Preparation and Properties of Various Magnetic Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
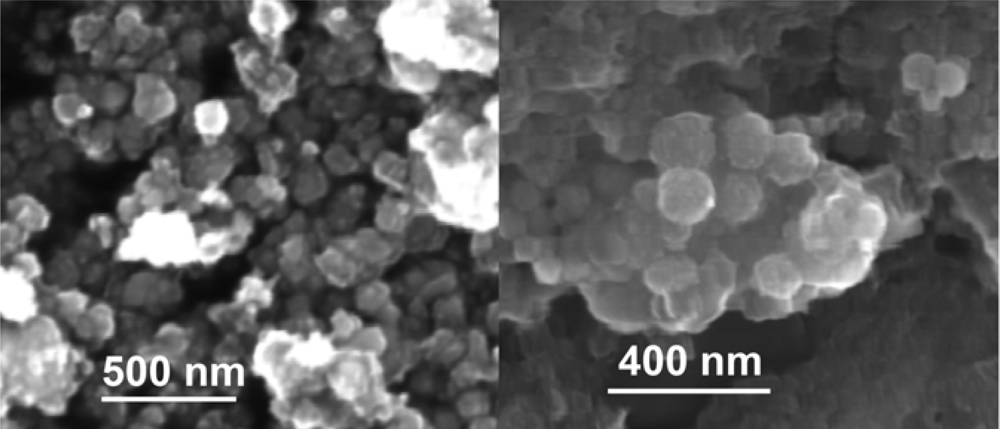
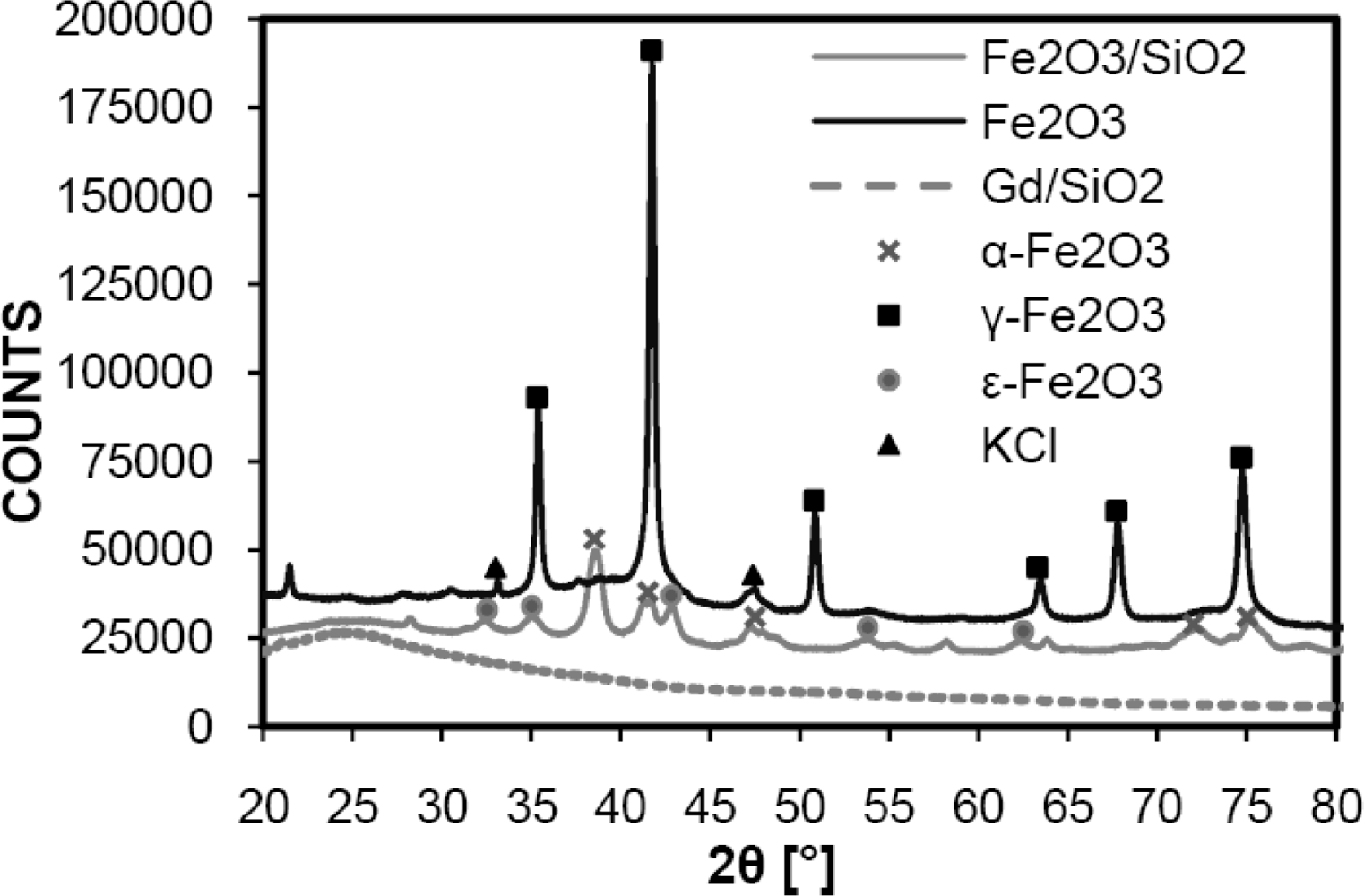
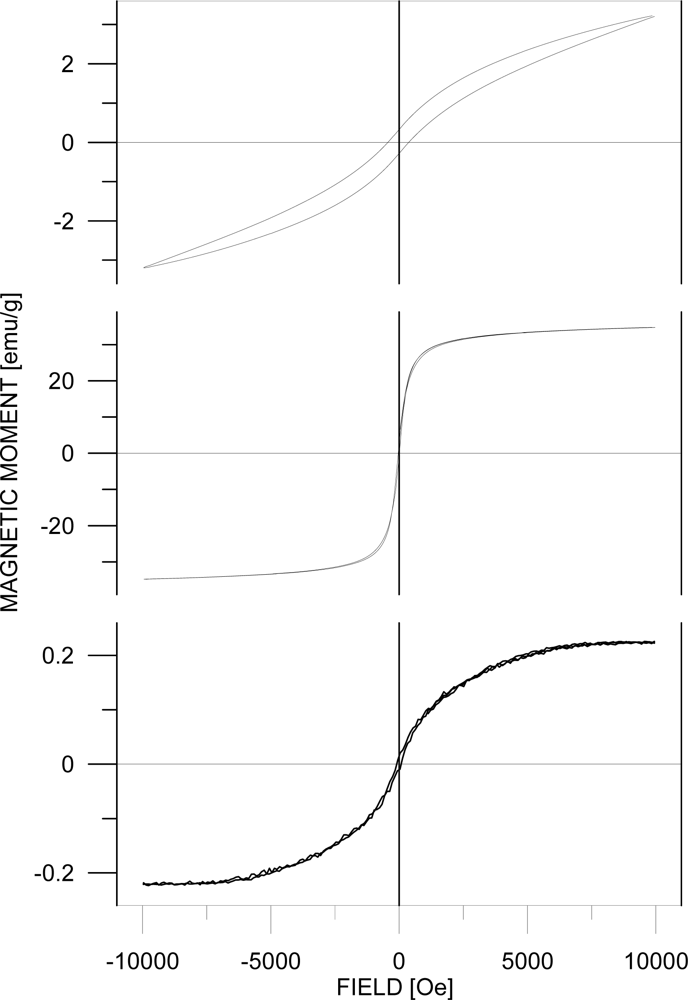
3. Results and Discussion
4. Conclusions
Acknowledgments
References and Notes
- Arruebo, M.; Fernandez-Pacheco, R.; Ibarra, M.R.; Santamaria, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem.-Int. Edit 2007, 46, 1222–1244. [Google Scholar]
- Kumar, C.S.S.R. Biofunctionalization of Nanomaterials; Wiley-VCH: Weinheim, 2005; Volume 1, pp. 72–98. [Google Scholar]
- Hutten, A.; Sudfeld, D.; Ennen, I.; Reiss, G.; Hachmann, W.; Heinzmann, U.; Wojczykowski, K.; Jutzi, P.; Saikaly, W.; Thomas, G. New magnetic nanoparticles for biotechnology. J. Biotechnol 2004, 112, 47–63. [Google Scholar]
- Tucek, J.; Zboril, R.; Petridis, D. Maghemite nanoparticles by view of Mossbauer spectroscopy. J. Nanosci. Nanotechnol 2006, 6, 926–947. [Google Scholar]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar]
- Xie, J.; Xu, C.J.; Xu, Z.C.; Hou, Y.L.; Young, K.L.; Wang, S.X.; Pourmond, N.; Sun, S.H. Linking hydrophilic macromolecules to monodisperse magnetite (Fe3O4) nanoparticles via trichloro-s-triazine. Chem. Mat 2006, 18, 5401–5403. [Google Scholar]
- Tang, D.P.; Yuan, R.; Chai, Y.Q. Direct electrochemical immunoassay based on immobilization of protein-magnetic nanoparticle composites on to magnetic electrode surfaces by sterically enhanced magnetic field force. Biotechnol. Lett 2006, 28, 559–565. [Google Scholar]
- Zboril, R.; Mashlan, M.; Petridis, D. Iron(III) oxides from thermal processes-synthesis, structural and magnetic properties, Mossbauer spectroscopy characterization, and applications. Chem. Mat 2002, 14, 969–982. [Google Scholar]
- Ortega, D.; Garitaonandia, J.S.; Barrera-Solano, C.; Ramirez-Del-Solar, M.; Blanco, E.; Dominguez, M. gamma-Fe2O3/SiO2 nanocomposites for magneto-optical applications: Nanostructural and magnetic properties. J. Non-Cryst. Solids 2006, 352, 2801–2810. [Google Scholar]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: magnetic and transport properties. J. Phys. D-Appl. Phys 2002, 35, R15–R42. [Google Scholar]
- Morales, M.A.; Finotelli, P.V.; Coaquira, J.A.H.; Rocha-Leao, M.H.M.; Diaz-Aguila, C.; Baggio-Saitovitch, E.M.; Rossi, A.M. In situ synthesis and magnetic studies of iron oxide nanoparticles in calcium-alginate matrix for biomedical applications. Mater. Sci. Eng. C-Biomimetic Supramol. Syst 2008, 28, 253–257. [Google Scholar]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W.H. Synthesis and characterization of silica-coated iron oxide nanoparticles in microemulsion: The effect of nonionic surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar]
- De Palma, R.; Trekker, J.; Peeters, S.; Van Bael, M.J.; Bonroy, K.; Wirix-Speetjens, R.; Reekmans, G.; Laureyn, W.; Borghs, G.; Maes, G. Surface modification of gamma-Fe2O3@ SiO2 magnetic nanoparticles for the controlled interaction with biomolecules. J. Nanosci. Nanotechnol 2007, 7, 4626–4641. [Google Scholar]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Kume, A.; Muramatsu, K.; Kiyohara, Y.; Akiyama, Y.; Osaka, T. Preparation of Human Immune Effector T Cells Containing Iron-Oxide Nanoparticles. Biotechnol. Bioeng 2008, 101, 1123–1128. [Google Scholar]
- Khalil, K.M.S.; Mahmoud, H.A.; Ali, T.T. Direct formation of thermally stabilized amorphous mesoporous Fe2O3/SiO2 nanocomposites by hydrolysis of aqueous iron (III) nitrate in sols of spherical silica particles. Langmuir 2008, 24, 1037–1043. [Google Scholar]
- Srinivasan, B.; Huang, X.F. Functionalization of magnetic nanoparticles with organic molecules: Loading level determination and evaluation of linker length effect on immobilization. Chirality 2008, 20, 265–277. [Google Scholar]
- Cannas, C.; Concas, G.; Gatteschi, D.; Musinu, A.; Piccaluga, G.; Sangregorio, C. How to tailor maghemite particle size in gamma-Fe2O3-SiO2 nanocomposites. J. Mater. Chem 2002, 12, 3141–3146. [Google Scholar]
- Ichiyanagi, Y.; Moritake, S.; Taira, S.; Setou, M. Functional magnetic nanoparticles for medical application. J. Magn. Magn. Mater 2007, 310, 2877–2879. [Google Scholar]
- Fabrik, I.; Krizkova, S.; Huska, D.; Adam, V.; Hubalek, J.; Trnkova, L.; Eckschlager, T.; Kukacka, J.; Prusa, R.; Kizek, R. Employment of electrochemical techniques for metallothionein determination in tumor cell lines and patients with a tumor disease. Electroanalysis 2008, 20, 1521–1532. [Google Scholar]
- Liu, H.L.; Sonn, C.H.; Wu, J.H.; Lee, K.M.; Kim, Y.K. Synthesis of streptavidin-FITC-conjugated core-shell Fe3O4-Au nanocrystals and their application for the purification of CD4(+) lymphocytes. Biomaterials 2008, 29, 4003–4011. [Google Scholar]
- Gerion, D.; Herberg, J.; Bok, R.; Gjersing, E.; Ramon, E.; Maxwell, R.; Kurhanewicz, J.; Budinger, T.F.; Gray, J.W.; Shuman, M.A.; Chen, F.F. Paramagnetic silica-coated nanocrystals as an advanced MRI contrast agent. J. Phys. Chem. C 2007, 111, 12542–12551. [Google Scholar]
- Ichiyanagi, Y.; Kimishima, Y. Structural, magnetic and thermal characterizations of Fe2O3 nanoparticle systems. J. Therm. Anal 2002, 69, 919–923. [Google Scholar]
- Santra, S.; Bagwe, R.P.; Dutta, D.; Stanley, J.T.; Walter, G.A.; Tan, W.; Moudgil, B.M.; Mericle, R.A. Synthesis and characterization of fluorescent radio-opaque and paramagnetic silica nanoparticles for multimodal bioimaging applications. Adv. Mater 2005, 17, 2165–2169. [Google Scholar]
- Ichiyanagi, Y.; Uozumi, T.; Kimishima, Y. Magnetic properties of Fe2O3 nanoparticles. Trans. Mat. Res. Soc. Jpn 2001, 26, 1097–1100. [Google Scholar]
- Ding, Y.; Morber, J.R.; Snyder, R.L.; Wang, Z.L. Nanowire structural evolution from Fe3O4 to epsilon-Fe2O3. Adv. Funct. Mater 2007, 17, 1172–1178. [Google Scholar]
- Long, G.J.; Grandjean, F. Mössbauer Spectroscopy Applied to Magnetism and Materials Science; Plenum Press: New York, 1993; Volume 1, pp. 115–159. [Google Scholar]
- Tronc, E.; Chaneac, C.; Jolivet, J.P. Structural and magnetic characterization of epsilon-Fe2O3. J. Solid State Chem 1998, 139, 93–104. [Google Scholar]
- Machala, L.; Zboril, R.; Gedanken, A. Amorphous iron(III) oxide - A review. J. Phys. Chem. B 2007, 111, 4003–4018. [Google Scholar]
- Popovici, M.; Gich, M.; Niznansky, D.; Roig, A.; Savii, C.; Casas, L.; Molins, E.; Zaveta, K.; Enache, C.; Sort, J.; de Brion, S.; Chouteau, G.; Nogues, J. Optimized synthesis of the elusive epsilon-Fe2O3 phase via sol-gel chemistry. Chem. Mat 2004, 16, 5542–5548. [Google Scholar]
- Tartaj, P.; Serna, C.J. Microemulsion-assisted synthesis of tunable superparamagnetic composites. Chem. Mat 2002, 14, 4396–4402. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses; VCH: New York, 1996; p. 117. [Google Scholar]
- Wohlfarth, E.P. Ferromagnetic Materials; North-Holland: Amsterdam, 1980; Volume 2, p. 442. [Google Scholar]
- Wohlfarth, E.P. Ferromagnetic Materials; North-Holland: Amsterdam, 1980; Volume 2, p. 512. [Google Scholar]
- O’Shea, M.J.; Perera, P. Influence of nanostructure (layers and particles) on the magnetism of rare-earth materials. J. Appl. Phys 1999, 85, 4322–4324. [Google Scholar]

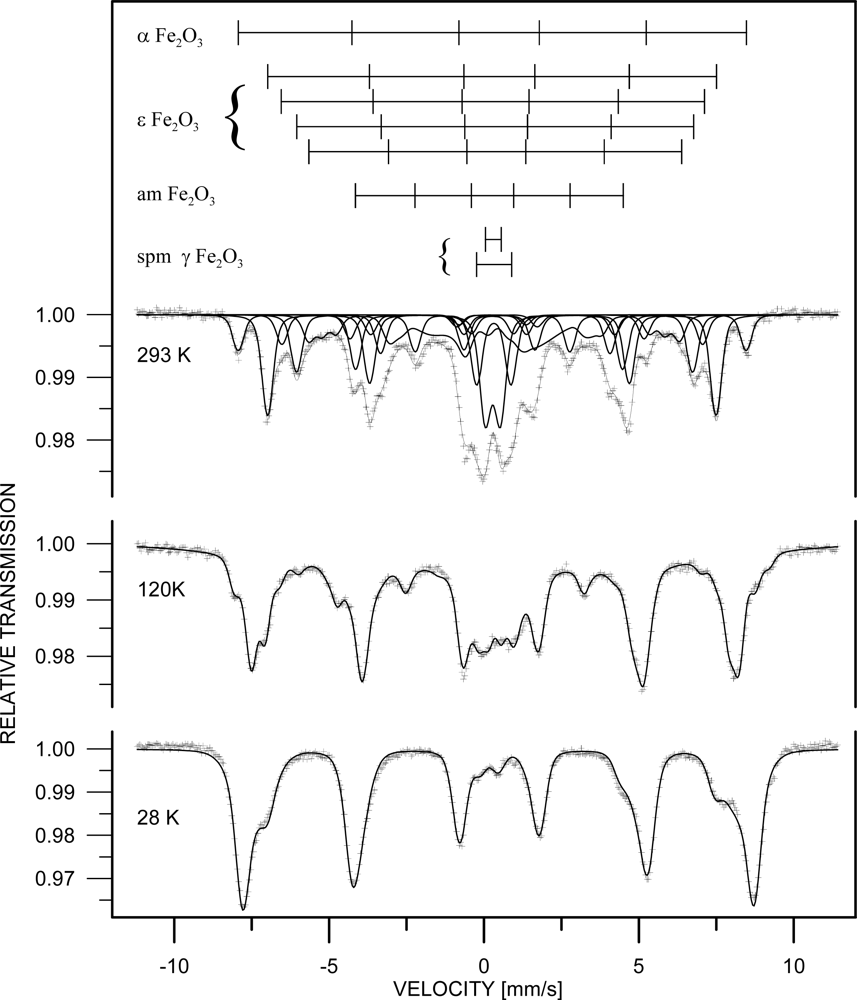
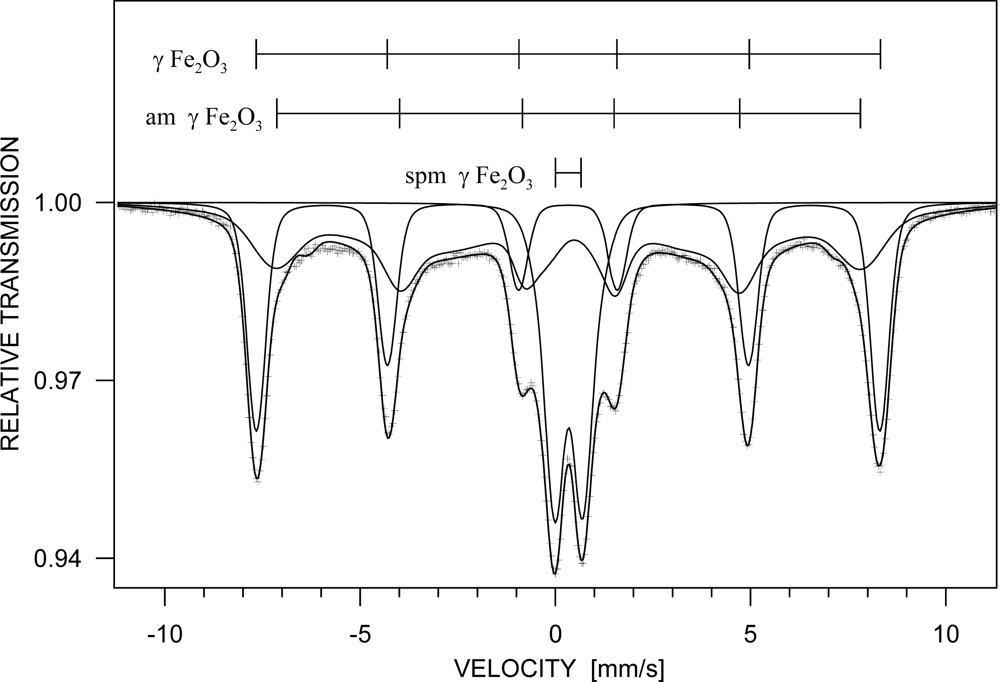
| Phase | c [%] | Component | I | Bhf [T] | δ [mm/s] | εQ [mm/s] | Δw [T] |
|---|---|---|---|---|---|---|---|
| ε-Fe2O3 | 51±1 | 1st sextet | 0.42±0.01 | 45.0±0.1 | 0.37±0.01 | −0.25±0.01 | - |
| 2nd sextet | 0.12±0.01 | 42.2±0.2 | 0.28±0.03 | −0.02±0.01 | - | ||
| 3rd sextet | 0.24±0.01 | 39.7±0.2 | 0.35±0.01 | −0.03±0.01 | - | ||
| 4th sextet | 0.22±0.01 | 26.8±0.1 | 0.22±0.01 | −0.11±0.01 | - | ||
| α-Fe2O3 | 8±1 | sextet | 1.00 | 51.3±0.1 | 0.34±0.01 | −0.17±0.02 | - |
| γ-Fe2O3 spm. | 19±1 | 1st doublet | 0.60±0.01 | - | 0.28±0.01 | 0.97±0.03 | - |
| 2nd doublet | 0.40±0.01 | - | 0.32±0.01 | 2.21±0.04 | - | ||
| am. | 22±1 | sextet | 1.00 | 11.9±0.3 | 0.35±0.04 | −0.20±0.06 | 7.3±0.8 |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Drbohlavova, J.; Hrdy, R.; Adam, V.; Kizek, R.; Schneeweiss, O.; Hubalek, J. Preparation and Properties of Various Magnetic Nanoparticles. Sensors 2009, 9, 2352-2362. https://doi.org/10.3390/s90402352
Drbohlavova J, Hrdy R, Adam V, Kizek R, Schneeweiss O, Hubalek J. Preparation and Properties of Various Magnetic Nanoparticles. Sensors. 2009; 9(4):2352-2362. https://doi.org/10.3390/s90402352
Chicago/Turabian StyleDrbohlavova, Jana, Radim Hrdy, Vojtech Adam, Rene Kizek, Oldrich Schneeweiss, and Jaromir Hubalek. 2009. "Preparation and Properties of Various Magnetic Nanoparticles" Sensors 9, no. 4: 2352-2362. https://doi.org/10.3390/s90402352





