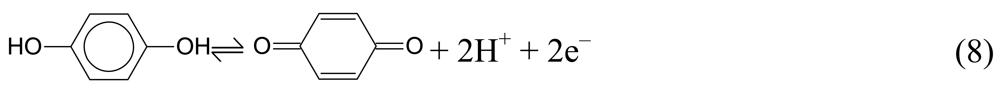
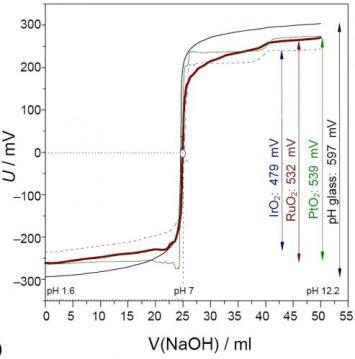
3.2. Metal-metal Oxide Electrodes
4. Platinum Metal Oxide Probes in Aqueous Solutions
- Hydrogen-sensitive: Co3O4, ZnO, SnO2, MoO3, WO3, MnO2
- Oxygen-sensitive: TiO2, SrTiO3, BaTiO3, ZrO2, Fe2O3, CoO, ZnO, SnO2, La2O3
4.1. Ruthenium Dioxide: Model System of a Novel Proton Probe
pH sensitivity
Consideration of adsorbed gases
- In acid solution, as well as it is known for the hydrogen oxidation at a hydrogen electrode, the electrode potential increases with rising pH. Protons are released by the dissociative adsorption of water and superacid OH groups. Simplified, by the help of rutile lattice sites [Ru], the potential determining surface process at the more negatively charged RuO2 electrode reads:
- In alkaline solution, as well as it is know for the oxygen reduction at an oxygen electrode, the electrode potential decreases with rising pH. By the dissociative adsorption of water, hydroxide sites are formed and bound in ruthenium cluster ions.

Cross sensitivity
Preparation
Non-aqueous solutions
4.2. Applications of Ruthenium Dioxide Sensors
Biosensors [69,70,71]
- Label-based immunosensors contain antibodies as analyte recognition parts. Horseradish peroxidase and alkaline phosphatase, e.g., catalyze reactions which produce electroactive products in immunoenzymatic biodevices. For example, at a polypyrrole coated screen-printed gold electrode, peroxidase may work as biocatalytic label converting o-phenylene-diamine into 2,3-diaminophenazine (in the presence of H2O2). Label-based analytical sensors with enzymatic, fluorescent, radiochemical, and nanoparticle markers rely on amperometric and optical detection rather than on potentiometry.
- Label-free bioaffinity sensors were realized by the aid of protein coated ISFETs, which dynamically measure the release or uptake of protons by biologically active protein molecules bound on the semiconductor. Antibodies can be adsorbed on colloidal nanoparticles (Au, Ag) in a polymer matrix (e.g. gelatin, nafion, polyvinyl butaral, thiosilane gel, poly(o-phenylenediamine)) on a metal support. Potentiometric genosensors – as field effect devices or membrane ion-selective electrodes, modified with oligonucleotides – shall detect complementary DNA sequences.
4.3. Iridium Dioxide
Redox chemistry
Preparation
Cross sensitivity [83,84]
Stability and recycling [85]
5. Tin Dioxide and Lead Dioxide
6. Transition Metal Oxides
7. Non-Oxidic Materials and Support Materials
8. Reference Electrodes
Silver-silver chloride electrode
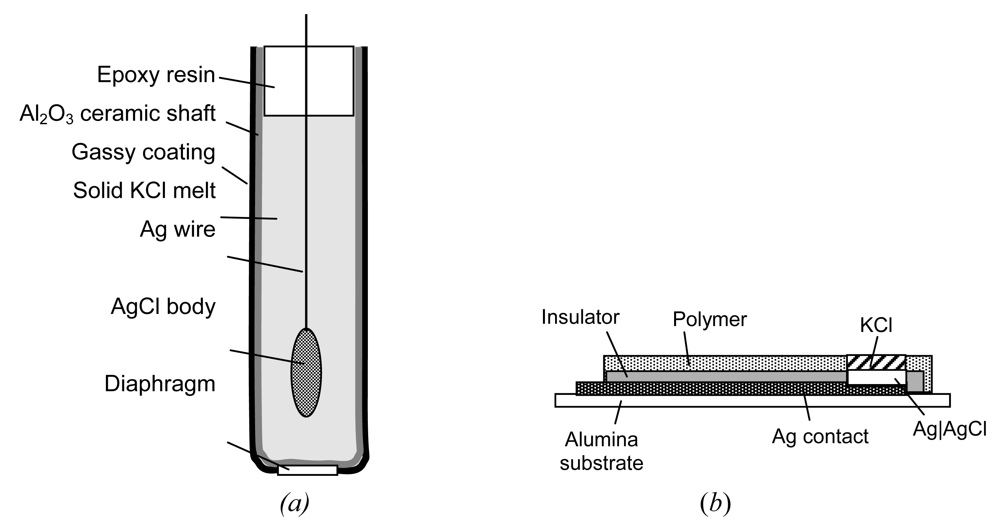
- At a porous AgCl coating in direct contact to the solution, redox reactions (e.g. O2/OH–) cause a mixed potential which deviates from the ideal response (+ 202 mV vs. SHE in sat. KCl solution, 20–25 °C). AgCl is a silver ion conductor. Voltage stability can be improved by small grains, and a thermal treatment of the AgCl coating below 455 °C [103]. As the chloride electrolyte is necessary, the Ag|AgCl|Cl– electrode requires a liquid junction across a diaphragm. All-solid electrodes for sensors are shown in Figure 7.
- Gel-like electrolytes are useful at temperatures up to 140 °C and pressures up to 15 bar, however, at cost of increased diffusion potentials, irreversible bleeding, biofouling and aging of the gel. Ag|AgCl on a flat support material can be coated by a hydrogel (e.g. polyacrylamide) and surrounded by a membrane. The long-term stability of such reference electrodes in ISFETs is poor.
- Polymer-electrolyte reference systems (e.g. solid KCl in polyester resin) and open junctions are commercially available, e.g., under the brand name XEROLYT®.
- All-solid-state reference electrodes [104] would be most favourable. In a cylindrical shaft of porous alumina ceramic, which additionally serves as a diaphragm for the liquid junction, molten KCl is filled around a centered Ag|AgCl electrode [105]. AgCl diffuses partly in the KCl phase. Humidity from the environment provides the necessary conductivity in the hygroscopic KCl phase.
- Thickfilms of noble metal filled glass (e.g. Corning 015) on oxide ceramic or steel supports show increased resistivity, response times in the range of minutes, and relatively short lifetime. The reference potential does often not obey the Nernst slope. Different thermal expansion coefficients between glass and ceramic support cause cracks.
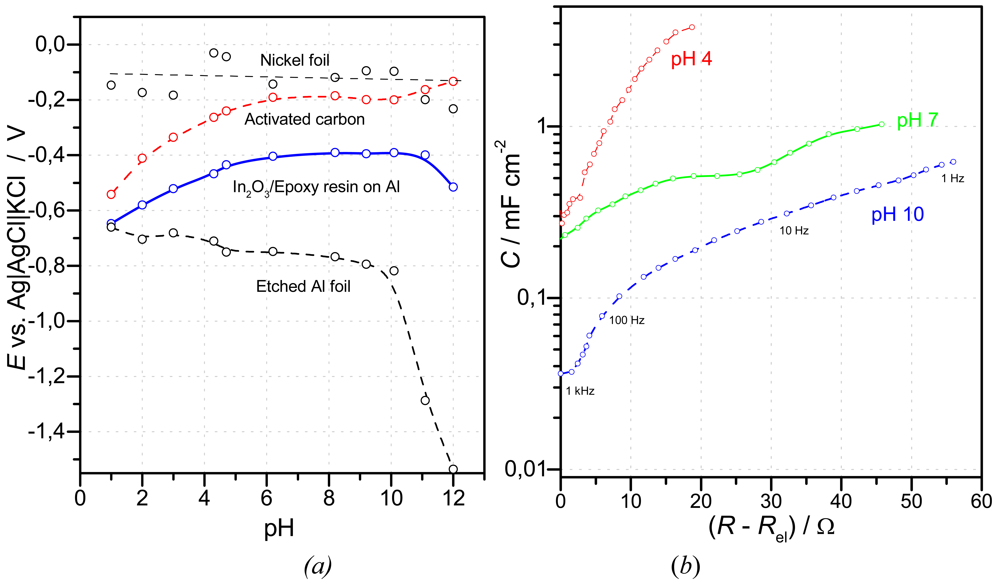
- The impact of a glassy carbon rod electrode under ambient conditions and the presence of dissolved oxygen, is shown in Figure 4-b. A linear function of cell voltage in the range between pH 2 to pH 12 was obtained by use of a plane gold counter-reference electrode; the total capacitance of the cell is dominated by the large capacitance of the rough RuO2 electrode, C = [CRuO2–1 + CAu–1]–1 ≈ CRuO2. Gold is known as an electrode material with negligible hydrogen sorption; the oxygen overpotential is higher than that of platinum.
- Rhodium foil, that is covered by a rhodium oxide layer, behaves nearby pH-insensitive (see Section 4.3).
- Molybdenum and tungsten bronzes [106], e.g. Li0.4Mo0.95W0.05O3 [107] do not significantly respond to pH changes, oxygen concentration and redox potential of the solution. However, they are sensitive to alkali ions (K+ < Na+ < Li+), but the preparation of single-crystalline electrodes is hardly reproducible.
- Manganese dioxide, HxMnO2 [108], suffers from poor reproducibility.
- Boron carbide might be useful as electrode material having a high hydrogen overvoltage.
- Prussian Blue as a reference in all-solid state pH glass electrodes was investigated in [109].
9. Measuring Techniques
10. Conclusions
- A common reference system valid for pH measurements in all media is still missing; as well, there is no simple pH reference besides the intricate standard hydrogen electrode and the Harned cell. The pH values in non-aqueous solutions cannot simply be compared with those in aqueous systems. A coulometric proton titrator might be the solution for this problem, once appropriate directly pH dependent materials are known.
- For the potentiometric pH determination in aqueous solutions, the glass electrode is still unsurpassed. For special applications in solutions containing fluoride or alkali, metal oxide electrodes have been introduced; whereby the antimony electrode is the most prominent example.
- The electrode-electrolyte interface at platinum metal oxides is able to exchange protons with the surrounding solution. RuO2 and IrO2 were successfully applied for disposable applications in technical solutions and biological media. ISFETs based on platinum metal oxides suffer from poor long-term stability yet.
- The redox pseudocapacitance of hydrous RuO2, in which protons are involved, is considered as a model system. Relative pH measurements based on standard buffer solutions are already possible by impedance spectroscopy. For absolute pH determination, the separation of interfacial surface charges and faradaic charges has still to be solved.
- Platinum metal oxides can easily be coated on nickel foil by thermal decomposition of precursor solutions. Powders, e.g. obtained by sol-gel processes, can be bound in an epoxy matrix.
- Activated carbon, glassy carbon, and possibly indium oxide seem to be useful as liquid-free reference systems in pH sensors at values between pH 5 and pH 10.
References and Notes
- Sørensen, S.P.L.; Linderstrøm-Lang, K. The determination and value of pH. Comp. Rend. Trav. Lab. Carlsberg 1909, 8, 1–168. [Google Scholar]
- Vonau, W.; Guth, U. pH monitoring: A review. J. Solid State Electrochem. 2006, 10, 746–752. [Google Scholar]
- Bates, R.G. Electrometric pH-Determinations, Theory and Practice; John Wiley and Sons: New York, NY, USA, 1954. [Google Scholar]
- Haber, F.; Klemensiewicz, Z. Ueber elektrische Phasengrenzkräfte. Zeitschr. f. Physik. Chem. 1909, 67, 385–431. [Google Scholar]
- Galster, H. pH Measurements.—Fundamentals, Methods, Applications, Instruments; VCH Publishers: New York, NY, USA, 1991. [Google Scholar]
- Ion Selective Electrodes; Koryta, J.; Stulik, K. (Eds.) Cambridge Univ. Press: Cambridge, UK, 1983.
- Covington, A.K.; Bates, R.G.; Durst, R.A. Definition of pH scales, standard reference values, measurement of pH, and related terminology (IUPAC Recommendations 1984). Pure Appl. Chem. 1985, 57, 531–542. [Google Scholar]
- Janata, J. Principles of Chemical Sensors; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- Glass Electrodes for Hydrogen and Other Cations; Eisenman, G. (Ed.) Marcel Dekker: New York, NY, USA, 1967.
- Covington, A.K.; Bütikofer, H.P.; Camoes, M.F.G.F.C.; Ferra, M.I.A.; Rebelo, M.J.F. Procedures for testing pH-responsive glass electrodes at 25, 37, 65 and 85 °C and determination of alkaline errors up to 1 mol/dm3 Na+, K+, Li+ (IUPAC Technical Report). Pure Appl. Chem. 1985, 57, 887–898. [Google Scholar]
- Harned, H.S.; Owen, B.B. The Physical Chemistry of Electrolytic Solutions; Reinhold: New York, NY, USA, 1958; Volume Chap. 14. [Google Scholar]
- Buck, R.P.; Rondinini, S.; Covington, A.K.; Baucke, F.G.K.; Brett, C.M.A.; Camoes, M.F.; Milton, M.J.T.; Mussini, T.; Naumann, R.; Pratt, K.W.; Spitzer, P.; Wilson, G.S. Measurement of pH. Definitions, standards, and procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 2169–2200. [Google Scholar]
- Rondinini, S.; Mussini, P.R.; Mussini, T.; Vertova, A. pH measurements in non-aqueous and mixed solvents: predicting pH(PS) of potassium hydrogen phthalate for alcohol-water mixtures (IUPAC Technical Report). Pure Appl. Chem. 1998, 70, 1419–1422. [Google Scholar]
- Kortüm, G. Lehrbuch der Elektrochemie.; Verlag Chemie: Weinheim, Germany, 1970; Volume Chap. XI.2. [Google Scholar]
- Kratz, L. Die Glaselektrode und ihre Anwendungen; D. Steinkopff: Frankfurt, Germany, 1950; pp. 199–200. [Google Scholar]
- Bergveld, P. Development of an ion-sensitive solid state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar]
- Sensors. A Comprehensive Survey; Göpel, W.; Hesse, J.; Zemel, J.N. (Eds.) VCH Publisher: Weinheim, Germany, 1991; Volume 2.
- Abe, H.; Esahi, M.; Matsuo, T. ISFET's using inorganic gate thin films. IEEE Trans. Electron Devices 1979, ED-26, 1939–1944. [Google Scholar]
- Liao, Y-H.; Chou, J.-C. Preparation and characteristics of ruthenium dioxide for pH array sensors with real-time measurement system. Sens. Actuat. B 2008, 128, 603–612. [Google Scholar]
- Manufacturer. Pfaudler Werke AG: Schwetzingen, Germany.
- Oesch, U.; Ammann, D.; Brzózka, Z.; Pham, H.V.; Pretsch, E.; Rusterholz, B.; Simon, W.; Suter, G.; lti, D.H.; Xu, A.P. Design of neutral hydrogen ion carriers for solvent polymeric membrane electrodes of selected pH range. Anal. Chem. 1986, 58, 2285–2289. [Google Scholar]
- Manufacturer: Orion Research; Cambridge, MA 02139, USA.
- Sensors Update; Göpel, W.; Baltes, H.; Hesse, J. (Eds.) VCH Publication: Weinheim, Germany, 2001; Vol. 8, Chap. 1.3.
- Sensoren; Schaumburg, H. Teubner: Stuttgart, Germany, 1992. [Google Scholar]
- Biilmann, E. L'electrode à quinhydrone et ses applications. Bull. Soc. Chim. France 1927, 41, 213–286. [Google Scholar]
- Kolthoff, I.M.; Hartong, B.D. On the antimony electrode. Rec. Trav. Chim. 1925, 44, 113–120. [Google Scholar]
- Uhl, A. Kestranek, Electrometric titration of acids and bases with an antimony indicator electrode. Monatsh. Chem. 1923, 44, 29–34. [Google Scholar]
- Schwabe, K. Die Wismutelektrode zur pH-Indikation. Z. Elektrochem. 1951, 55, 411. [Google Scholar]
- Sensors. A Comprehensive Survey; Göpel, W.; Hesse, J.; Zemel, J.N. (Eds.) VCH Publisher: Weinheim, Germany, 1991; Volume 2.
- Liu, J.H.; Zhang, Y.H.; Zhang, Z.Y.; Ni, L.; Li, H.X. Study of thick-film pH sensors. Sens. Actuat. B 1993, 14, 566–567. [Google Scholar]
- Trasatti, S.; Lodi, G. Conductive Metal Oxides; Elsevier: Amsterdam, The Netherlands, 1980; Vol. A. [Google Scholar]
- Beer, H.B. The Invention and industrial development of metal anodes. J. Electrochem. Soc. 1980, 127, 303C. [Google Scholar]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuat. 1984, 5, 137–146. [Google Scholar]
- Olthuis, W.; Robben, M.A.; Bergveld, P.; Bos, M.; van der Linden, W.E. pH sensor properties of electrochemically grown iridium oxide. Sens. Actuat. B 1990, 2, 247–256. [Google Scholar]
- Ardizzone, S.; Daghetti, A.; Franceschi, L.; Trasatti, S. The point of zero charge of hydrous RuO2. Colloids Surf. 1989, 35, 85–96. [Google Scholar]
- Trasatti, S. Physical electrochemistry of ceramic oxides. Electrochim. Acta 1991, 36, 225–241. [Google Scholar]
- Trasatti, S.; Kurzweil, P. Electrochemical supercapacitors as versatile energy stores. Platinum Met. Rev. 1994, 38, 46–56. [Google Scholar]
- Kurzweil, P. Electrochemical Capacitors. Metal Oxide. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE-Cebelcor: Brussels, Belgium, 1974. [Google Scholar]
- Kurzweil, P. Precious metal oxides for electrochemical energy converters: Pseudocapacitance and pH dependence of redox processes. J. Power Sources 2009, 190, 189–200. [Google Scholar]
- Conway, B.E. Electrochemical Supercapacitors; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; Volume Chap. 11. [Google Scholar]
- Doblhofer, K.; Metikos, M.; Ogumi, Z.; Gerischer, H. Electrochemical oxidation and reduction of the RuO2/Ti electrode surface. Ber. Bunsenges. Phys. Chem. 1978, 82, 1046–50. [Google Scholar]
- Patel, A.; Richens, D.T. The tetranuclear ruthenium(IV) aqua ion: evidence in support of its formulation as Hn[Ru4O6(OH2)12](4+n)+ (n = 0-4). Inorg. Chem. 1991, 30, 3789–3792. [Google Scholar]
- Pourbaix, M. Atlas of electrochemical equilibria in aqueous solutions; NACE-Cebelcor: Brussels, Belgium, 1974. [Google Scholar]
- Romain, S.; Bozoglian, F.; Sala, X.; Llobet, A. Oxygen-oxygen bond formation by the Ru-Hbpp water oxidation catalyst occurs solely via an intramolecular reaction pathway. J. Am. Chem. Soc. 2009, 131, 2768–2769. [Google Scholar]
- Soto, J.; Labrador, R.H.; Marcos, M.D.; Martinez-Manez, R.; Coll, C.; Garcia-Breijo, E.; Gil, L. A model for the assessment of interfering processes in Faradic electrodes. Sens. Actuat. A 2008, 142, 56–60. [Google Scholar]
- Sarangapani, S.; Lessner, P.; Forchione, J.; Griffith, A.; Laconti, A.B. Advanced double layer capacitors. J. Power Sources 1990, 29, 355–364. [Google Scholar]
- Roginskaya, Y.E.; Morozova, O.V. The role of hydrated oxides in formation and structure of DSA-type oxide electrocatalysts. Electrochim. Acta 1995, 40, 817–822. [Google Scholar]
- Mihell, J.A.; Atkinson, J.K. Planar thick-film pH electrodes based on ruthenium dioxide hydrate. Sens. Actuat. B 1998, 48, 505–511. [Google Scholar]
- Wöhler, L.; Balz, P.; Metz, L. Die oxyde des rutheniums. Z. Anorg. Allgem. Chem. 1924, 139, 205–219. [Google Scholar]
- Armelao, L.; Barreca, D.; Moraru, B. A molecular approach to RuO2-based thin films: sol-gel synthesis and characterization. J. Non-Cryst. Solids 2003, 316, 364–371. [Google Scholar]
- Kurzweil, P. Long time stable electrode European patent EP 0622815, DE 4313474, 1994.
- Kurzweil, P. Precious metal oxides for electrochemical energy converters: Pseudocapacitance and pH dependence of redox processes. J. Power Sources 2009, 190, 189–200. [Google Scholar]
- Takasu, Y.; Onoue, S.; Kameyama, K.; Murakami, Y.; Yahikozawa, K. Preparation of ultrafine RuO2-IrO2-TiO2 oxide particles by a sol-gel process. Electrochim. Acta 1994, 39, 1993–1997. [Google Scholar]
- Kim, H.; Popov, B.N. Characterization of hydrous ruthenium oxide/carbon nanocomposite supercapacitors prepared by a colloidal method. J. Power Sources 2002, 104, 52–61. [Google Scholar]
- Jang, J.H.; Kato, A.; Machida, K.; Naoi, K. Supercapacitor performance of hydrous ruthenium oxide electrodes prepared by electrophoretic deposition. J. Electrochem. Soc. 2006, 153, A321–A328. [Google Scholar]
- Kurzweil, P. Unpublished results. University of Applied Sciences: Amberg, Germany, 2009.
- Kreider, K.G.; Tarlov, M.J.; Cline, J.P. Sputtered thin-film pH electrodes of platinum, palladium, ruthenium, and iridium oxides. Sens. Actuat. B 1995, 28, 167–172. [Google Scholar]
- Vonau, W.; Enseleit, U.; Gerlach, F.; Herrmann, S. Conceptions, materials, processing technologies and fields of application for miniaturized electrochemical sensors with planar membranes. Electrochim. Acta 2004, 49, 3745–3750. [Google Scholar]
- Allen, M.D.; Livesley, D.J.; Potter, R.J.; Pratt, A.S. (JohnsonMatthey) pH-meter with constant current applied between the transition metal-based sensor electrode and the reference electrode. Patent WO/2000/004379, 2000. [Google Scholar]
- Koncki, R.; Mascini, M. Screen-printed ruthenium dioxide electrodes for pH measurements. Anal. Chim. Acta 1997, 351, 143–149. [Google Scholar]
- Mihell, J.A.; Atkinson, J.K. Planar thick-film pH electrodes based on ruthenium hydrate. Sens. Actuat. B 1998, 48, 505–511. [Google Scholar]
- Gac, A.; Atkinson, J.K.; Zhang, Z.; Sion, R.P. A comparison of thick-film chemical sensor characteristics in laboratory and on-line industrial process applications. Meas. Sci. Technol. 2002, 13, 2062–2073. [Google Scholar]
- Liao, Y-H.; Chou, J.-C. Preparation and characteristics of ruthenium dioxide for pH array sensors with real-time measurement system. Sens. Actuat. B 2008, 128, 603–612. [Google Scholar]
- Colombo, C.; Kappes, T.; Hauser, P.C. Coulometric micro-titrator with a ruthenium dioxide pH-electrode. Anal.Chim. Acta 2000, 412, 69–75. [Google Scholar]
- Cagnini, A.; Palchettia, I.; Liontia, I.; Mascinia, M. Turnerbet A.P.F. Disposable ruthenized screen-printed biosensors for pesticides monitoring. Sens. Actuat. B 1995, 24, 85–89. [Google Scholar]
- Tymecki, L.; Koncki, R. Thick-film potentiometric biosensor for bloodless monitoring of hemodialyis. Sens. Actuat. B 2006, 113, 782–786. [Google Scholar]
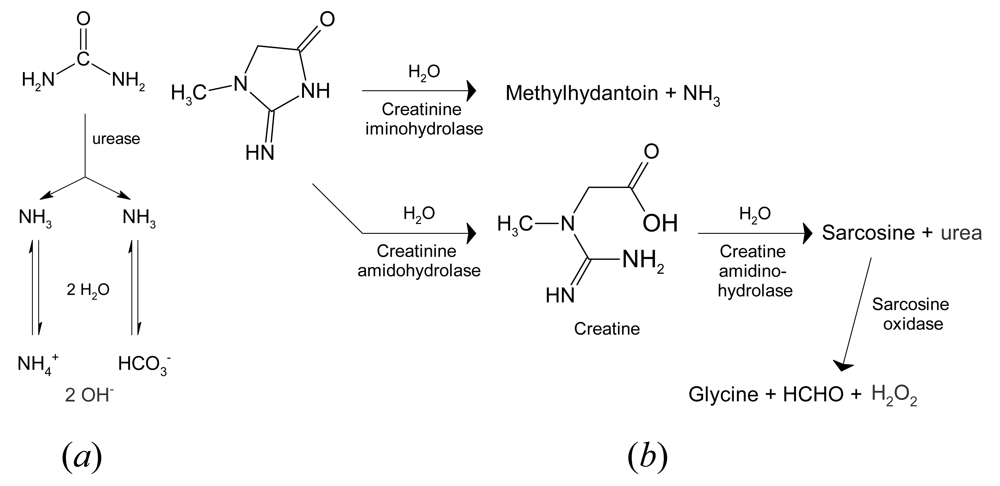
- Ogonczyk, D.; Tymecki, L.; Wyzkiewicz, I.; Koncki, R.; Glab, S. Screen-printed disposable urease-based biosensors for inhibitive detection of heavy metal ions. Sens. Actuat. B 2005, 106, 450–454. [Google Scholar]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: recommended definitions and classification (IUPAC Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar]
- Koncki, R. Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta 2007, 599, 7–15. [Google Scholar]
- Kotzian, P.; Brazdilova, P.; Kalcher, K.; Handlir, K.; Vytras, K. Oxides of platinum metal group as potential catalysts in carbonaceous amperometric biosensors based on oxidases. Sens. Actuat. B 2007, 124, 297–302. [Google Scholar]
- Schwarz, J.; Kaden, H.; Kutschke, S.; Glombitza, F. Potentiometrische Biosensoren zur Bestimmung von Metallkationen. GIT Labor-Fachzeitschrift 2007, 51, 408–409. [Google Scholar]
- Perley, G.A.; Godshalk, J.B. Cell for pH measurements. US 2 416 949 1947. [Google Scholar]
- Widera, J.; Riehl, B.L.; Johnson, J.M.; Hansen, D.C. State-of-the-art monitoring of fuel acidity. Sens. Actuat. B 2008, 130, 871–881. [Google Scholar]
- Kress-Rogers, E. Solid-state pH sensors for food applications. Trends Food Sci. Technol. 1991, 12, 320–324. [Google Scholar]
- de Rooij, N.F.; Bergvelt, P. Monitoring Vital Parameters during Extracorporeal Circulation; Kimmich, H.P., Ed.; Kaerge: Basel, Switzerland, 1981. [Google Scholar]
- Glab, S.; Hulanicki, A.; Edwall, G.; Ingman, F. Metal-metal oxide and metal oxide electrodes as pH sensors. Crit. Rev. Anal. Chem. 1989, 21, 29–47. [Google Scholar]
- Kinlen, P.J.; Heider, J.E.; Hubbard, D.E. A solid-state pH sensor based on a Nafion-coated iridium oxide indicator electrode and a polymer-based silver chloride reference electrode. Sens. Actuat. B 1994, 22, 13–25. [Google Scholar]
- Radiometer (electrode maker). In Růžička electrode.; Radiometer: Copenhagen, Denmark, 1990.
- Oelßner, W.; Kaden, H. Iridiumdioxid-pH-Elektroden in Dickfilmtechnik. In Elektrochemie der Ionenleiter; Beck, E., Ed.; GDCh-Monographie: Frankfurt/Main, Germany, 1995; Volume 3, pp. 390–392. [Google Scholar]
- Kessel, R.; Drägerwerk, A.G. Amperometric sensor. US Pat. 5518602, 1996. [Google Scholar]
- West, W.; Buehler, M.; Keymeulen, D. Metal/Metal Oxide Differential Electrode pH Sensors. NASA's Jet Propulsion Laboratory: Pasadena, CA, 2007. Available online: www.techbriefs.com/component/content/article/2287 (accessed on 19 May 2009).
- Kinoshita, E.; Ingman, F.; Edwal, G.; Thulin, S.; Glab, S. Polycrystalline and monocrystalline antimony, iridium and palladium as electrode material for pH-sensing electrodes. Talanta 1986, 33, 125–134. [Google Scholar]
- Katsube, T.; Lauks, I.; Zemel, J.N. pH-Sensitive sputtered iridium oxide films. Sens. Actuat. B 1982, 2, 399–410. [Google Scholar]
- El-Giar, E.E.M.; Wipf, D.O. Microparticle-based iridium oxide ultramicroelectrodes for pH sensing and imaging. J. Electroanal. Chem. 2007, 609, 147–154. [Google Scholar]
- Pan, C.W.; Chou, J.C.; Sun, T.P.; Hsiung, S.K. Development of the tin oxide pH electrode by the sputtering method. Sens. Actuat. B 2005, 108, 863–869. [Google Scholar]
- Tsai, C.N.; Chou, J.C.; Sun, T.P.; Hsiung, S.K. Study on the sensing characteristics and hysteresis effect of the tin oxide pH electrode. Sens. Actuat. B 2005, 108, 877–882. [Google Scholar]
- Yin, L.T.; Chou, J.C.; Chung, W.Y.; Sun, T.P.; Hsiung, S.K. Study on separate structure extended gate H+-ion sensitive field effect transistor on a glass substrate. Sens. Actuat. B 2000, 71, 106–111. [Google Scholar]
- Eftekhari, A. pH Sensor based on deposited film of lead dioxide on aluminium substrate electrode. Sens. Actuat. B 2003, 88, 234–238. [Google Scholar]
- Kinoshita, K.; Madou, M.J. Electrochemical measurement on Pt, Ir and Ti oxides as pH probes. J. Electrochem. Soc. 1984, 131, 1089–1094. [Google Scholar]
- Pocrifka, L.A.; Goncalves, C.; Grossi, P.; Colpa, P.C.; Pereira, E.C. Development of RuO2-TiO2 (70-30) mol% for pH measurements. Sens. Actuat. B 2006, 113, 1012–1016. [Google Scholar]
- Qingwen, L.; Yiming, W.; Guoan, L. pH-response of nanosized MnO2 prepared with solid state reaction route at room temperature. Sens. Actuat. B 1999, 59, 42–47. [Google Scholar]
- Kinoshita, E.; Ingman, F.; Edwall, G.; Glab, S. An examination of the palladium/palladium oxide system and its utility for pH-sensing electrodes. Electrochim. Acta 1986, 31, 29–35. [Google Scholar]
- Niedrach, L.W. Oxygen ion-conducting ceramics: A new application in High-Temperature-High-Pressure pH sensors. Science 1980, 207, 1200–1202. [Google Scholar]
- Seyfried, W.E.; Zhang, Z.; Ding, K. metal/metal oxide electrode as pH-sensor and methods of production. Patent WO 02095386, 2002. [Google Scholar]
- Gao Pengtao, M.L.J.; Dos Santos, M.P.; Teixeira, V.; Andritschky, M. Characterization of ZrO2 films prepared by rf reactive sputtering at different O2 concentrations in the sputtering gases. Vacuum 2000, 56, 143–148. [Google Scholar]
- Chiang, J.L.; Chen, Y.C.; Choul, J.C.; Cheng, C.C. Temperature effect on AlN/SiO2 gate pH-ion-sensitive field-effect transistor devices study on the pH-sensing characteristics of ISFET with aluminium nitride membrane. Jpn. J. Appl. Phys. 2002, 41, 541–545. [Google Scholar]
- Starr, S. Novel materials for pH sensors. Master thesis., University of Applied Sciences, Amberg, Germany, 2009. [Google Scholar]
- Winsel, A. Hydrogen rod electrode with integrated hydrogen source. US Pat. 5,407,555, 1995. [Google Scholar]
- Ives, D.J.G.; Janz, G.J. Reference Electrodes; Academic Press: New York, NY, USA, 1961. [Google Scholar]
- Huang, I.Y.; Huang, R.S.; Lo, L.H. Improvement of integrated Ag/AgCl thin-film electrodes by KCl-gel coating for ISFET applications. Sens. Actuat. B 2003, 94, 53–64. [Google Scholar]
- Cranny, A.; Atkinson, J.K. Thick film silver-silver chloride reference electrodes. Meas. Sci. Technol. 1998, 9, 1557–65. [Google Scholar]
- Arevalo, A.; Souto, R.M.; Arevalo, M.C. Preparation and reproducibility of a silver-silver chloride electrode. J. Applied Electrochem. 1985, 15, 727–735. [Google Scholar]
- Nikolskii, B.P.; Materova, E.A. Solid contact in membrane ion-selective electrodes. Ion Sel. Electrode Rev. 1985, 7, 3–39. [Google Scholar]
- Vonau, W.; Oelßner, W.; Guth, U.; Henze, J. An all-solid-state reference electrode. Sens. Actuat. B 2009. [Google Scholar] [CrossRef]
- Shuk, P.; Guth, U.; Greenblatt, M. Ion selective sensors based on molybdenum bronzes. Solid State Electrochem. 2002, 8, 374–383. [Google Scholar]
- Gabel, J.; Vonau, W.; Shuk, P.; Guth, U. New reference electrodes based on tungsten-substituted molybdenum bronzes. Solid State Ionics 2004, 169, 75–80. [Google Scholar]
- Guitton, J.; Forestier, M.; Kahil, H. New positive electrode for a rechargeable electrochemical generator and process for its manufacture. French patent, FR 2547678, 1984. [Google Scholar]
- Noll, A.; Rudolf, V.; Grabner, E.W. A glass electrode with solid internal contact based on Prussian blue. Electrochim. Acta 1998, 44, 415–419. [Google Scholar]
- Kurzweil, P.; Fischle, H.-J. A new monitoring method for electrochemical aggregates by impedance spectroscopy. J. Power Sources 2004, 127, 331–340. [Google Scholar]
- Rezvani, B.; Lomibao, J.; Feng, C.D. Impedance measurement of a pH electrode. WO 2008021546 2008. [Google Scholar]
Symbols and Abbreviations
| a | activity, a = γc(c/c0) = γm(m/m0) |
| C | capacitance (F), C = dQ/dU |
| c | molar concentration (mol/L), amount of a substance solved per liter of solution |
| c0 | standard concentration: c0 ≡ 1 mol/L |
| CE | counter electrode |
| CVD | chemical vapour deposition |
| E | potential of an electrode versus a reference electrode (in V vs. ref) |
| E0 | standard potential of an electrode or half-reaction (V NHE): at 25 °C, 101325 Pa, 1-active solution |
| ΔE0 | difference in standard potential of two half-cells (V), electromotive force |
| F | Faraday constant; charge on one mole of electrons: F = 96485 C mol–1 |
| I | electric current (A) |
| j | imaginary operator, √(–1) |
| Q | electric charge (C = A s) |
| M | molar mass of a compund (kg mol–1) |
| m | molality (mol kg–1), amount of substance solved per kilogram of solvent: m = c/(ρ – Mc) |
| m0 | standard molality: m0 ≡ 1 mol kg–1 |
| NHE | standard hydrogen electrode, see Section 2.2 |
| p | pressure (1 Pa = 10–5 bar) |
| p0 | standard pressure: 101325 Pa |
| R | electric resistance (Ω) |
| Ref | Reference (electrode) |
| RE | reference electrode |
| RHE | reversible hydrogen electrode: ENHE = ERHE – 0.05916pH (25 °C) |
| (s) | solid phase |
| SCE | saturated calomel electrode |
| SHE | standard hydrogen electrode, see Section 2.2 |
| T | absolute temperature (in K) |
| TRIS | tris(hydroxymethyl) aminomethane |
| U | electric voltage (V), potential difference between two electrodes |
| Δφ | electric potential difference between two phases (V) |
| ρ | density of a liquid (1 kg m–3 = 1 g/L = 0.001 g cm–1) |
| v | scan rate, v = dU/dt (in V s–1) |
| WE | working electrode |
| Z | ac, impedance (Ω): Z = Re Z + j Im Z |
| z | charge number of an ion; number of electrons transferred in the half-reaction equation |
| γ | activity coefficient |



| Gate | Sensitivity | Response and stability |
|---|---|---|
| SiO2 | 20–40 mV pH–1 | non-linear response |
| SiO2 (40…110 nm, thermally grown) + Si3N4 (∼100 nm, CVD) on silicon [18]. Channel: 20 μm × 100 μm. Reference electrode: Ag|AgCl|NaCl | ∼53 mV pH–1 | Slow response; sensitivity decreases with time (formation of oxynitride) |
| Al2O3 | 53–57 mV pH–1 | linear response, very low drift |
| Ta2O5 | 55–59 mV pH–1 | linear response, undesired light sensitivity |
| Range of application | Challenges | |
|---|---|---|
| Glass electrode | Temperature: < 80…130 °C Pressure: < 60 bar (with counter pressure) Stability: ± 1 mV week–1 |
|
| ISFET | Temperature: < 85 °C Pressure: < 2 bar |
|
| Antimony electrode Optical sensors | e.g. strong caustic solutions (no sodium error), fluoride containing waste water |
|
| Transparent liquids Small and flexible (fiber sensors) No reference element required Signal transmittance over large distances |
|
| Anode reaction (electrochemical oxidation) |  | Cathode reaction (electrochemical reduction) | ||
| Ag | 2Ag + HCl ⇌ 2AgCl + H+ + 2 e– 2Ag + H2S ⇌ Ag2S + 2H+ + 2e– 2Ag + HCN ⇌ 2AgCN + 2H+ + 2e– | O2 + 4H+ + 4e–⇌2H2O (in acid solution) | Ag Pt | |
| Au | SO2 +2H2O ⇌ H2SO4 + 2H+ + 2e– | |||
| O2 + 2H2O + 4e–⇌4OH– (in alkaline solution) | Au C | |||
| Pt | CO + H2O ⇌ CO2 + 2H+ + 2e– | |||
| 2H2O ⇌ O2 + 4H+ + 4e– | Cl2 + 2e–⇌2Cl– | Au |
| Group | Material | Redox equilibrium: Ox + ze–⇌Red | E0 / V (pH 0) | E0′ / V (pH 14) | |
|---|---|---|---|---|---|
| IVa | Tin | SnO2 + 4H+ + 4e–⇌Sn + 2H2O | –0.117 | –0.945 | |
| Lead | HPbO2– + H2O + 2e–⇌Pb + 3OH– | (–0.36) | –0.537 | ||
| Va | Arsenic | As2O3 + 6H+ + 6e–⇌2As + 3H2O | +0.234 | –0.68 | |
| Antimony | Sb2O3 + 6H+ + 6e–⇌2Sb + 3H2O | +0.152 | –0.639 | ||
| Bismuth | Bi2O3 + 3H2O + 6e–⇌2Bi + 6OH– | +0.317 | –0.46 | *) | |
| Ib | Copper | Cu2O + H2O + 2e–⇌2Cu + 2OH– | (+0.34) | –0.36 | *) |
| Silver | Ag2O + H2O + 2e–⇌2Ag + 2OH– | (+0.80) | +0.342 | ||
| Gold | H2AuO3– + H2O + 3e–⇌Au + 4OH– | +1.50 | +0.70 | ||
| IIb | Zinc | ZnO + H2O + 2e–⇌Zn + 2OH– | (–0.497) | (–1.260) | *) |
| Mercury | HgO + H2O + 2e–⇌Hg + 2OH– | +0.860 | +0.098 | ||
| Vb | Tantalum | Ta2O5 + 10H+ + 10e–⇌2Ta + 5H2O | –0.750 | –1.578 | |
| VIb | Tungsten | WO2 + 4H+ + 4e–⇌W + 2H2O | –0.119 | (–0.946) | |
| VIIb | Rhenium | Re2O3 + 6H+ + 6e–⇌2Re+ 3H2O | +0.227 | –0.600 | |
| VIIIb | Iron | Fe3O4 + 8H+ + 8e–⇌3Fe + 4H2O | (–0.085) | –0.912 | *) |
| Nickel | NiO + 2H+ + 2e–⇌Ni + H2O | (+0.110) | –0.717 | *) | |
| Osmium | OsO4 + 8H+ + 8e–⇌Os + 4H2O | +0.838 | (≈ 0.00) | ||
| Rhodium | RhOH2+ + H+ + 3e–⇌Rh + H2O | +0.83 | ≈ 0.00 | ||
| Iridium | Ir2O3 + 3H2O + 6e–⇌2Ir + 6OH– | +0.923 | +0.098 | ||
| Platinum | PtO2 + 4H+ + 4e–⇌Pt + 2H2O | +1.0 | +0.14 | ||
| Construction of the sensor: WE = working electrode, RE = reference, CE = counter | Applications and properties | Ref. |
|---|---|---|
| Film layers Screen-printed layers of graphite-based conducting inks containing 10% RuO2 | Lemonades, wine and milk.Sensitivity: −51 mV pH–1Response time: < 5 min | [61] |
| Planar thick-film of RuO2·xH2O in a polymer matrix on a current collector on a alumina substrate Thick-film fabricated chemical sensor: RuO2 in a polymer binder on gold back contact. 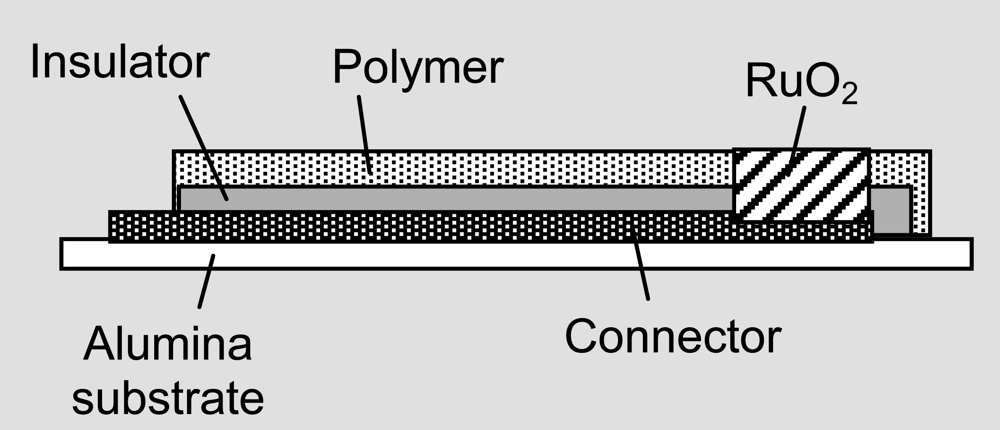 | 651 mV vs. Ag|AgCl (pH 0)–52 mV pH–1 (pH 2–10) | [62] |
| Application in water-based inks:Sensitivity: −47 mV pH–1 (pH 4–10)pH sensitivity drift: 50 μV pH–1 d–1Previous calibration is needed.Drift of thick-film Ag|AgCl reference electrode: dU/dpH = [−0.070 ln (t/d) + 0.125] mV | [63] | |
| ISFET RuO2 sensing membrane on a p-type silicon wafer substrate by radio frequency sputtering (Ru metal, 1.3 Pa, in Ar/O2; 10 W, 13.56 MHz). Drain-source voltage 0.2 V; gate voltage UG = 0–6 V; while the drain-source current IDS is measured. | Applications: lemonades, vinegar, milk, water.Sensitivity: ∼57 ± 1 mV pH–1 (IDS = 200 μA)Response time: < 1 sDrift rate: 0.13 mV pH–1 (pH 4)0.38 mV pH–1 (pH 7)7.31 mV pH–1 (pH 10),Hysteresis width: 4.4 mV (pH 7–4–7–10–7)2.2 mV (pH 7–10–7–4–7)Loop time: ∼13 minInterfering ions: K+, Na+ (k ≈ 4·10–6, Equation 4)The IDS(UG) curve is shifted positively as the pH value increases (see Figure 3). | [64] |
| Coulometric micro-tritrator: Actuator for the coulometric production: two gold electrodes (on copper support)End-point detection: Ru/RuO2 (WE), Ag|AgCl (RE), Au (CE) Amperometric Biosensor: 5% Ru/carbon/enzyme (WE) on a silver-conductive layer (CE) on a polyester support Potentiometric biosensor: RuO2/urease (WE) and RuO2/bovine serum albumin (CE) on silver current collector 59.5% RuO2, 40% graphite paste, 0.5% urease, screen-printed on a current collector. | Acid-base titration, e.g. 0.01 molar acetic acid: ΔE = ∼ 200 mV at 6.8 μA applied current | [65] |
| Pesticides monitoring by help of acetylcholine esterase and choline oxidase at 700 mV vs. SCE: Acetylcholine + H2O → Acetate + Choline Choline + O2 → Betaine aldehyde + H2O2 The measured current is proportional to choline concentration in phosphate buffer (pH 7). | [66] | |
| Flow injection system: Dialysate fluid and buffer are continously droped on the sensor by help of a peristalic pump. | [67] | |
| Detection of silver and copper ions, which inhibit urease, by a change of potential: ∼50 mV mmol–1 | [68] |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kurzweil, P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors 2009, 9, 4955-4985. https://doi.org/10.3390/s90604955
Kurzweil P. Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook. Sensors. 2009; 9(6):4955-4985. https://doi.org/10.3390/s90604955
Chicago/Turabian StyleKurzweil, Peter. 2009. "Metal Oxides and Ion-Exchanging Surfaces as pH Sensors in Liquids: State-of-the-Art and Outlook" Sensors 9, no. 6: 4955-4985. https://doi.org/10.3390/s90604955