Interpretation of Absorption Bands in Airborne Hyperspectral Radiance Data
Abstract
:1. Introduction
2. Data and Methods
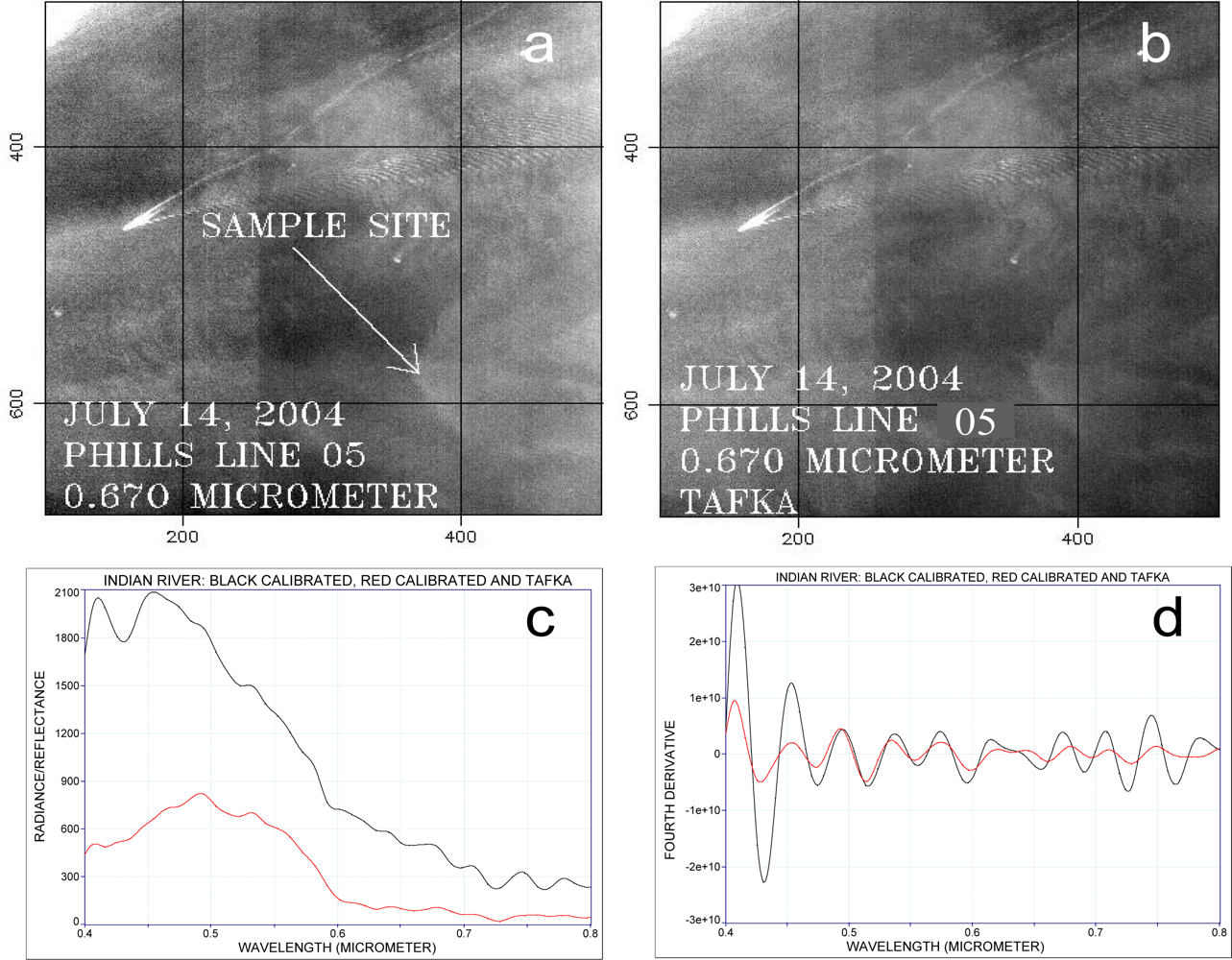
2.1. Remote Sensing Data Acquisition
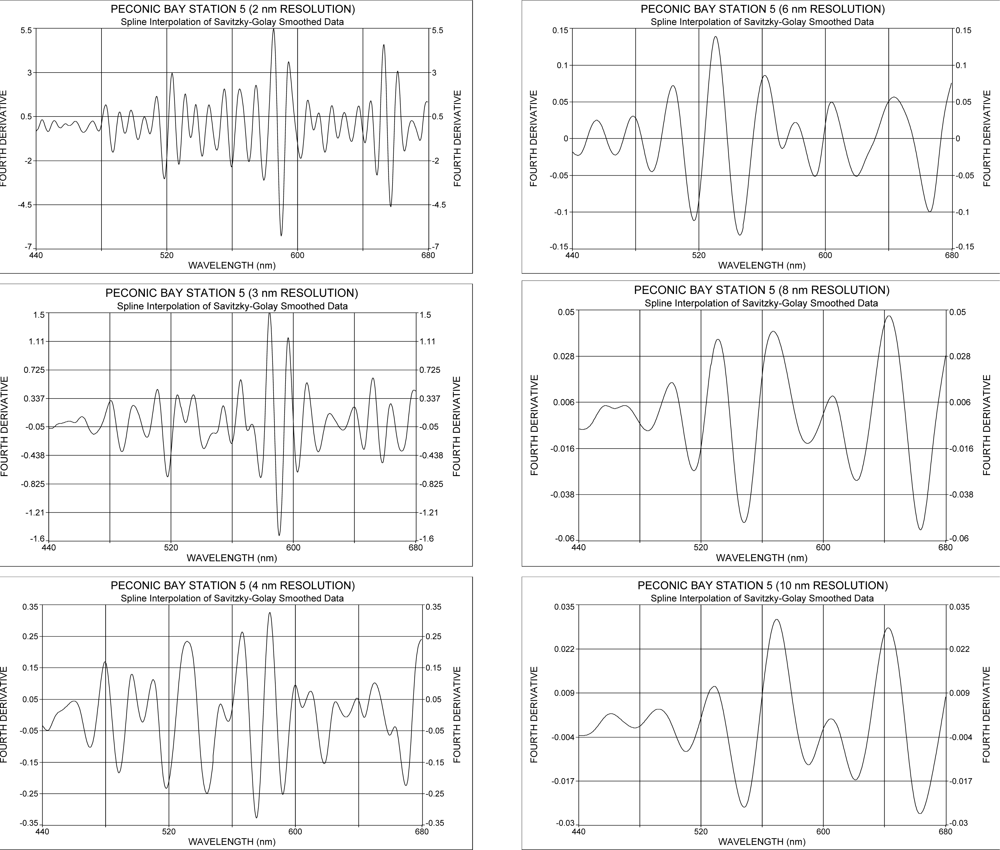
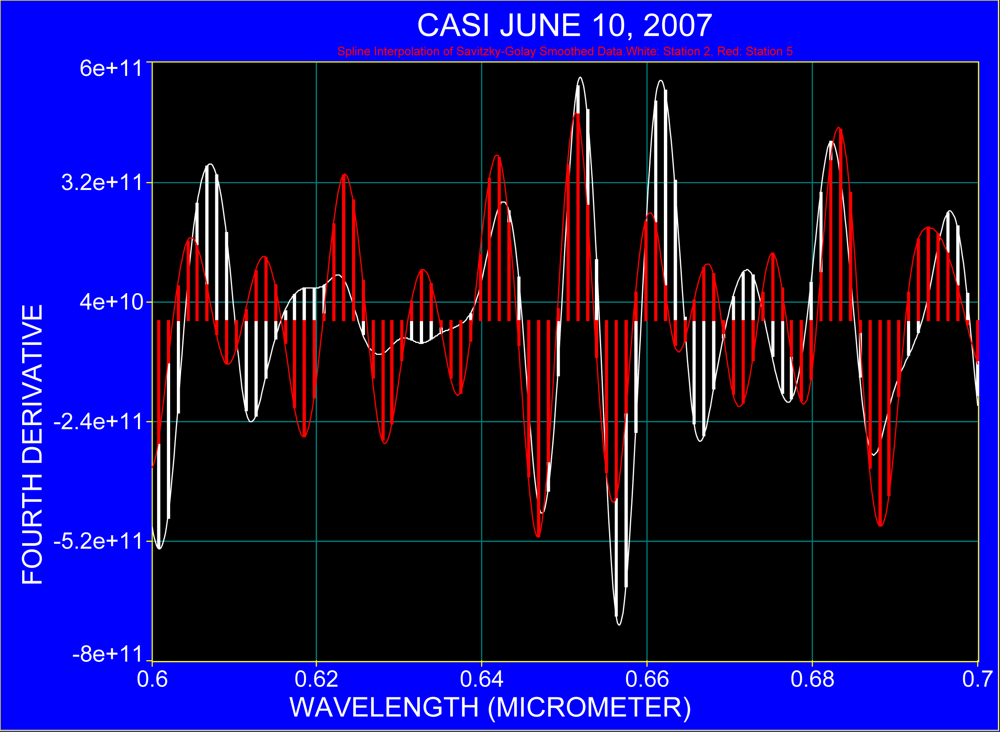
2.2. Use of the Fourth Derivative
2.3. Continuum Removal
3. Results and Discussion
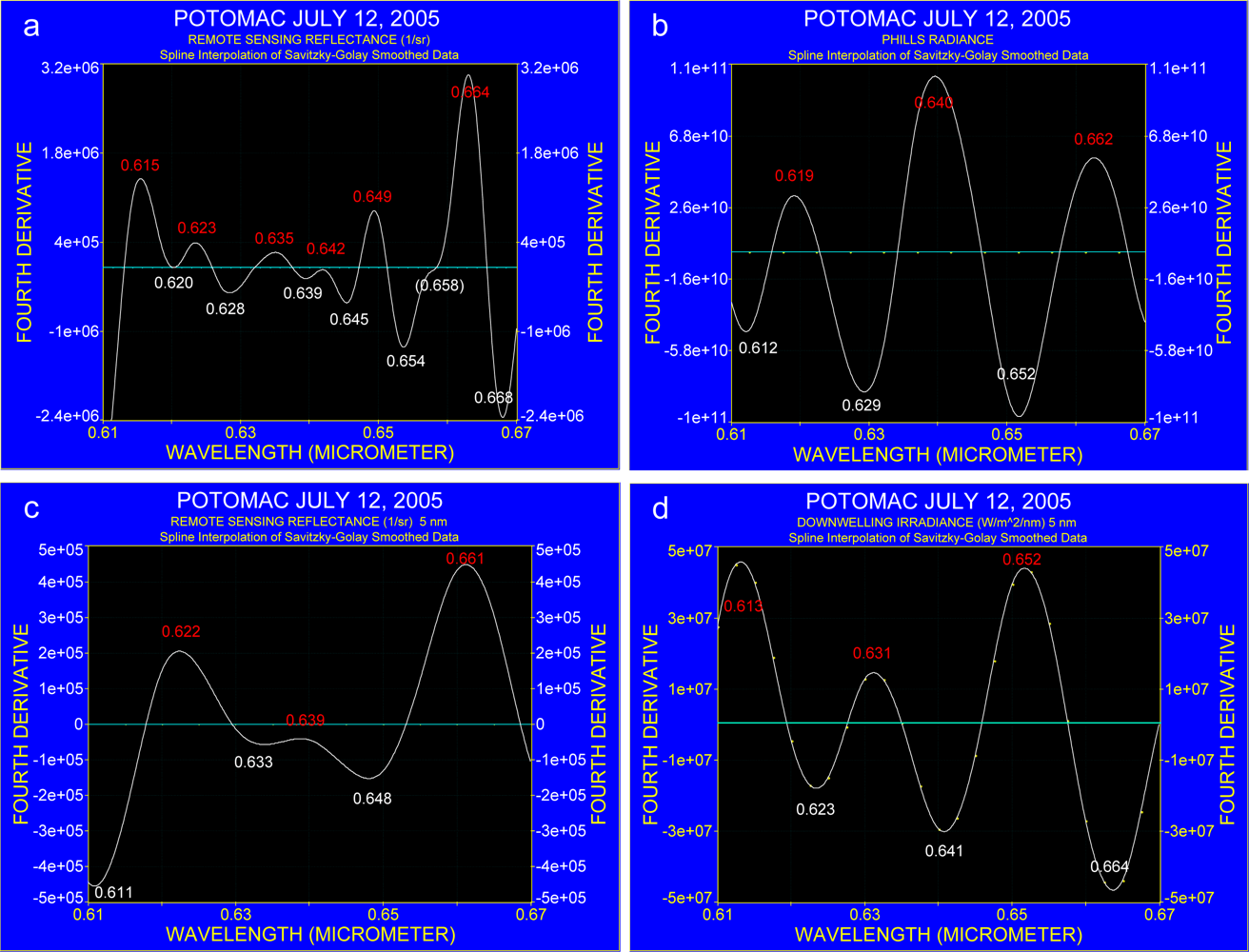
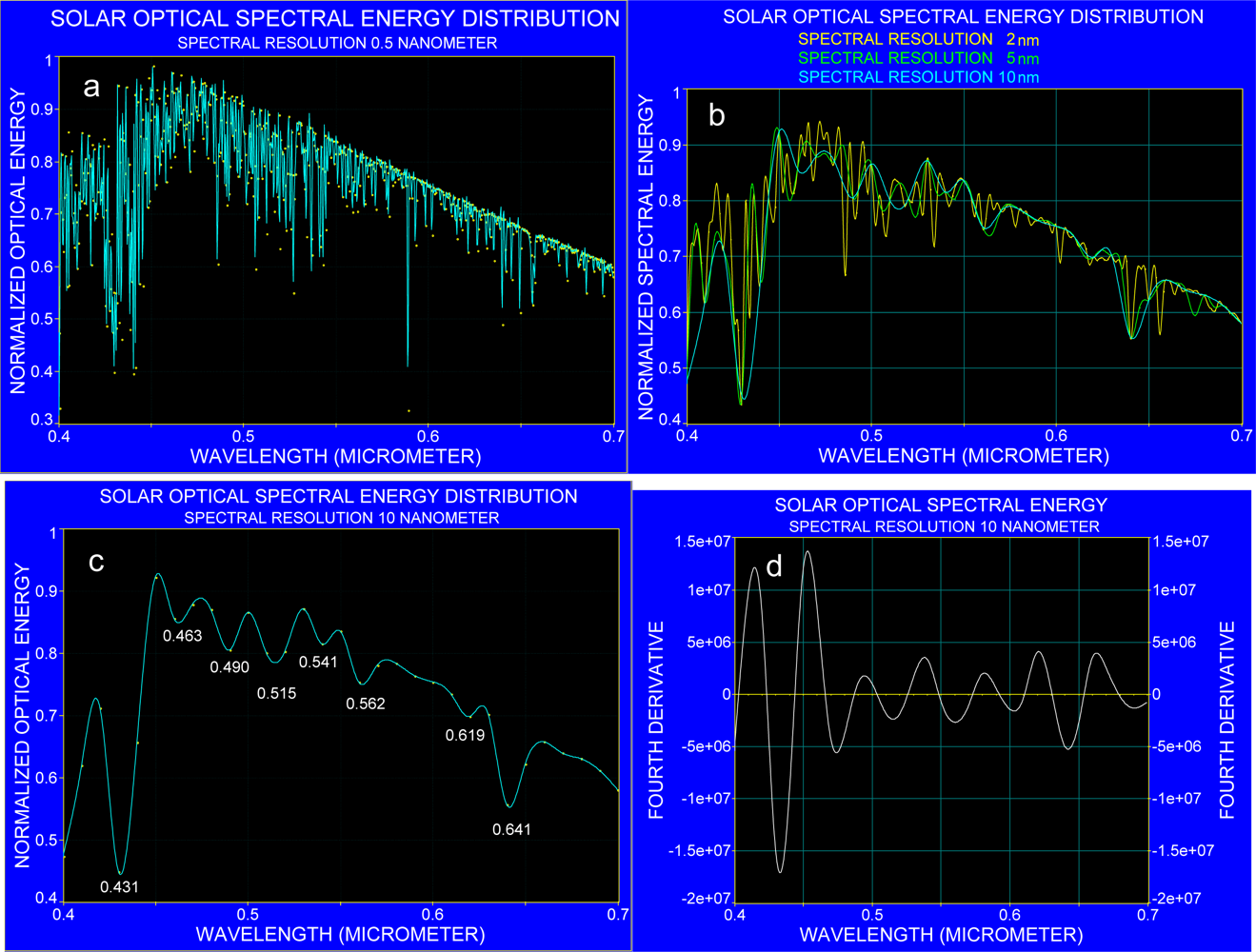
3.1. The Effect of Spectral Resolution on Absorption Band Recognition
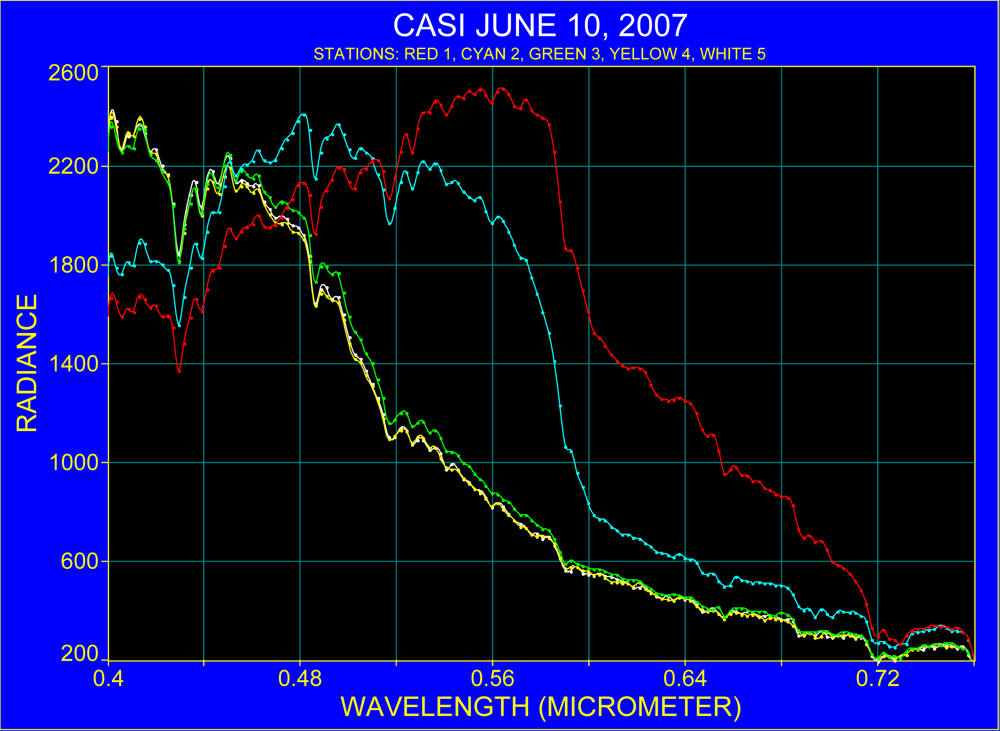
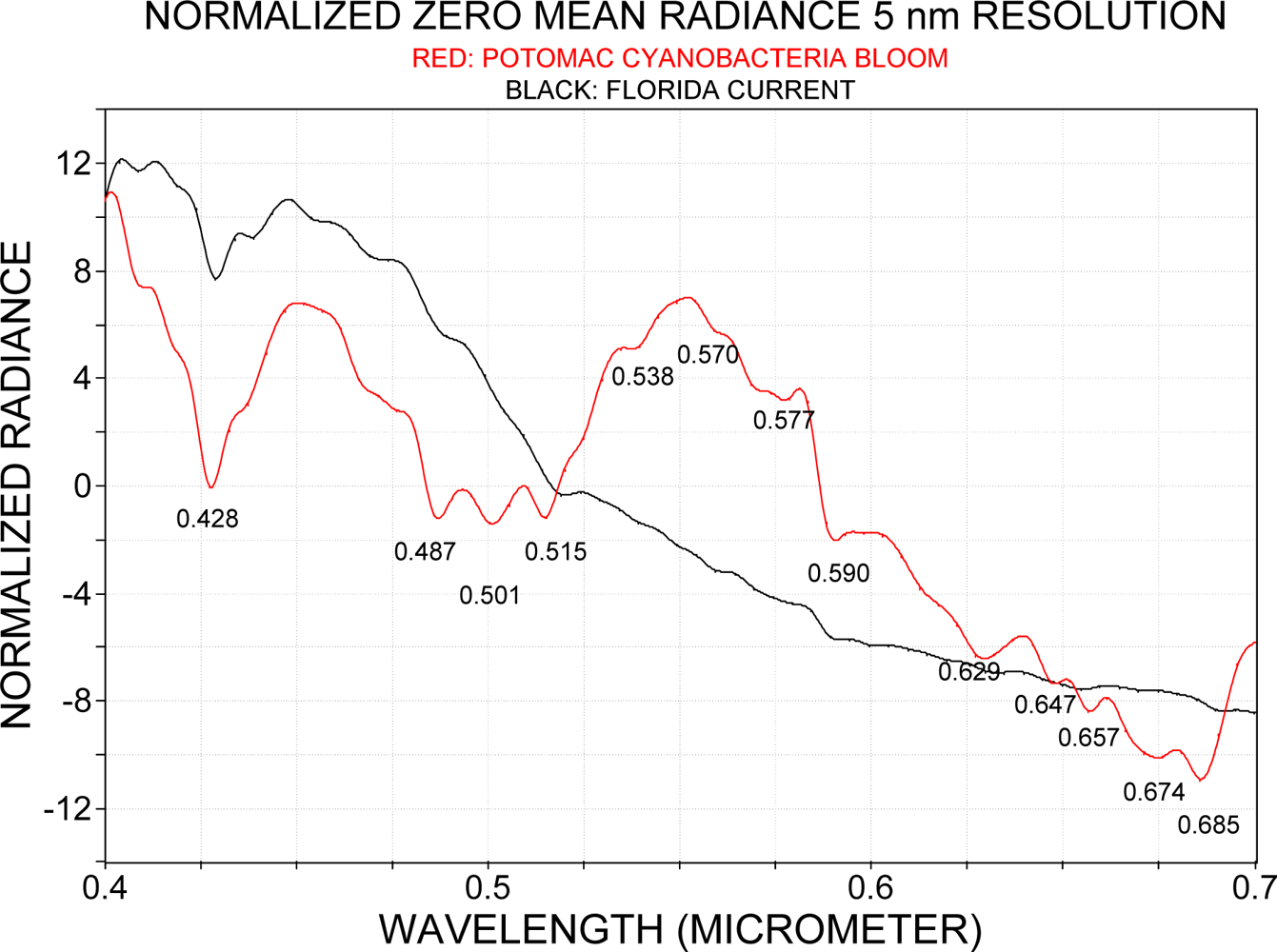
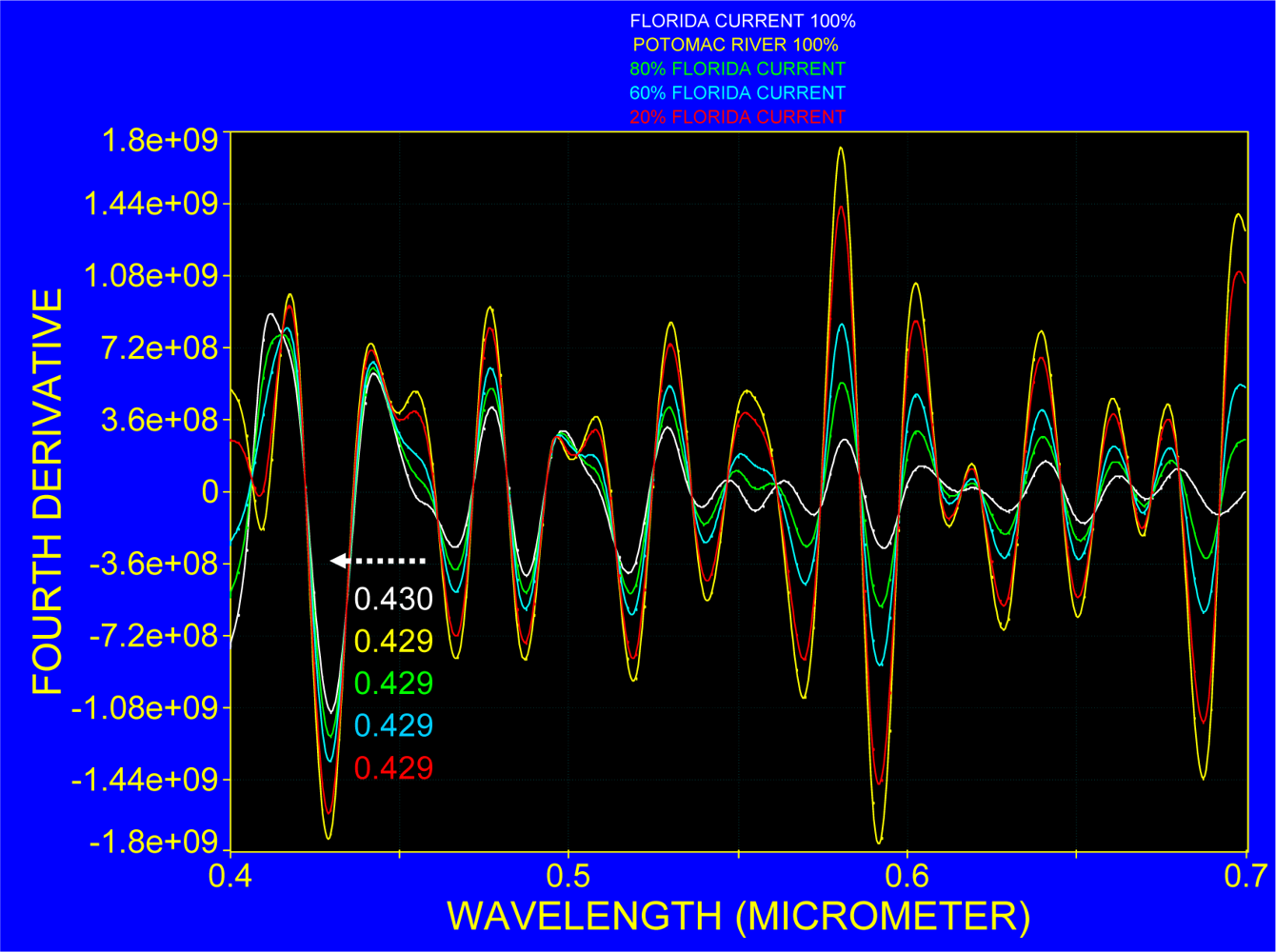
3.2. Spectra over Coastal Waters and the Florida Current
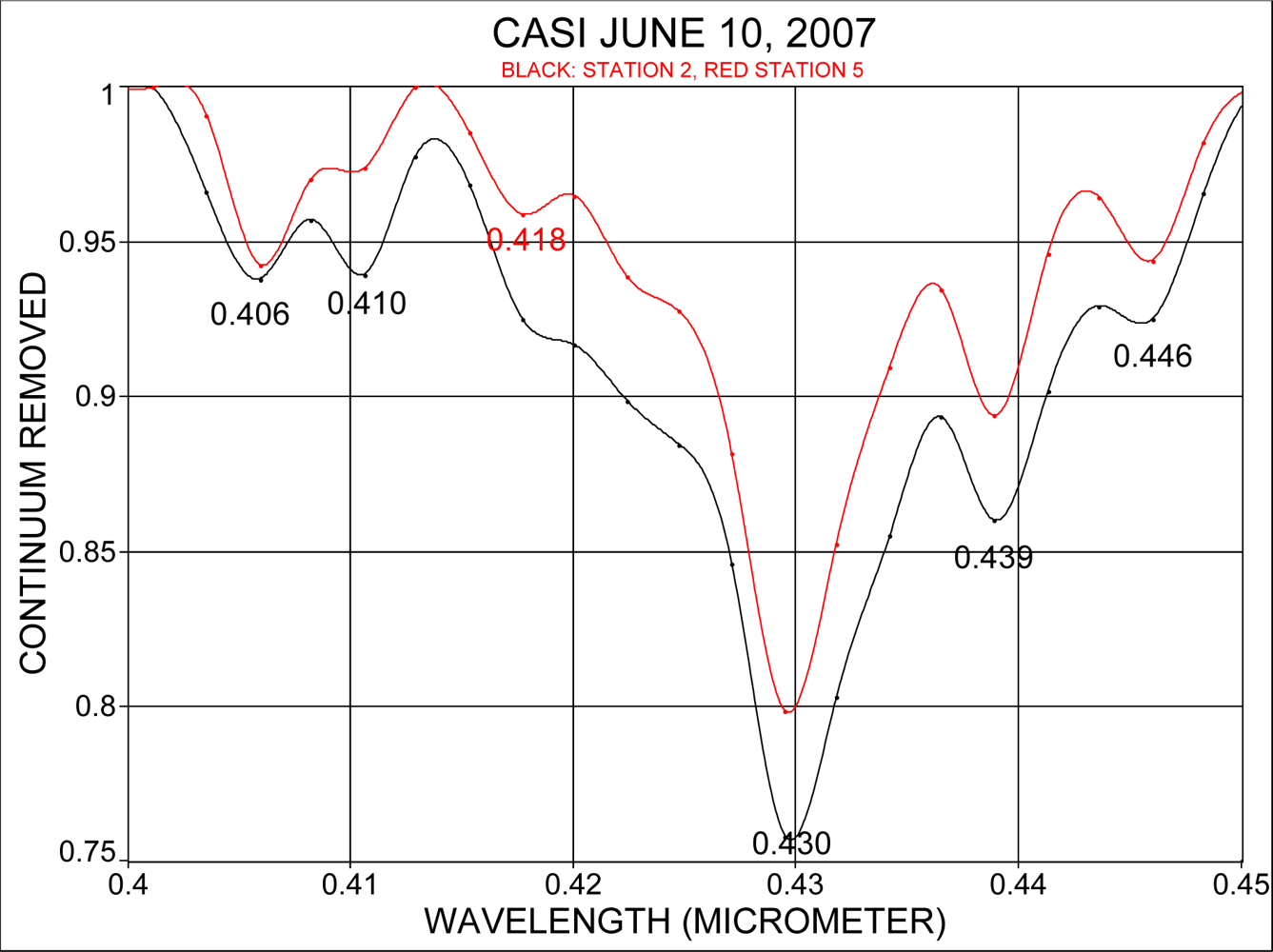
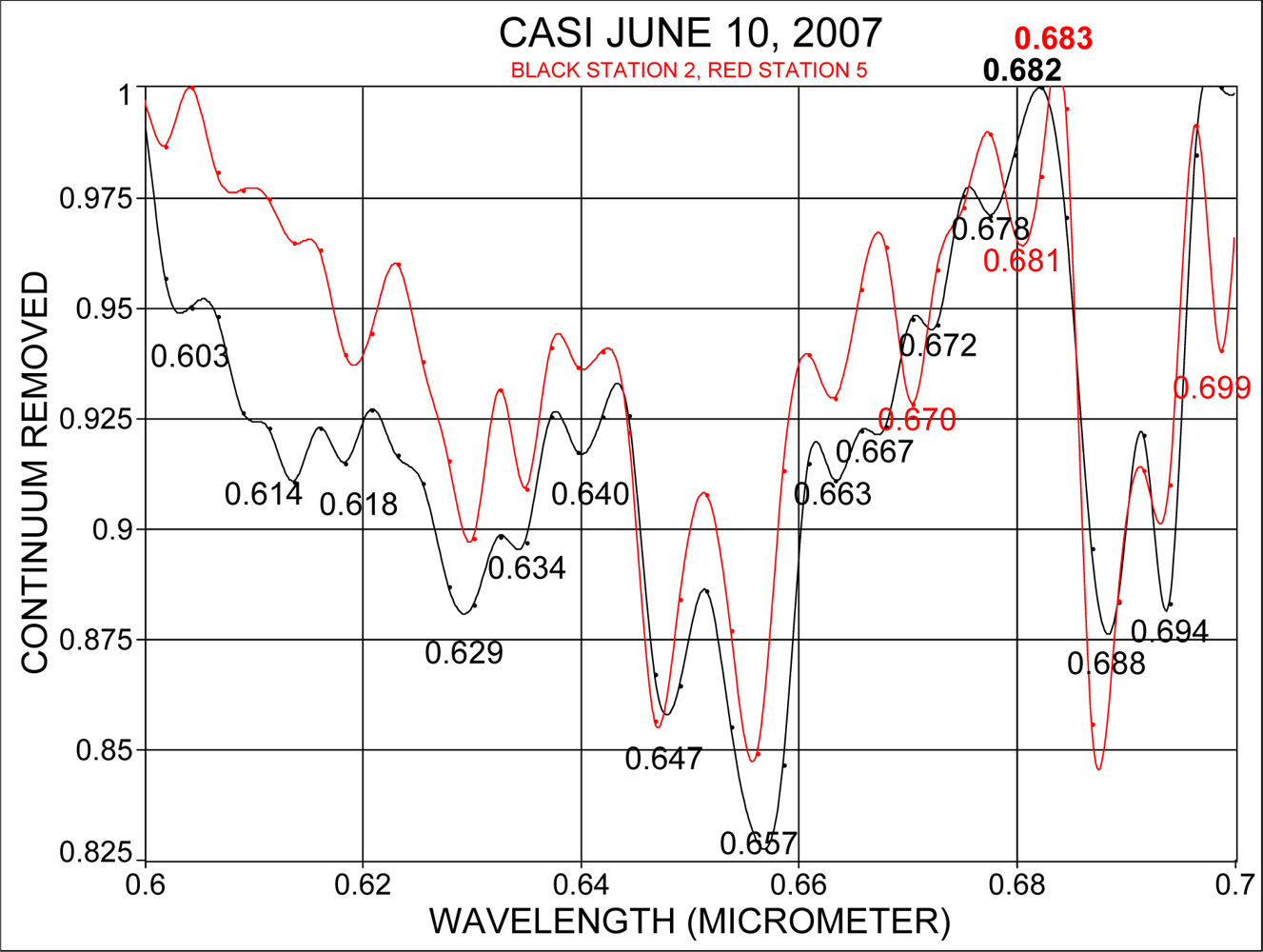
3.3. Estimates of Accuracy of Absorption Band Locations in CASI Data
3.4. Interpretation of Derivative Spectra at 2.4 nm Resolution
3.5. Spectral Interpretation of a Cyanobacteria Bloom Conditions with Reference to Case 2 Water
3.6. Summary and Conclusions
Acknowledgments
References and Notes
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Andréfouet, S.; Hochberg, C.; Che, L.M.; Atkinson, M.J. Airborne hyperspectral detection of microbial mat pigmentation in Rangiroa atoll (French Polynesia). Limnol. Oceanogr 2003, 48, 426–430. [Google Scholar]
- Richardson, L.L.; Buisson, D.; Liu, C.J.; Ambrosia, V. The detection of algal photosynthetic accessory pigments using airborne visible-infrared imaging spectrometer (AVIRIS) spectral data. Mar. Technol. Soc. J 1994, 28, 10–21. [Google Scholar]
- Quibell, G. Estimating chlorophyll concentrations using upwelling radiance for freshwater algal genera. Int. J. Remote Sens 1992, 13, 2611–2621. [Google Scholar]
- Kutser, T. Quantitative detection of chlorophyll in cyanobacterial blooms by satellite remote sensing. Limnol. Oceanogr 2004, 49, 2179–2189. [Google Scholar]
- Kutser, T.; Metsamaa, L.; Strömbeck, N.; Vahtmäe, E. Monitoring cyanobacterial blooms by satellite remote sensing. Estuar. Coast. Shelf Sci 2006, 67, 303–312. [Google Scholar]
- Reinart, A; Kutser, T. Comparison of different satellite sensors in detecting cyanobacterial bloom events in the Baltic Sea. Remote Sens. Environ 2006, 10, 74–85. [Google Scholar]
- Butler, W.L.; Hopkins, D.W. Higher derivative analysis of complex absorption spectra. Photochem. Photobiol 1970, 12, 439–450. [Google Scholar]
- Tsai, T.; Philpot, W. Derivative analysis of hyperspectral data. Remote Sens. Environ 1998, 66, 41–51. [Google Scholar]
- Torrecilla, E.; Aymerich, I.F.; Pons, S.; Piera, J. Effect of spectral resolution in hyperspectral data analysis. IEEE Int. Geosci. Remote Sens. Symp 2007, 910–913. [Google Scholar]
- Roelke, D.L.; Kennedy, C.D.; Weideman, A.D. Use of discriminant and fourth-derivative analyses with high-resolution absorption spectra for phytoplankton research: Limitations at varied signal-to-noise ratio and spectral resolution. Gul. Mex. Sci 1999, 2, 75–86. [Google Scholar]
- Szekielda, K.H.; Gobler, C.; Moshary, F.; Gross, B.; Ahmed, S. Spectral reflectance measurements of estuarine waters. Ocean Dyn 2003, 53, 98–102. [Google Scholar]
- Garver, S.A.; Siegel, D.A. Variability in near-surface particulate absorption spectra: What can a satellite ocean color imager see? Limnol. Oceanogr 1994, 39, 1349–1367. [Google Scholar]
- Hoge, F.; Wright, C.W.; Lyon, P.E.; Swift, R.N.; Yungel, J.K. Satellite retrieval of the absorption coefficient of phytoplankton phycoerythrin pigment: Theory and feasibility status. Appl. Opt 1999, 38, 7431–7441. [Google Scholar]
- Bidigare, R.R.; Ondrusek, M.E.; Morrow, J.H.; Kiefer, D.A. In vivo absorption properties of algal pigments. Proc. SPIE 1990, 1302, 290–302. [Google Scholar]
- Smith, C.M.; Alberte, R.S. Characterization of in vivo absorption features of chlorophyte, phaeophyte and rhodophyte algal species. Mar. Biol 1994, 118, 511–521. [Google Scholar]
- Millie, D.F.; Kirkpatrick, G.J.; Vinyard, B.T. Relating photosynthetic pigments and in vivo optical density spectra to irradiance for the Florida red-tide dinoflagellate Gymnodinium Breve. Mar. Ecol. Prog. Ser 1995, 120, 65–75. [Google Scholar]
- Carra, P.O. Purification and N-terminal analysis of algal biliproteins. Biochem. J 1965, 94, 171–174. [Google Scholar]
- Johnsen, G.; Samset, O.; Granskog, L.; Sakshaug, E. In vivo absorption characteristics in 10 classes of bloom-forming phytoplankton: taxonomic characteristics and responses to photoadaptation by means of discriminant and HPLC analysis. Mar. Ecol. Prog. Ser 1994, 105, 149–157. [Google Scholar]
- Aguirre-Gomez, R.; Weeks, A.R.; Boxall, S.R. The identification of phytoplankton pigments from absorption spectra. Int. J. Remote Sens 2001, 22, 315–338. [Google Scholar]
- Davis, C.O.; J. Bowles, J.; Leathers, R.A.; Korwan, D.; Downes, T.; Snyder, W.A.; Rhea, W.J.; Chen, W.; Fisher, J.; Bissett, W.P.; et al. Ocean PHILLS hyperspectral imager: design, characterization, and calibration. Opt. Express 2002, 10, 210–221. [Google Scholar]
- Hoge, F.; Swift, R. Ocean color spectral variability studies using solar induced chlorophyll fluorescence. Appl. Opt 1987, 26, 18–21. [Google Scholar]
- Gower, J.F.R.; Doerfer, R.; Borstad, G.A. Interpretation of the 685 nm peak in water-leaving radiance spectra in terms of fluorescence, absorption and scattering, and its observation by MERIS. Int. J. Remote Sens 1999, 9, 1771–1786. [Google Scholar]
- Gitelson, A. The peak near 700 nm on radiance spectra of algae and water: relationship of its magnitude and position with chlorophyll concentration. Int. J. Remote Sens 1992, 13, 3367–3373. [Google Scholar]
- Dekker, A.G.; Malthus, T.J.; Goddijn, L.M. Monitoring cyanobacteria in eutrophic waters using airborne imaging spectroscopy and multispectral remote sensing systems. Proceedings of Sixth Australasian Remote Sensing Conference, Wellington, New Zealand, November 2–6, 1992; 1, pp. 204–214.
- Simis, G.H.; Tijden, M.; Hoogveld, H.L.; Gons, H.J. Optical changes associated with cyanobacteria bloom termination by viral lysis. J. Plankton Res 2005, 27, 937–949. [Google Scholar]
- Simis, G.H.; A. Ruiz-Verdú, A.; Dominguez-Gomez, J.A.; Pena-Martines, R.; Peters, S.W.M.; Gons, H.J. Influence of phytoplankton pigment composition on remote sensing of cyanobacterial biomass. Remote Sens. Environ 2007, 106, 414–427. [Google Scholar]
- Lobel, A. SpectroWeb: oscillator strength measurements of atomic absorption lines in the Sun and Procyon. J. Phys. Conf. Ser 2008, 130, 012015. [Google Scholar] [CrossRef]




| Pigment | AbsorptionMAX (μm) | Pigment | AbsorptionMAX (μm) |
|---|---|---|---|
| Chlorophyll a | 0.44116) | B-Phycoerythrin | 0.56518) |
| Chlorophyll c | 0.45420) | Phycoerythrobilin (PE) | 0.56514) |
| Carotenoids | 0.46215) | R-Phycoerythrin (RPE) | 0.566A) |
| Chlorophyll c1 | 0.46617) | 0.566B) | |
| Diadinoxanthin | 0.5681) | ||
| Chlorophyll b | 0.47020) | R-Phycoerythrin I | 0.56818) |
| 0.47015) | Phycoerythrin maxima | 0.570–0.57516) | |
| 0.47116) | Phycoerythrocyanin | 0.575A) | |
| Zeaxanthin | 0.48820) | Chlorophyll c | 0.58917) |
| Fucoxanthin | 0.49015) | 0.58820) | |
| 19’-acylfucoxanthin, Diadinoxanthin | 0.49019) | R-Phycoerythrin | 0.5981) |
| C-Phycocyanin | 0.615A) | ||
| Phycoerythrin rich in PUB | 0.49215) | 0.6151) 0.616B) | |
| 0.49517) | 0.61518) | ||
| Phycourobilin (PUB) | 0.49514) | R-Phycocyanin | 0.61518) |
| R-Phycoerythrin | 0.495A) | Phycocyanin | 0.6306) |
| 0.49818) | 0.6301) | ||
| PE maxima | 0.49916) | R-Phycocyanin | 0.63016) |
| Fucoxanthin | 0.51520) | Chlorophyll c | 0.63520) |
| Fucoxanthin | 0.53520) | Chlorophyll c1, | |
| Phycoerythrin | 0.53816) | Fucoxanthin, | 0.63917) |
| 0.5401) | 19’-acylofucoxanthin | ||
| 0.54018) | Chlorophyll b | 0.65215) | |
| 0.5411) | Cyanobacteria | 0.65013) | |
| 0.5441) | Allophycocyanin | 0.652A) | |
| 0.545A) | 0.6501) | ||
| 0.5451) | 0.652B) | ||
| B-Phycoerythrin (BPE) | 0.545A) | Chlorophyll) | 0.67112) |
| 0.546B) | 0. 67513) | ||
| R-Phycocyanin | 0.55318) | 0.6761) | |
| 0.555A) | Chlorophyll a | 0.67415) | |
| Fucoxanthin | 0.55420) | 0.68316) | |
| C-Phycoerythrin | 0.56318) | 0.67717) | |
| R-Phycoerythrin II | 0.56418) | 0.67520) |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szekielda, K.H.; Bowles, J.H.; Gillis, D.B.; Miller, W.D. Interpretation of Absorption Bands in Airborne Hyperspectral Radiance Data. Sensors 2009, 9, 2907-2925. https://doi.org/10.3390/s90402907
Szekielda KH, Bowles JH, Gillis DB, Miller WD. Interpretation of Absorption Bands in Airborne Hyperspectral Radiance Data. Sensors. 2009; 9(4):2907-2925. https://doi.org/10.3390/s90402907
Chicago/Turabian StyleSzekielda, Karl H., Jeffrey H. Bowles, David B. Gillis, and W. David Miller. 2009. "Interpretation of Absorption Bands in Airborne Hyperspectral Radiance Data" Sensors 9, no. 4: 2907-2925. https://doi.org/10.3390/s90402907




