Standard CMOS Fabrication of a Sensitive Fully Depleted Electrolyte-Insulator-Semiconductor Field Effect Transistor for Biosensor Applications
Abstract
:1. Introduction
2. Experimental
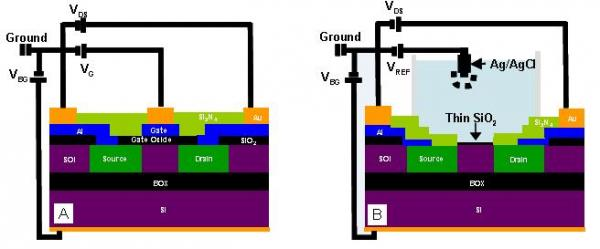
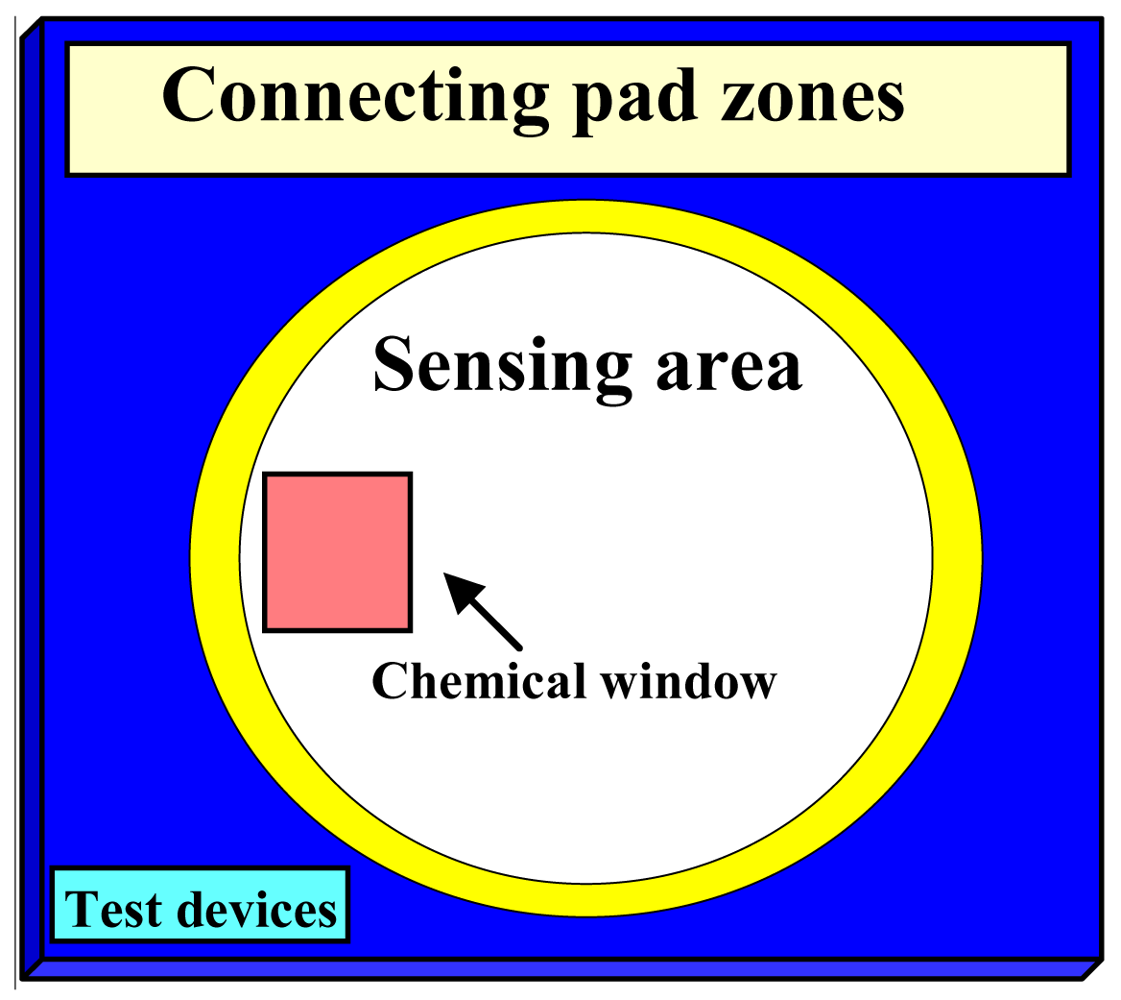
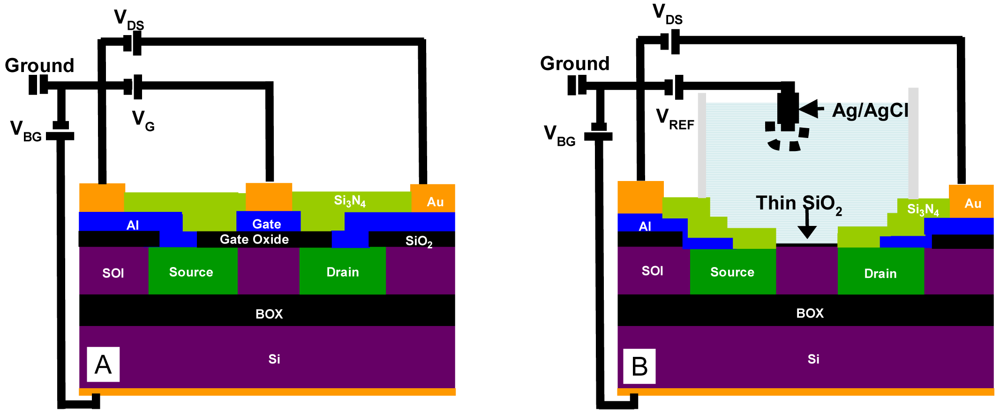
2.1. Device Fabrication
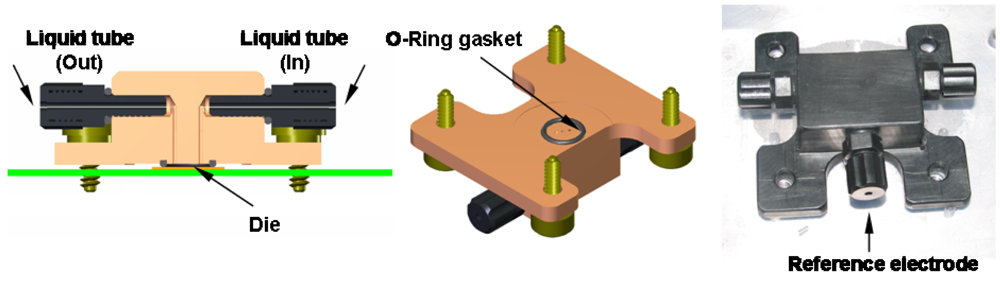
2.2. Electronic Measurements
2.3. Device Functionalization
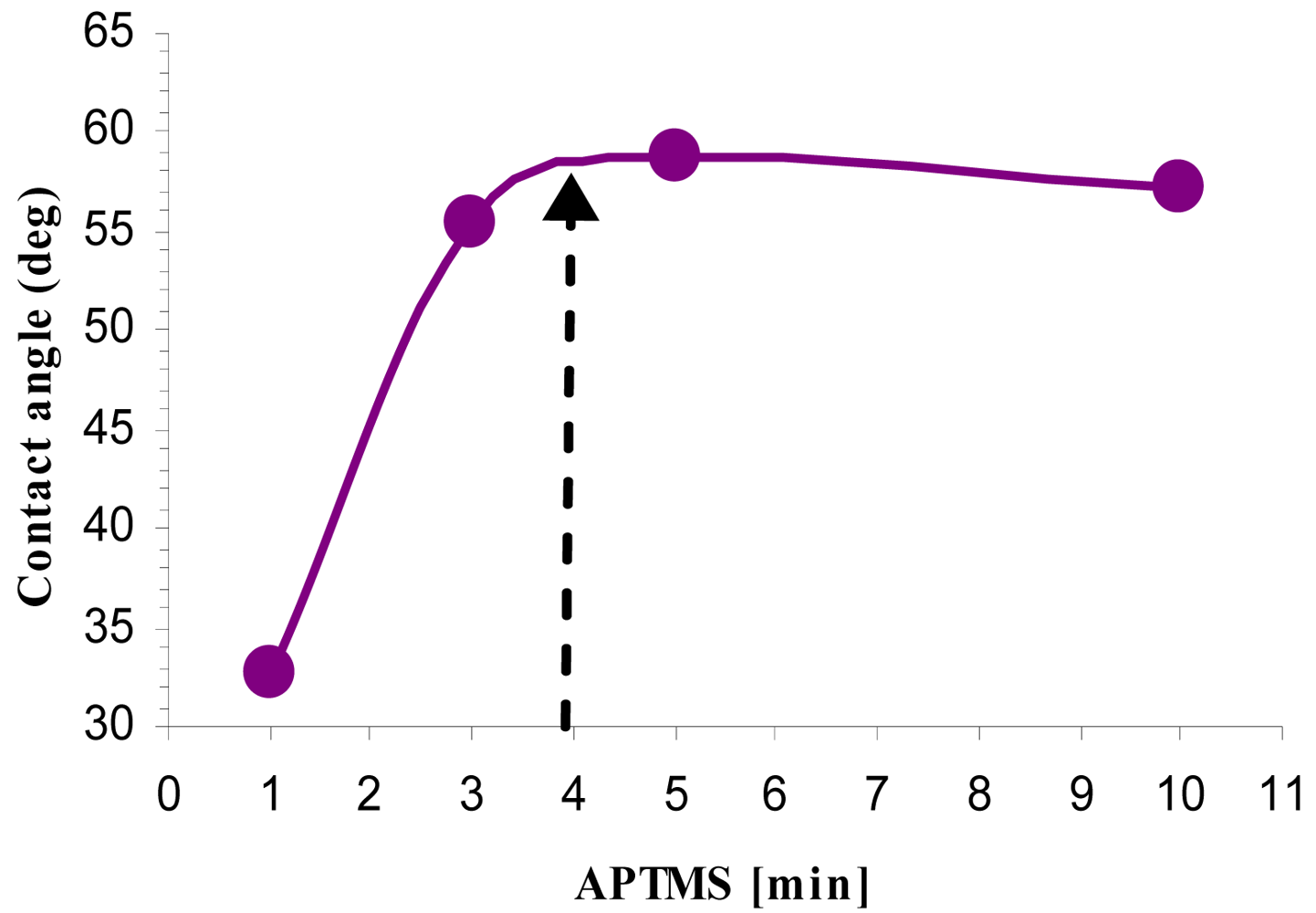
2.4. Contact Angle Analysis
2.5. pH Measurements
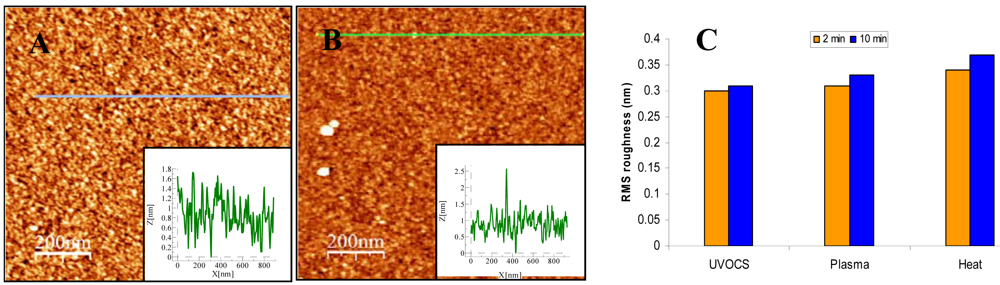
2.6. AFM Surface Analysis
3. Results and Discussion
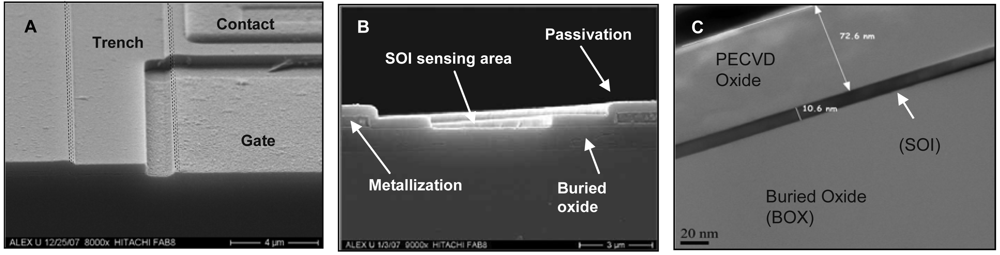
3.1. Fabrication
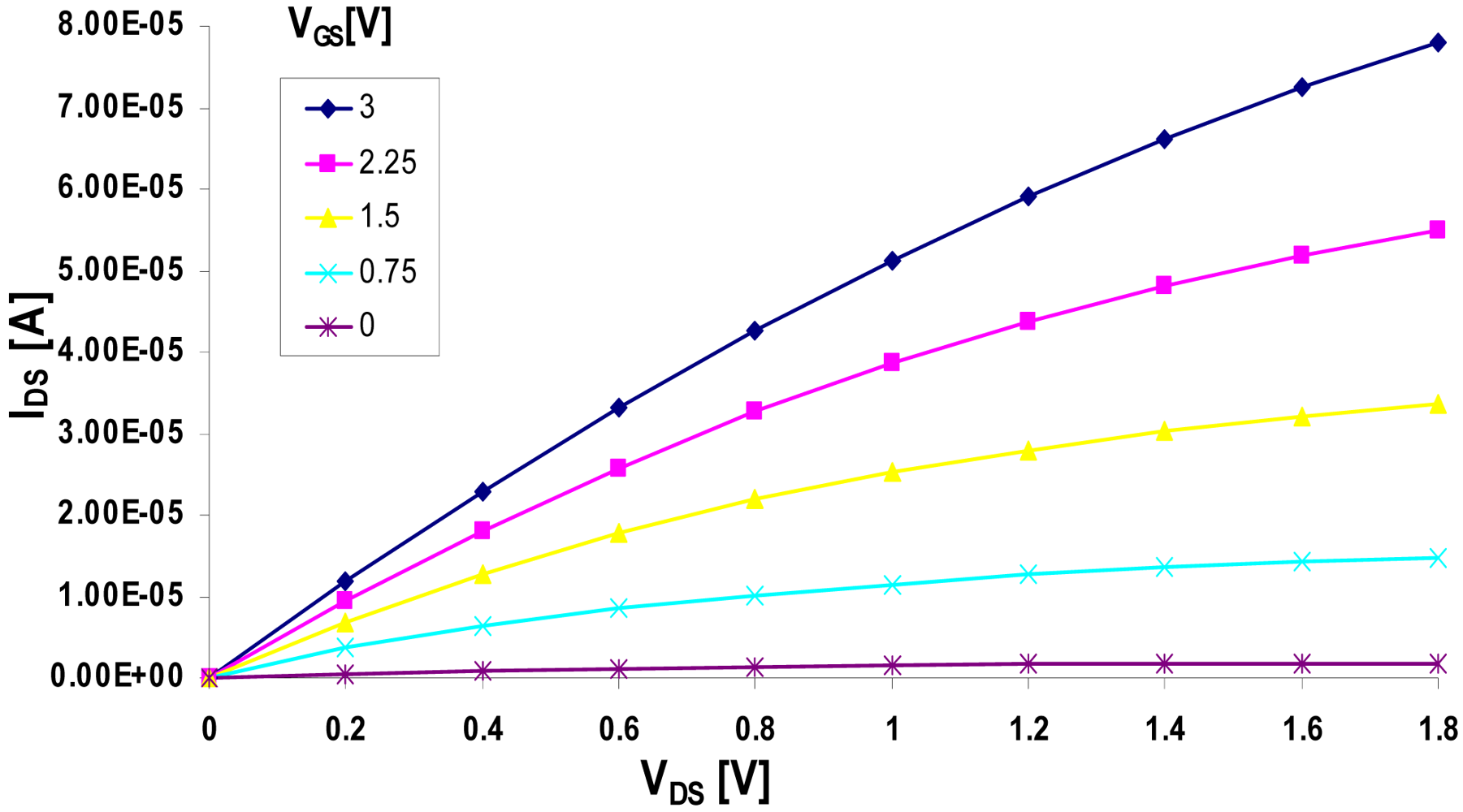
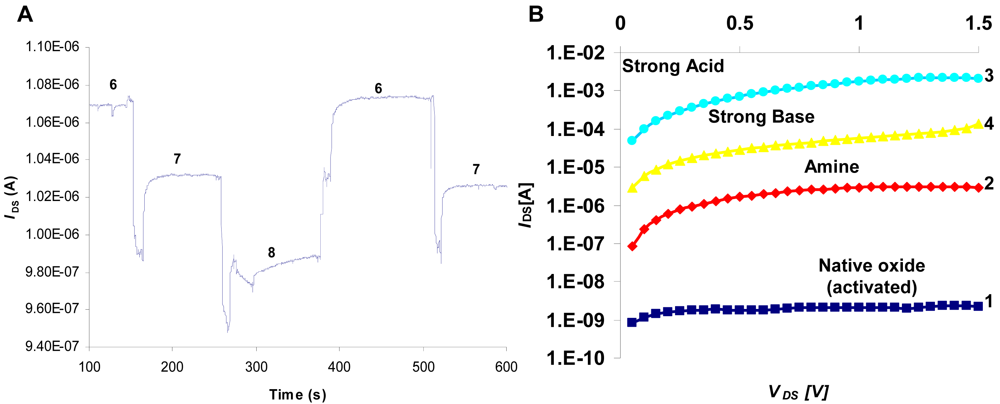
3.2. Electrical Testing
3.3. Activation
3.4. pH Sensing
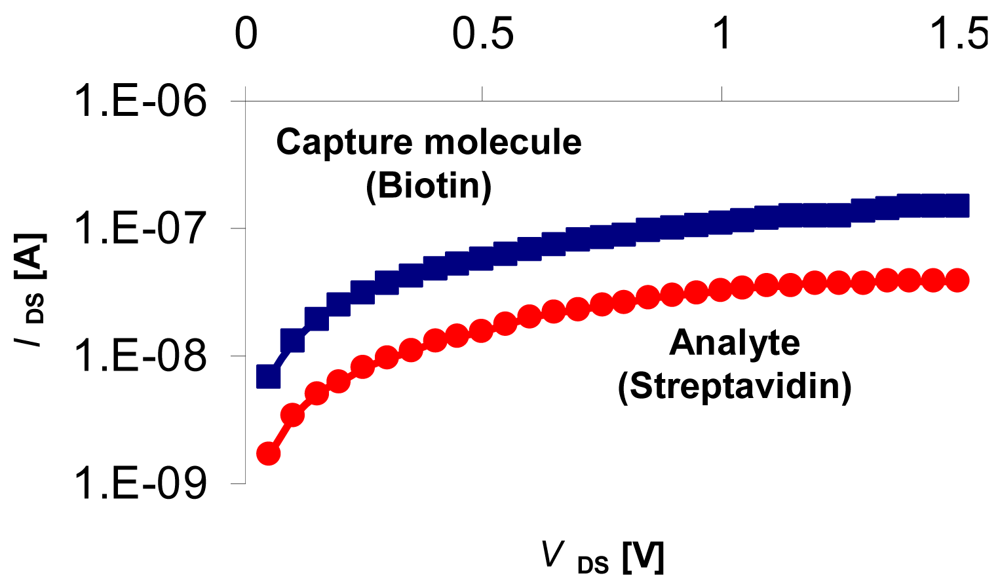
3.5. Sensing Biomolecular Interactions
4. Conclusions
References
- Pejcic, B.; De Marco, R.; Parkinson, G. The role of biosensors in the detection of emerging infectious diseases. Analyst 2006, 131, 1079–1090. [Google Scholar]
- Rasooly, A.; Herold, K.E. Biosensors for the analysis of food and waterborne pathogens and their toxins. J. AOAC Int. 2006, 89, 873–883. [Google Scholar]
- Soper, S.A.; Brown, K.; Ellington, A.; Frazier, B.; Garcia-Manero, G.; Gau, V.; Gutmang, S.I.; Hayes, D.F.; Brenda, K.; Landers, J.L.; Larson, D.; Ligler, F.; Majumdar, A.; Mascini, M.; Nolte, D.; Rosenzweig, Z.; Wang, J.; Wilson, D. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens. Bioelectron. 2006, 21, 1932–1942. [Google Scholar]
- Yang, C.H.; Liao, Y.W. An ISFET interface circuitry for biomedical applications. Proceedings of IEEE Conference on Electron Devices and Solid-State Circuits, Tainan, Taiwan, December 20-22, 2007; pp. 1083–1086.
- Killard, A.J.; Smyth, M.R. Creatinine biosensors: principles and designs. Trends Biotechnol. 2000, 18, 433–437. [Google Scholar]
- Xu, J.J.; Luo, X.L.; Chen, H.Y. Analytical aspects of FET-based biosensors. Front. Biosci. 2005, 10, 420–430. [Google Scholar]
- Machauf, A.; Cohen, A.; Doron, A.; Beraha, M.; Weinfeld, B.; Levy, I. Fabrication of a sensitive Field Effect Device (FED) for biosensor application. Proceedings of ISIE 2007, Vigo, Spain, June 4-7, 2007; pp. 2820–2823.
- Kal, S.; Bhanu Priya, V. Design and modeling of ISFET for pH sensing. Proceedings of TENCON 2007, Taipei, Taiwan, October 30 - November 2, 2007; pp. 1–4.
- Shinwari, M.W.; Deen, M.J.; Landheer, D. Study of the electrolyte-insulator-semiconductor field-effect transistor (EISFET) with applications in biosensor design. Microelectron. Reliab. 2007, 47, 2025–2057. [Google Scholar]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar]
- Pan, T.M.; Liao, K.M.; Yen, L.C.; Hsieh, Y.Y.; Chen, Y.Z. Effects of oxygen content on the structural and sensing properties of Y203 sensing membrane for pH-ISFET application. Proceedings of International Semiconductor Device Research Symposium, Maryland, USA, December 12-14, 2007; pp. 1–2.
- Bergveld, P. Thirty years of ISFETOLOGY. What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuat. B 2003, 88, 1–20. [Google Scholar]
- Yuqing, M.; Jianguo, G.; Jianrong, C. Ion sensitive field effect transducer-based biosensors. Biotechnol. Adv. 2003, 21, 527–534. [Google Scholar]
- Schoning, M.J.; Poghossian, A. Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst. 2002, 127, 1137–1151. [Google Scholar]
- Park, K.; Choi, S.; Lee, M.; Sohn, B.; Choi, S. ISFET glucose sensor system with fast recovery characteristics by employing electrolysis. Sens Actuat. B 2002, 83, 90–97. [Google Scholar]
- Starodub, N.F.; Dzantiev, B.B.; Starodub, V.M.; Zherdev, A.V. Immunosensor for the determination of the herbicide simazine based on an ion-selective field-effect transistor. Anal. Chim. Acta. 2000, 424, 37–43. [Google Scholar]
- Estrela, P.; Migliorato, P.; Takiguchi, H.; Fukushima, H.; Nebashi, S. Electrical detection of biomolecular interactions with metal–insulator–semiconductor diodes. Biosens. Bioelectron. 2005, 20, 1580–1586. [Google Scholar]
- Offenhausser, A.; Knoll, W. Cell-transistor hybrid systems and their potential applications. Trends Biotechnol. 2001, 19, 62–66. [Google Scholar]
- Nair, P.R.; Alam, M.A. Design considerations of silicon nanowire biosensors. IEEE Trans. Electron. Dev. 2007, 54, 3400–3408. [Google Scholar]
- Thelander, C.; Fröbergfroberg, L.E.; Rehnstedt, C.; Samuelson, L.; Wernersson, L.E. Vertical enhancement-mode InAs nanowire field-effect transistor with 50- nm wrap gate. IEEE Electron. Dev. Lett. 2008, 29, 206–208. [Google Scholar]
- Chen, Z.; Farmer, D.; Xu, S.; Gordon, R.; Avouris, P.; Appenzeller, J. Externally assembled gate-all-around carbon nanotube field-effect transistor. IEEE Electron. Dev. Lett. 2008, 29, 183–185. [Google Scholar]
- Shalev, G.; Doron, A.; Virobnik, U.; Cohen, A.; Sanhedrai, Y.; Levy, I. Gain optimization in ISFET based sensor with fully depleted silicon-on-insulator. Appl. Phys. Lett. 2008, 93, 1–3. [Google Scholar]
- Shaya, O.; Shaked, M.; Doron, A.; Cohen, A.; Levy, I.; Rosenwaks, Y. Distinguishing between dipoles and field effects in molecular gated transistors. App. Phys. Lett. 2008, 93, 043509. 1–3. [Google Scholar]
- Adamson, A. Physical Chemistry of Surfaces; John Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Lim, H.K.; Fossum, J.G. Threshold voltage of thin-film siliconon insulator (SOI) MOSFET's. IEEE Trans. Electron. Dev. 1983, ED-30, 1244–1251. [Google Scholar]
- Shaya, O.; Shaked, M.; Usherenko, Y.; Halperin, E.; Shalev, G.; Doron, A.; Levy, I.; Rosenwaks, Y. Tracing the mechanism of molecular gated transistors. J. Phys. Chem. 2009, 113, 6163–6168. [Google Scholar]
- Aswal, D.K.; Lenfant, S.; Guerin, D.; Yakhmi, J.V.; Vuillaume, D. Self assembled monolayers on silicon for molecular electronics. Anal. Chim. Acta. 2006, 568, 84–108. [Google Scholar]
- Brzoska, J.B.; Azouz, I.B.; Rondelez, F. Silanization of Solid Substrates: A step toward reproducibility. Langmuir 1994, 10, 4367–4373. [Google Scholar]
- Wasserman, S.R.; Tao, Y.T.; Whitesides, G.M. Structure and reactivity of alkylsiloxane monolayers formed by reaction of alkyltrichlorosilanes on silicon substrates. Langmuir 1989, 5, 1074–1087. [Google Scholar]
- Schwartz, D.K.; Steinberg, S.; Israelachvili, J.; Zasadzinski, J.A.N. Growth of a self-assembled monolayer by fractal aggregation. Phys. Rev. Lett. 1992, 69, 3354–3357. [Google Scholar]
- Denoyel, R.; Glez, J.C.; Trens, P. Grafting γ-aminopropyl triethoxysilane onto silica: consequence on polyacrylic acid adsorption. Colloid Surf. A. 2002, 197, 213–223. [Google Scholar]
- Horr, T.J.; Arora, P.S. Determination of the acid-base properties for 3-amino, 3-chloro and 3-mercaptopropyltrimethoxysilane coatings on silica surfaces by XPS. Colloid Surf. A. 1997, 126, 113–121. [Google Scholar]
- Allen, G.C.; Sorbello, F.; Altavilla, C.; Castorina, A.; Ciliberto, E. Macro-, micro- and nano-investigations on 3-aminopropyltrimethoxysilane self-assembly-monolayers. Thin Solid Films 2005, 483, 306–311. [Google Scholar]
- Petri, D.F.; Wenz, G.; Schunk, P.; Schimmel, T. An improved method for the assembly of amino-terminated monolayers on SiO2 and the vapor deposition of gold layers. Langmuir 1999, 15, 4520–4523. [Google Scholar]
- Siqueira Petri, D.F.; Wenz, G.; Schunk, P.; Schimmel, T. An improved method for the assembly of amino-terminated monolayers on SiO2 and the vapor deposition of gold layers. Langmuir 1999, 15, 4520–4523. [Google Scholar]
- van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A general model to describe the electrostatic potential at electrolyte oxide interfaces. Adv. Coll. Interf. Sci. 1996, 69, 31–62. [Google Scholar]
- Iler, R.K. The Chemistry of Silica; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Notsu, H.; Fukazawa, T.; Tatsuma, T.; Tryk, D.A.; Fujiwara, Y. Hydroxyl groups on boron-doped diamond electrodes and their modification with a silane coupling agent. Electrochem. Solid-State Lett. 2001, 4, H1–H3. [Google Scholar]
- Song, K.S.; Nakamura, Y.; Sasaki, Y.; Degawa, M.; Yang, J.H.; Kawarada, H. pH-sensitive diamond field-effect transistors (FETs) with directly aminated channel surface. Anal. Chim. Acta. 2006, 573, 3–8. [Google Scholar]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar]
- Wilchek, M.; Bayer, E.A. Biotin-binding proteins: overview and prospects. Methods Enzymol. 1990, 184, 49–51. [Google Scholar]
- Shahriar, J. An analytical technique for counteracting drift in ion-selective field effect transistors (ISFETs). IEEE Sens. J. 2004, 4, 795–801. [Google Scholar]


© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shalev, G.; Cohen, A.; Doron, A.; Machauf, A.; Horesh, M.; Virobnik, U.; Ullien, D.; Levy, I. Standard CMOS Fabrication of a Sensitive Fully Depleted Electrolyte-Insulator-Semiconductor Field Effect Transistor for Biosensor Applications. Sensors 2009, 9, 4366-4379. https://doi.org/10.3390/s90604366
Shalev G, Cohen A, Doron A, Machauf A, Horesh M, Virobnik U, Ullien D, Levy I. Standard CMOS Fabrication of a Sensitive Fully Depleted Electrolyte-Insulator-Semiconductor Field Effect Transistor for Biosensor Applications. Sensors. 2009; 9(6):4366-4379. https://doi.org/10.3390/s90604366
Chicago/Turabian StyleShalev, Gil, Ariel Cohen, Amihood Doron, Andrew Machauf, Moran Horesh, Udi Virobnik, Daniela Ullien, and Ilan Levy. 2009. "Standard CMOS Fabrication of a Sensitive Fully Depleted Electrolyte-Insulator-Semiconductor Field Effect Transistor for Biosensor Applications" Sensors 9, no. 6: 4366-4379. https://doi.org/10.3390/s90604366