Recent Advances in Nanotechnology Applied to Biosensors
Abstract
:1. Introduction
2. The Use of Nanomaterials in Biosensors
2.1. The Use of Gold Nanoparticles in Biosensors
2.2. The Use of CNTs in Biosensors
2.3. The Use of Magnetic Nanoparticales in Biosensor
2.4. The Use of QDs in Biosensors
2.5. The Use of Other Nanomaterials in Biosensors
3. Potential Application of Nanomaterials-Based Biosensors
3.1. Nanomaterials-Based Biosensors for the Detection of Glucose
3.2. Nanomaterials-Based Biosensors for the Detection of DNA and Protein
3.3. Nanomaterials-Based Biosensors for the Detection of Other Molecules
4. Challenges and Prospects
Acknowledgments
References and Notes
- Turner, A.P.F.; Karube, I.; Wilson, G.S. Biosensors - Fundamentals and Applications.; Oxford University Press: New York, NY, USA, 1987; pp. 719–800. [Google Scholar]
- Kricka, L.J. Molecular and Ionic Recognition by Biological Systems. In Chemical Sensors; Edmonds, T.E., Ed.; Blackie and Sons: Glasgow, U.K., 1988; pp. 3–14. [Google Scholar]
- Buch, R.M.; Rechnitz, G.A. Intact chemoreceptor-based biosensors: responses and analytical limits. Biosensors 1989, 4, 215–230. [Google Scholar]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdev. 2008, 10, 321–328. [Google Scholar]
- Cui, D. Advances and prospects on biomolecules functionalized carbon nanotubes. J. Nanosci. Nanotechnol. 2007, 7, 1298–1314. [Google Scholar]
- Pan, B.; Cui, D.; Ozkan, C.S.; Ozkan, M.; Xu, P.; Huang, T.; Liu, F.; Chen, H.; Li, Q.; He, R.; Gao, F. Effects of carbon nanotubes on photoluminescence properties of quantum dots. J. Phys. Chem. C 2008, 112, 939–944. [Google Scholar]
- Pan, B.; Cui, D.; Xu, P.; Li, Q.; Huang, T.; He, R.; Gao, F. Study on interaction between gold nanorod and bovine serum albumin. Colloids Surface A 2007, 295, 217–222. [Google Scholar]
- Cui, D.; Tian, F.; Coyer, S.R.; Wang, J.; Pan, B.; Gao, F.; He, R.; Zhang, Y. Effects of antisense-myc-conjugated single-walled carbon nanotubes on HL-60 cells. J. Nanosci. Nanotechnol. 2007, 7, 1639–1646. [Google Scholar]
- Pan, B.; Cui, D.; Sheng, Y.; Ozkan, C.; Gao, F.; He, R.; Li, Q.; Xu, P.; Huang, T. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007, 67, 8156–8163. [Google Scholar]
- You, X.; He, R.; Gao, F.; Shao, J.; Pan, B.; Cui, D. Hydrophilic high-luminescent magnetic nanocomposites. Nanotechnology 2007, 18, 035701:1–035701:5. [Google Scholar]
- Yang, D.; Cui, D. Advances and Prospects of Gold Nanorods. Chem. Asian J. 2008, 3, 2010–2022. [Google Scholar]
- Bryant, G.W.; de Abajo, F.J.G.; Aizpurua, J. Mapping the plasmon resonances of metallic nanoantennas. Nano Lett. 2008, 8, 631–636. [Google Scholar]
- Patolsky, F.; Timko, B.P.; Zheng, G.; Lieber, C.M. Nanowire-based nanoelectronic devices in the life sciences. MRS Bull. 2007, 32, 142–149. [Google Scholar]
- Jena, B.K.; Raj, C.R. Optical sensing of biomedically important polyionic drugs using nano-sized gold particles. Biosens. Bioelectron. 2008, 23, 1285–1290. [Google Scholar]
- Zhao, W.; Chiuman, W.; Lam, J.C.F.; Brook, M.A.; Li, Y. Simple and rapid colorimetric enzyme sensing assays using non-crosslinking gold nanoparticle aggregation. Chem. Commun. 2007, 3729–3731. [Google Scholar]
- Wei, H.; Li, B.; Li, J.; Wang, E.; Dong, S. Simple and sensitive aptamer-based colorimetric sensing of protein using unmodified gold nanoparticle probes. Chem. Commun. 2007, 3735–3737. [Google Scholar]
- Liang, K.Z.; Qi, J.S.; Mu, W.J.; Chen, Z.G. Biomolecules/gold nanowires-doped sol-gel film for label-free electrochemical immunoassay of testosterone. J. Biochem. Biophys. Meth. 2008, 70, 1156–1162. [Google Scholar]
- He, X.; Yuan, R.; Chai, Y.; Shi, Y. A sensitive amperometric immunosensor for carcinoembryonic antigen detection with porous nanogold film and nano-Au/chitosan composite as immobilization matrix. J. Biochem. Biophys. Meth. 2008, 70, 823–829. [Google Scholar]
- Chai, R.; Yuan, R.; Chai, Y.; Ou, C.; Cao, S.; Li, X. Amperometric immunosensors based on layer-by-layer assembly of gold nanoparticles and methylene blue on thiourea modified glassy carbon electrode for determination of human chorionic gonadotrophin. Talanta 2008, 74, 1330–1336. [Google Scholar]
- Li, N.B.; Park, J.H.; Park, K.; Kwon, S.J.; Shin, H.; Kwak, J. Characterization and electrocatalytic properties of Prussian blue electrochemically deposited on nano-Au/PAMAM dendrimer-modified gold electrode. Biosens. Bioelectron. 2008, 23, 1519–1526. [Google Scholar]
- Gao, F.; Yuan, R.; Chai, Y.; Chen, S.; Cao, S.; Tang, M. Amperometric hydrogen peroxide biosensor based on the immobilization of HRP on nano-Au/Thi/poly (p-aminobenzene sulfonic acid)-modified glassy carbon electrode. J. Biochem. Biophys. Meth. 2007, 70, 407–413. [Google Scholar]
- Shi, A.W.; Qu, F.L.; Yang, M.H.; Shen, G.L.; Yu, R.Q. Amperometric H2O2 biosensor based on poly-thionine nanowire/HRP/nano-Au-modified glassy carbon electrode. Sens. Actuat. B 2008, 129, 779–783. [Google Scholar]
- Deng, L.; Wang, Y.; Shang, L.; Wen, D.; Wang, F.; Dong, S. A sensitive NADH and glucose biosensor tuned by visible light based on thionine bridged carbon nanotubes and gold nanoparticles multilayer. Biosens. Bioelectron. 2008, 24, 957–963. [Google Scholar]
- Cui, R.; Huang, H.; Yin, Z.; Gao, D.; Zhu, J.J. Horseradish peroxidase-functionalized gold nanoparticle label for amplified immunoanalysis based on gold nanoparticles/carbon nanotubes hybrids modified biosensor. Biosens. Bioelectron. 2008, 23, 1666–1673. [Google Scholar]
- Wang, M.; Li, Z. Nano-composite ZrO2/Au film electrode for voltammetric detection of parathion. Sens. Actuat. B 2008, 133, 607–612. [Google Scholar]
- Wasowicz, M.; Viswanathan, S.; Dvornyk, A.; Grzelak, K.; Kłudkiewicz, B.; Radecka, H. Comparison of electrochemical immunosensors based on gold nano materials and immunoblot techniques for detection of histidine-tagged proteins in culture medium. Biosens. Bioelectron. 2008, 24, 284–289. [Google Scholar]
- Nusz, G.J.; Marinakos, S.M.; Curry, A.C.; Dahlin, A.; Hook, F.; Wax, A.; Chilkoti, A. Label-free plasmonic detection of biomolecular binding by a single gold nanorod. Anal. Chem. 2008, 80, 984–989. [Google Scholar]
- York, J.; Spetzler, D.; Xiong, F.; Frasch, W.D. Single-molecule detection of DNA via sequence-specific links between F 1-ATPase motors and gold nanorod sensors. Lab Chip 2008, 8, 415–419. [Google Scholar]
- Pan, B.; Cui, D.; He, R.; Gao, F.; Zhang, Y. Covalent attachment of quantum dot on carbon nanotubes. Chem. Phys. Lett. 2006, 417, 419–424. [Google Scholar]
- Cui, D.; Tian, F.; Kong, Y.; Titushikin, I.; Gao, H. Effects of single-walled carbon nanotubes on the polymerase chain reaction. Nanotechnology 2004, 15, 154–157. [Google Scholar]
- Joshi, P.P.; Merchant, S.A.; Wang, Y.; Schmidtke, D.W. Amperometric biosensors based on redox polymer-carbon nanotube-enzyme composites. Anal. Chem. 2005, 77, 3183–3188. [Google Scholar]
- Teh, K.S.; Lin, L. MEMS sensor material based on polypyrrole-carbon nanotube nanocomposite: Film deposition and characterization. J. Micromech. Microeng. 2005, 15, 2019–2027. [Google Scholar]
- Qi, P.; Vermesh, O.; Grecu, M.; Javey, A.; Wang, Q.; Dai, H.; Peng, S.; Cho, K.J. Toward large arrays of multiplex functionalized carbon nanotube sensors for highly sensitive and selective molecular detection. Nano Lett. 2003, 3, 347–351. [Google Scholar]
- Li, G.; Xu, H.; Huang, W.; Wang, Y.; Wu, Y.; Parajuli, R. A pyrrole quinoline quinone glucose dehydrogenase biosensor based on screen-printed carbon paste electrodes modified by carbon nanotubes. Meas. Sci. Technol. 2008, 19–26. [Google Scholar]
- Chen, X.; Chen, J.; Deng, C.; Xiao, C.; Yang, Y.; Nie, Z.; Yao, S. Amperometric glucose biosensor based on boron-doped carbon nanotubes modified electrode. Talanta 2008, 76, 763–767. [Google Scholar]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Wong Shi Kam, N.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 4984–4989. [Google Scholar]
- Zeng, J.; Wei, W.; Liu, X.; Wang, Y.; Luo, G. A simple method to fabricate a Prussian Blue nanoparticles/carbon nanotubes/poly(1,2-diaminobenzene) based glucose biosensor. Microchim. Acta 2008, 160, 261–267. [Google Scholar]
- Wu, L.; Lei, J.; Zhang, X.; Ju, H. Biofunctional nanocomposite of carbon nanofiber with water-soluble porphyrin for highly sensitive ethanol biosensing. Biosens. Bioelectron. 2008, 24, 644–649. [Google Scholar]
- Bai, H.P.; Lu, X.X.; Yang, G.M.; Yang, Y.H. Hydrogen peroxide biosensor based on electrodeposition of zinc oxide nanoflowers onto carbon nanotubes film electrode. Chin. Chem. Lett. 2008, 19, 314–318. [Google Scholar]
- Zhao, Y.; Liu, H.; Kou, Y.; Li, M.; Zhu, Z.; Zhuang, Q. Structural and characteristic analysis of carbon nanotubes-ionic liquid gel biosensor. Electrochem. Commun. 2007, 9, 2457–2462. [Google Scholar]
- Muguruma, H.; Shibayama, Y.; Matsui, Y. An amperometric biosensor based on a composite of single-walled carbon nanotubes, plasma-polymerized thin film, and an enzyme. Biosens. Bioelectron. 2008, 23, 827–832. [Google Scholar]
- Abe, M.; Murata, K.; Ataka, T.; Matsumoto, K. Calibration method for a carbon nanotube field-effect transistor biosensor. Nanotechnology 2008, 19, 045505:1–045505:4. [Google Scholar]
- Weeks, M.L.; Rahman, T.; Frymier, P.D.; Islam, S.K.; McKnight, T.E. A reagentless enzymatic amperometric biosensor using vertically aligned carbon nanofibers (VACNF). Sens. Actuat. B 2008, 133, 53–59. [Google Scholar]
- Cui, D.; Pan, B.; Zhang, H.; Gao, F.; Wu, R.; Wang, J.; He, R.; Asahi, T. Self-assembly of quantum dots and carbon nanotubes for ultrasensitive DNA and antigen detection. Anal. Chem. 2008, 80, 7996–8001. [Google Scholar]
- Kim, D.H.; Lee, S.H.; Kim, K.N.; Kim, K.M.; Shim, I.B.; Lee, Y.K. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J. Magn. Magn. Mater. 2005, 293, 287–292. [Google Scholar]
- Lee, H.; Lee, E.; Kim, D.K.; Jang, N.K.; Jeong, Y.Y.; Jon, S. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J. Am. Chem. Soc. 2006, 128, 7383–7389. [Google Scholar]
- Ito, A.; Ino, K.; Kobayashi, T.; Honda, H. The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials 2005, 26, 6185–6193. [Google Scholar]
- Sincai, M.; Ganga, D.; Ganga, M.; Argherie, D.; Bica, D. Antitumor effect of magnetite nanoparticles in cat mammary adenocarcinoma. J. Magn. Magn. Mater. 2005, 293, 438–441. [Google Scholar]
- Morishita, N.; Nakagami, H.; Morishita, R.; Takeda, S.I.; Mishima, F.; Terazono, B.; Nishijima, S.; Kaneda, Y.; Tanaka, N. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 2005, 334, 1121–1126. [Google Scholar]
- Guedes, M.H.A.; Sadeghiani, N.; Peixoto, D.L.G.; Coelho, J.P.; Barbosa, L.S.; Azevedo, R.B.; Kückelhaus, S.; Da Silva, M.D.F.; Morais, P.C.; Lacava, Z.G.M. Effects of AC magnetic field and carboxymethyldextran-coated magnetite nanoparticles on mice peritoneal cells. J. Magn. Magn. Mater. 2005, 293, 283–286. [Google Scholar]
- Rife, J.C.; Miller, M.M.; Sheehan, P.E.; Tamanaha, C.R.; Tondra, M.; Whitman, L.J. Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens. Actuat. A 2003, 107, 209–218. [Google Scholar]
- Zhang, H.L.; Lai, G.S.; Han, D.Y.; Yu, A.M. An amperometric hydrogen peroxide biosensor based on immobilization of horseradish peroxidase on an electrode modified with magnetic dextran microspheres. Anal. Bioanal. Chem. 2008, 390, 971–977. [Google Scholar]
- Lai, G.S.; Zhang, H.L.; Han, D.Y. A novel hydrogen peroxide biosensor based on hemoglobin immobilized on magnetic chitosan microspheres modified electrode. Sens. Actuat. B 2008, 129, 497–503. [Google Scholar]
- Janssen, X.J.A.; Schellekens, A.J.; van Ommering, K.; van Ijzendoorn, L.J.; Prins, M.W.J. Controlled torque on superparamagnetic beads for functional biosensors. Biosens. Bioelectron. In press. [CrossRef]
- Loh, K.S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2,4-dichlorophenoxyacetic acid. Sensors 2008, 8, 5775–5791. [Google Scholar]
- Tamanaha, C.R.; Mulvaney, S.P.; Rife, J.C.; Whitman, L.J. Magnetic labeling, detection, and system integration. Biosens. Bioelectron. 2008, 24, 1–13. [Google Scholar]
- Park, S.H.; Soh, K.S.; Hwang, D.G.; Rhee, J.R.; Lee, S.S. Detection of magnetic nanoparticles and Fe-hemoglobin inside red blood cells by using a highly sensitive spin valve device. J. Magnetics 2008, 13, 30–33. [Google Scholar]
- Mujika, M.; Arana, S.; Castaño, E.; Tijero, M.; Vilares, R.; Ruano-López, J.M.; Cruz, A.; Sainz, L.; Berganza, J. Magnetoresistive immunosensor for the detection of Escherichia coli O157:H7 including a microfluidic network. Biosens. Bioelectron. 2009, 24, 1253–1258. [Google Scholar]
- Jun, Y.W.; Seo, J.W.; Cheon, J. Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc. Chem. Res. 2008, 41, 179–189. [Google Scholar]
- Jeong, H.; Chang, A.M.; Melloch, M.R. The Kondo effect in an artificial quantum dot molecule. Science 2001, 293, 2221–2223. [Google Scholar]
- Li, X.; Wu, Y.; Steel, D.; Gammon, D.; Stievater, T.H.; Katzer, D.S.; Park, D.; Piermarocchi, C.; Sham, L.J. An all-optical quantum gate in a semiconductor quantum dot. Science 2003, 301, 809–811. [Google Scholar]
- Lu, W.; Ji, Z.; Pfeiffer, L.; West, K.W.; Rimberg, A.J. Real-time detection of electron tunnelling in a quantum dot. Nature 2003, 423, 422–425. [Google Scholar]
- Kaul, Z.; Yaguchi, T.; Kaul, S.C.; Hirano, T.; Wadhwa, R.; Taira, K. Mortalin imaging in normal and cancer cells with quantum dot immuno-conjugates. Cell Res. 2003, 13, 503–507. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar]
- Hoshino, A.; Fujioka, K.; Manabe, N.; Yamaya, S.I.; Goto, Y.; Yasuhara, M.; Yamamoto, K. Simultaneous multicolor detection system of the single-molecular microbial antigen with total internal reflection fluorescence microscopy. Microbiol. Immunol. 2005, 49, 461–470. [Google Scholar]
- Han, M.; Gao, X.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar]
- Huang, X.; Li, L.; Qian, H.; Dong, C.; Ren, J. A resonance energy transfer between chemiluminescent donors and luminescent quantum-dots as acceptors (CRET). Angew. Chem., Int. Ed. 2006, 45, 5140–5143. [Google Scholar]
- Du, D.; Chen, S.; Song, D.; Li, H.; Chen, X. Development of acetylcholinesterase biosensor based on CdTe quantum dots/gold nanoparticles modified chitosan microspheres interface. Biosens. Bioelectron. 2008, 24, 475–479. [Google Scholar]
- Deng, Z.; Zhang, Y.; Yue, J.; Tang, F.; Wei, Q. Green and orange CdTe quantum dots as effective pH-sensitive fluorescent probes for dual simultaneous and independent detection of viruses. J. Phys. Chem. B 2007, 111, 12024–12031. [Google Scholar]
- Ansari, A.A.; Kaushik, A.; Solanki, P.R.; Malhotra, B.D. Sol-gel derived nanoporous cerium oxide film for application to cholesterol biosensor. Electrochem. Commun. 2008, 10, 1246–1249. [Google Scholar]
- Zhang, F.; Ulrich, B.; Reddy, R.K.; Venkatraman, V.L.; Prasad, S.; Vu, T.Q.; Hsu, S.T. Fabrication of submicron IrO2 nanowire array biosensor platform by conventional complementary metal-oxide-semiconductor process. Jpn. J. Appl. Phys., Pt. 1 2008, 47, 1147–1151. [Google Scholar]
- Ghanbari, K.; Bathaie, S.Z.; Mousavi, M.F. Electrochemically fabricated polypyrrole nanofiber-modified electrode as a new electrochemical DNA biosensor. Biosens. Bioelectron. 2008, 23, 1825–1831. [Google Scholar]
- Shan, D.; Zhu, M.; Han, E.; Xue, H.; Cosnier, S. Calcium carbonate nanoparticles: A host matrix for the construction of highly sensitive amperometric phenol biosensor. Biosens. Bioelectron. 2007, 23, 648–654. [Google Scholar]
- Ekanayake, E.M.I.M.; Preethichandra, D.M.G.; Kaneto, K. Polypyrrole nanotube array sensor for enhanced adsorption of glucose oxidase in glucose biosensors. Biosens. Bioelectron. 2007, 23, 107–113. [Google Scholar]
- Dai, Z.; Bai, H.; Hong, M.; Zhu, Y.; Bao, J.; Shen, J. A novel nitrite biosensor based on the direct electron transfer of hemoglobin immobilized on CdS hollow nanospheres. Biosens. Bioelectron. 2008, 23, 1869–1873. [Google Scholar]
- Li, J.; Lin, X. Simultaneous determination of dopamine and serotonin on gold nanocluster/overoxidized-polypyrrole composite modified glassy carbon electrode. Sens. Actuat. B 2007, 124, 486–493. [Google Scholar]
- Miao, X.M.; Yuan, R.; Chai, Y.Q.; Shi, Y.T.; Yuan, Y.Y. Direct electrocatalytic reduction of hydrogen peroxide based on Nafion and copper oxide nanoparticles modified Pt electrode. J. Electroanal. Chem. 2008, 612, 157–163. [Google Scholar]
- Liu, A. Towards development of chemosensors and biosensors with metal-oxide-based nanowires or nanotubes. Biosens. Bioelectron. 2008, 24, 167–177. [Google Scholar]
- Cheng, J.; Di, J.; Hong, J.; Yao, K.; Sun, Y.; Zhuang, J.; Xu, Q.; Zheng, H.; Bi, S. The promotion effect of titania nanoparticles on the direct electrochemistry of lactate dehydrogenase sol-gel modified gold electrode. Talanta 2008, 76, 1065–1069. [Google Scholar]
- Xia, C.; Wang, N.; Lidong, L.; Lin, G. Synthesis and characterization of waxberry-like microstructures ZnO for biosensors. Sens. Actuat. B 2008, 129, 268–273. [Google Scholar]
- Huang, Z.B.; Yan, D.H.; Yang, M.; Liao, X.M.; Kang, Y.Q.; Yin, G.F.; Yao, Y.D.; Hao, B.Q. Preparation and characterization of the biomineralized zinc oxide particles in spider silk peptides. J. Colloid Interface Sci. 2008, 325, 356–362. [Google Scholar]
- Lu, X.; Zhang, H.; Ni, Y.; Zhang, Q.; Chen, J. Porous nanosheet-based ZnO microspheres for the construction of direct electrochemical biosensors. Biosens. Bioelectron. 2008, 24, 93–98. [Google Scholar]
- Chen, Y.; Wang, X.; Hong, M.; Erramilli, S.; Mohanty, P. Surface-modified silicon nano-channel for urea sensing. Sens. Actuat. B 2008, 133, 593–598. [Google Scholar]
- Zhang, Z.L.; Asano, T.; Uno, H.; Tero, R.; Suzui, M.; Nakao, S.; Kaito, T.; Shibasaki, K.; Tominaga, M.; Utsumi, Y.; Gao, Y.L.; Urisu, T. Fabrication of Si-based planar type patch clamp biosensor using silicon on insulator substrate. Thin Solid Films 2008, 516, 2813–2815. [Google Scholar]
- Wang, D.; Sun, G.; Xiang, B.; Chiou, B.S. Controllable biotinylated poly(ethylene-co-glycidyl methacrylate) (PE-co-GMA) nanofibers to bind streptavidin-horseradish peroxidase (HRP) for potential biosensor applications. Eur. Polym. J. 2008, 44, 2032–2039. [Google Scholar]
- Kusakari, A.; Izumi, M.; Ohnuki, H. Preparation of an enzymatic glucose sensor based on hybrid organic-inorganic Langmuir-Blodgett films: Adsorption of glucose oxidase into positively charged molecular layers. Colloids Surf., A 2008, 321, 47–51. [Google Scholar]
- Kim, G.Y.; Kang, M.S.; Shim, J.; Moon, S.H. Substrate-bound tyrosinase electrode using gold nanoparticles anchored to pyrroloquinoline quinone for a pesticide biosensor. Sens. Actuat. B 2008, 133, 1–4. [Google Scholar]
- Lu, B.W.; Chen, W.C. A disposable glucose biosensor based on drop-coating of screen-printed carbon electrodes with magnetic nanoparticles. J. Magn. Magn. Mater. 2006, 304, e400–e402. [Google Scholar]
- Yu, J.; Yu, D.; Zhao, T.; Zeng, B. Development of amperometric glucose biosensor through immobilizing enzyme in a Pt nanoparticles/mesoporous carbon matrix. Talanta 2008, 74, 1586–1591. [Google Scholar]
- Manesh, K.M.; Kim, H.T.; Santhosh, P.; Gopalan, A.I.; Lee, K.P. A novel glucose biosensor based on immobilization of glucose oxidase into multiwall carbon nanotubes-polyelectrolyte-loaded electrospun nanofibrous membrane. Biosens. Bioelectron. 2008, 23, 771–779. [Google Scholar]
- Zou, Y.; Xiang, C.; Sun, L.X.; Xu, F. Glucose biosensor based on electrodeposition of platinum nanoparticles onto carbon nanotubes and immobilizing enzyme with chitosan-SiO2 sol-gel. Biosens. Bioelectron. 2008, 23, 1010–1016. [Google Scholar]
- Cao, C.; Kim, J.H.; Yoon, D.; Hwang, E.S.; Kim, Y.J.; Baik, S. Optical detection of DNA hybridization using absorption spectra of single-walled carbon nanotubes. Mater. Chem. Phys. 2008, 112, 738–741. [Google Scholar]
- Zhang, W.; Yang, T.; Huang, D.; Jiao, K.; Li, G. Synergistic effects of nano-ZnO/multi-walled carbon nanotubes/chitosan nanocomposite membrane for the sensitive detection of sequence-specific of PAT gene and PCR amplification of NOS gene. J. Membr. Sci. 2008, 325, 245–251. [Google Scholar]
- Zhang, W.; Yang, T.; Huang, D.M.; Jiao, K. Electrochemical sensing of DNA immobilization and hybridization based on carbon nanotubes/nano zinc oxide/chitosan composite film. Chin. Chem. Lett. 2008, 19, 589–591. [Google Scholar]
- Galandova, J.; Ziyatdinova, G.; Labuda, J. Disposable electrochemical biosensor with multiwalled carbon nanotubes - Chitosan composite layer for the detection of deep DNA damage. Anal. Sci. 2008, 24, 711–716. [Google Scholar]
- García, T.; Casero, E.; Revenga-Parra, M.; Martín-Benito, J.; Pariente, F.; Vázquez, L.; Lorenzo, E. Architectures based on the use of gold nanoparticles and ruthenium complexes as a new route to improve genosensor sensitivity. Biosens. Bioelectron. 2008, 24, 184–190. [Google Scholar]
- McKenzie, F.; Faulds, K.; Graham, D. Sequence-specific DNA detection using high-affinity LNA-functionalized gold nanoparticles. Small 2007, 3, 1866–1868. [Google Scholar]
- Ma, Y.; Jiao, K.; Yang, T.; Sun, D. Sensitive PAT gene sequence detection by nano-SiO2/p-aminothiophenol self-assembled films DNA electrochemical biosensor based on impedance measurement. Sens. Actuat. B 2008, 131, 565–571. [Google Scholar]
- Maki, W.C.; Mishra, N.N.; Cameron, E.G.; Filanoski, B.; Rastogi, S.K.; Maki, G.K. Nanowire-transistor based ultra-sensitive DNA methylation detection. Biosens. Bioelectron. 2008, 23, 780–787. [Google Scholar]
- Vamvakaki, V.; Chaniotakis, N.A. Pesticide detection with a liposome-based nano-biosensor. Biosens. Bioelectron. 2007, 22, 2848–2853. [Google Scholar]
- Zhang, W.; Tang, H.; Geng, P.; Wang, Q.; Jin, L.; Wu, Z. Amperometric method for rapid detection of Escherichia coli by flow injection analysis using a bismuth nano-film modified glassy carbon electrode. Electrochem. Commun. 2007, 9, 833–838. [Google Scholar]
- Seo, S.; Dobozi-King, M.; Young, R.F.; Kish, L.B.; Cheng, M. Patterning a nanowell sensor biochip for specific and rapid detection of bacteria. Microelectron. Eng. 2008, 85, 1484–1489. [Google Scholar]
- Medley, C.D.; Smith, J.E.; Tang, Z.; Wu, Y.; Bamrungsap, S.; Tan, W. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal. Chem. 2008, 80, 1067–1072. [Google Scholar]
- Prow, T.W.; Bhutto, I.; Grebe, R.; Uno, K.; Merges, C.; McLeod, D.S.; Lutty, G.A. Nanoparticle-delivered biosensor for reactive oxygen species in diabetes. Vision Res. 2008, 48, 478–485. [Google Scholar]
- Li, B.; Du, Y.; Wei, H.; Dong, S. Reusable, label-free electrochemical aptasensor for sensitive detection of small molecules. Chem. Commun. 2007, 3780–3782. [Google Scholar]
- Kerman, K.; Saito, M.; Tamiya, E.; Yamamura, S.; Takamura, Y. Nanomaterial-based electrochemical biosensors for medical applications. TrAC-Trend. Anal. Chem. 2008, 27, 585–592. [Google Scholar]
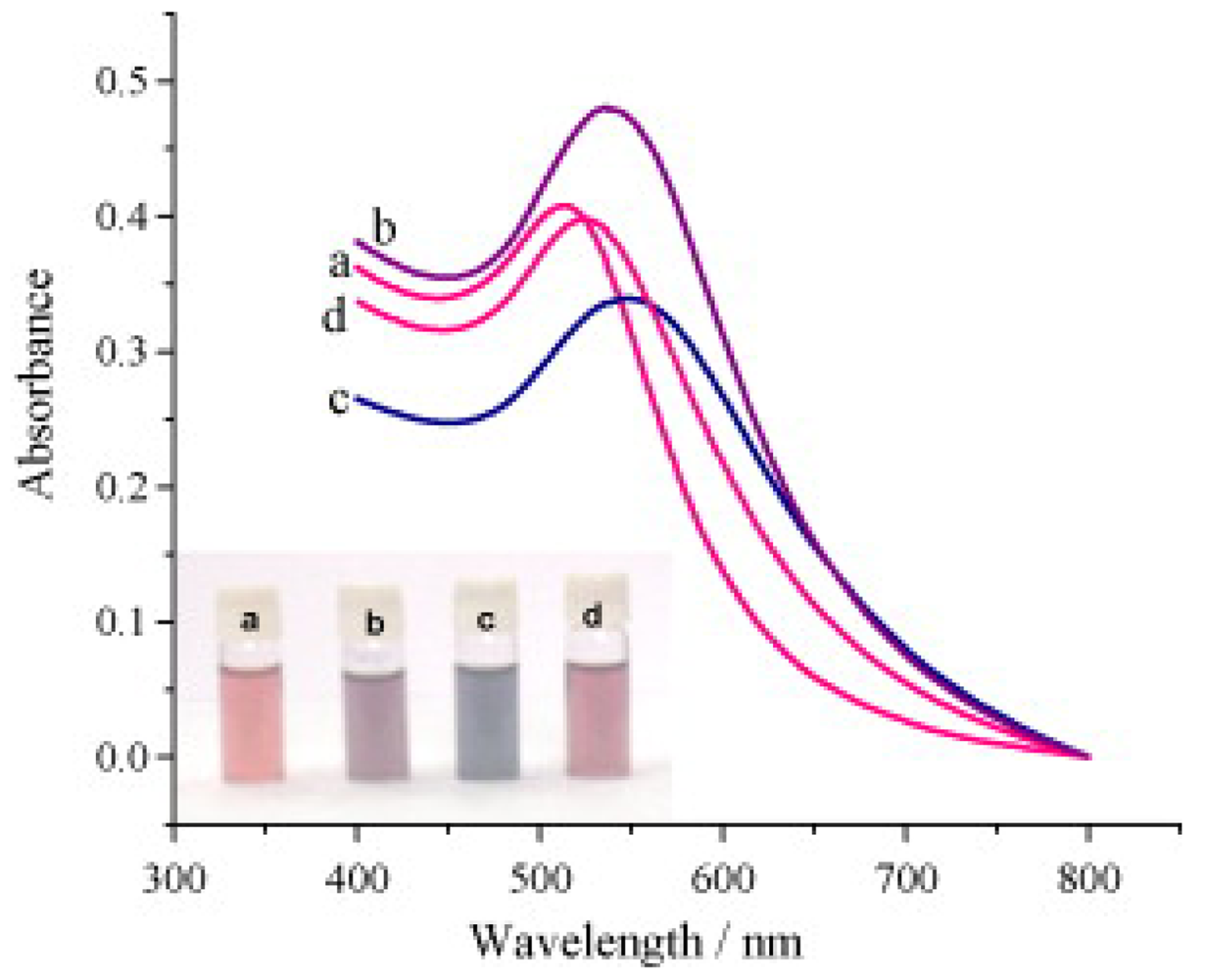

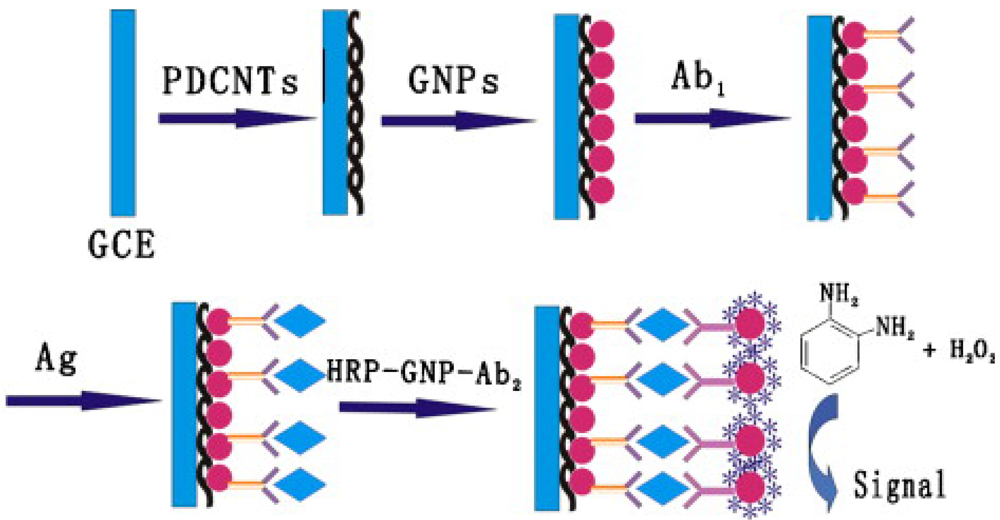
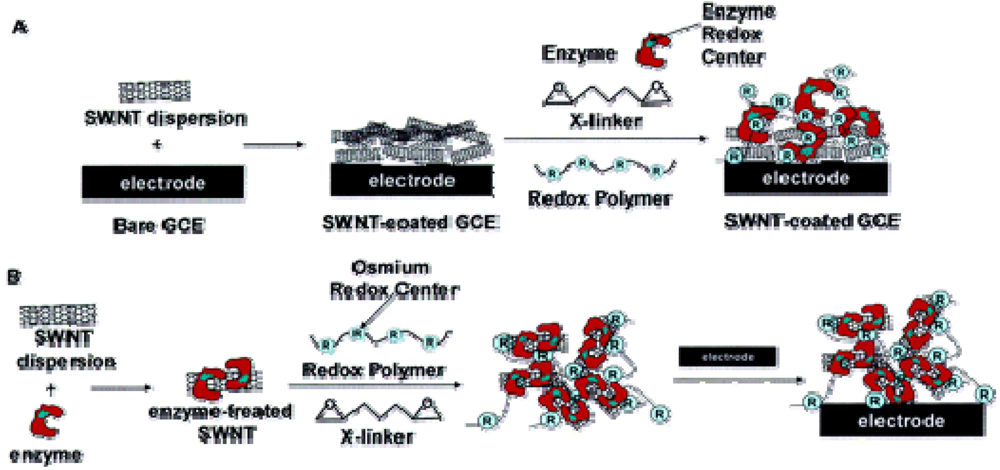
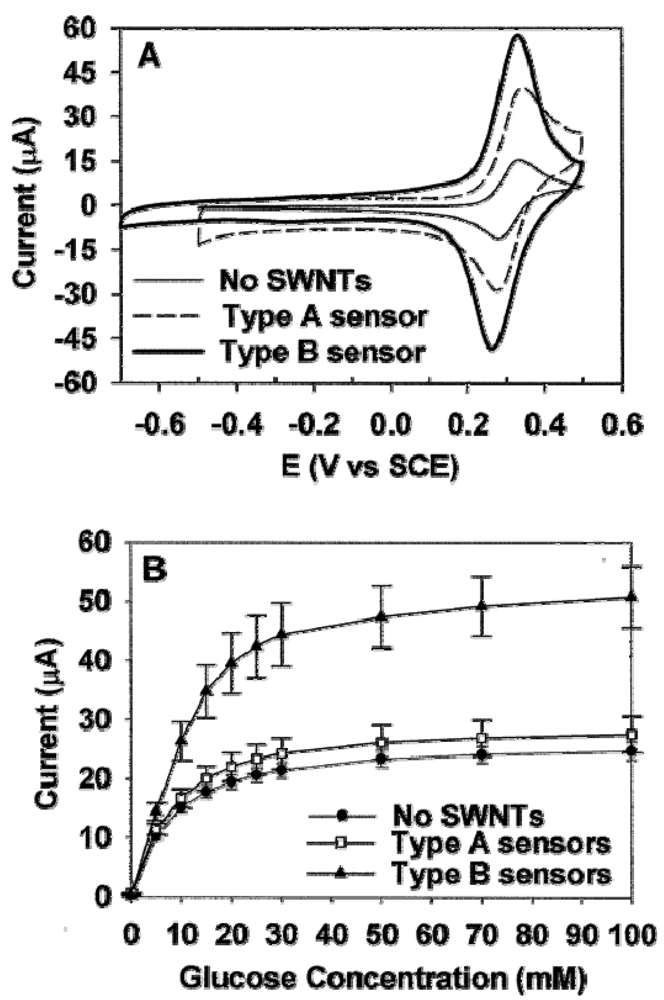


© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, X.; Guo, Q.; Cui, D. Recent Advances in Nanotechnology Applied to Biosensors. Sensors 2009, 9, 1033-1053. https://doi.org/10.3390/s90201033
Zhang X, Guo Q, Cui D. Recent Advances in Nanotechnology Applied to Biosensors. Sensors. 2009; 9(2):1033-1053. https://doi.org/10.3390/s90201033
Chicago/Turabian StyleZhang, Xueqing, Qin Guo, and Daxiang Cui. 2009. "Recent Advances in Nanotechnology Applied to Biosensors" Sensors 9, no. 2: 1033-1053. https://doi.org/10.3390/s90201033



