Breath Analysis Using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits
Abstract
:1. Introduction
2. 35 Identified Breath Biomarkers and Their Related Physiological Symptoms
3. Laser Spectroscopic Techniques for Breath Analysis
4. Breath Biomarkers Detected by the Laser Spectroscopic Techniques
Acetaldehyde (C2H4O)
Acetone ((CH3)2CO)
Ammonia (NH3)
Carbon dioxide (CO2) and 13C-isotope
Carbon monoxide (CO)
Carbonyl sulfide (OCS)
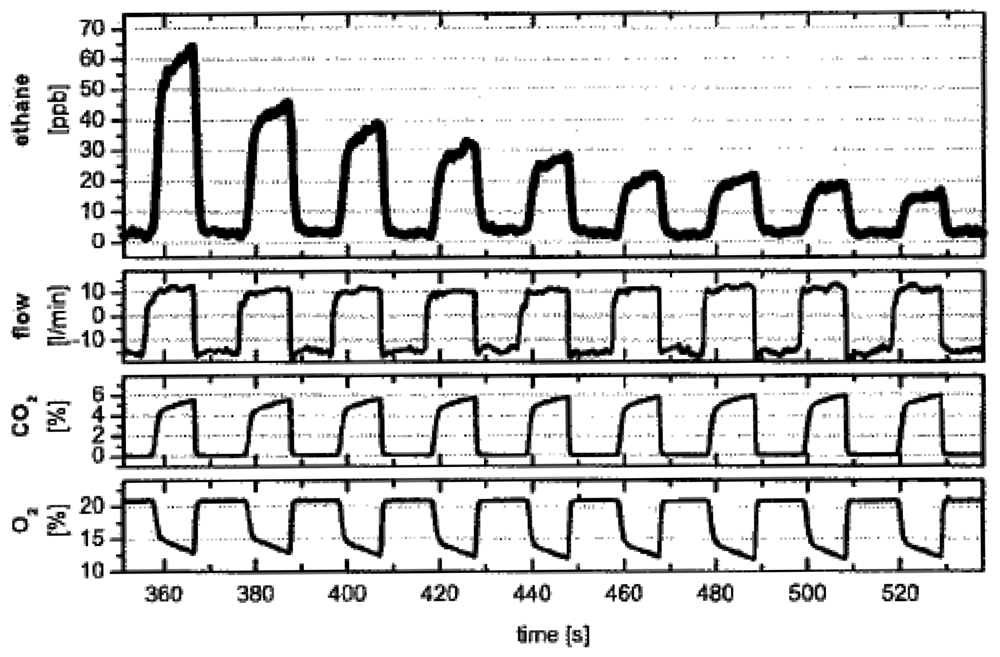
Ethane (C2H6)
Ethylene (C2H4)
Formaldehyde (HCHO)
D/H isotope
Methane (CH4)
Methylated amines (MAs)
Nitric oxide (NO)
5. Current Status of Prototype and commercialized Breath Sensors
6. Challenges and Perspectives
7. Summary
Acknowledgments
References and Notes
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374–2384. [Google Scholar]
- Jansson, B.O.; Larsson, B.T.J. Analysis of organic compounds in human breath by gas chromatography-mass spectrometry lab. Clin. Med. 1969, 74, 961–966. [Google Scholar]
- Gordon, S.M.; Szidon, J.P.; Krotoszynski, B.K.; Gibbons, R.D.; O'Neill, H.J. Volatile organic compounds in exhaled air from patients with lung cancer. Clin. Chem. 1985, 31, 1278–1282. [Google Scholar]
- O'Neill, H.; Gordon, S.M.; O'Neill, M.; Gibbons, R.D.; Szidon, J.P. A computerized classification technique for screening for the presence of breath biomarkers in lung cancer. Clin. Chem. 1988, 34, 1613–1618. [Google Scholar]
- Buszewski, B.; Kesy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. chromatogr: BMC 2007, 21, 553–566. [Google Scholar]
- Grote, C.; Pawliszyn, J. Solid-phase microextraction for the analysis of human breath. Anal. Chem. 1997, 69, 587–596. [Google Scholar]
- Lord, H.; Yu, Y.F.; Segal, A.; Pawliszyn, J. Breath analysis and monitoring by membrane extraction with sorbent interface. Anal. Chem. 2002, 74, 5650–5657. [Google Scholar]
- Anderson, J.C.; Lamm, W.J.E.; Hlasatala, M.P. Measuring airway exchange of endogenous acetone using a single-exhalation breathing maneuver. J. Appl. Physiol. 2005, 100, 880–889. [Google Scholar]
- Raymer, J.H.; Thomas, K.W.; Cooper, S.D.; Whitaker, D.A.; Pellizzari, E.D. A device for sampling of human alveolar breath for the measurement of expired volatile organic compounds. J. Anal. Toxicol. 1990, 14, 337–344. [Google Scholar]
- Mendis, S.; Sobotka, P.A.; Euler, D.E. Pentane and isoprene in expired air from humans: gas-chromatographic analysis of single breath. Clin. Chem. 1994, 40, 1485–1488. [Google Scholar]
- Henderson, M.J.; Karger, B.A.; Wrenshall, G.A. Acetone in the breath; A study of acetone exhalation in diabetic and nondiabetic human subjects. Diabetes 1952, 1, 188–193. [Google Scholar]
- Phillips, M. Method for the collection and assay of volatile organic compounds in breath. Anal. Biochem. 1997, 247, 272–278. [Google Scholar]
- Miekisch, W.; Schubert, J.K.; Vagts, D.A.; Geiger, K. Analysis of volatile disease markers in blood. Clin. Chem. 2001, 47, 1053–1060. [Google Scholar]
- Moser, B.; Bodrogi, F.; Eibl, G.; Lechner, M.; Rieder, J.; Lirk, P. Mass spectrometric profile of exhaled breath-field study by PTR-MS. Respir. Physiol. Neurobiol. 2005, 145, 295–300. [Google Scholar]
- Harrison, G.R.; Critchley, A.D.; Mayhew, C.A.; Thompson, J.M. Real-time breath monitoring of propane and its volatile metabolites during surgery using a novel mass spectrometric technique: a feasibility study. Br. J. Anaesth. 2003, 91, 797–799. [Google Scholar]
- Yuan, H.; Mester, Z.; Lord, H.; Pawliszyn, J. Automated in-tube solid phase microextraction coupled with liquid chromatography-electrospray ionization mass spectrometry for the determination of selected benzodiazepines. J. Anal. Toxicol. 2000, 24, 718–725. [Google Scholar]
- Ruzsanyi, V.; Baumbach, J.; Litterst, P.; Westhoff, M.; Freitag, L. Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J. Chromatogr. A 2005, 1084, 145–151. [Google Scholar]
- Smith, D.; Wang, T.; Spanel, P. On-line, simultaneous quantification of ethanol, some metabolites and water vapour in breath following the ingestion of alcohol. Physiol. Meas. 2002, 23, 477–479. [Google Scholar]
- Diskin, A.M.; Spanel, P.; Smith, D. Time variation of ammonia, acetone, isoprene and ethanol in breath: a quantitative SIFT-MS study over 30 days. Physiol. Meas. 2003, 24, 107–120. [Google Scholar]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry SIFT-MS. Physiol. Meas. 2006, 27, 321–337. [Google Scholar]
- Smith, D.; Spanel, P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005, 24, 661–700. [Google Scholar]
- Di Natale, C.; Macagnano, A.; Martinelli, E.; Paolesse, R.; D'Arcangelo, G.; Roscioni, C.; Finazzi-Agro, A.; D'Amico, A. Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosens. Bioelectron. 2003, 18, 1209–1218. [Google Scholar]
- Fleischer, M.; Simon, E.; Rumpel, E.; Ulmer, H.; Harbeck, M.; Wandel, M.; Fietzek, C.; Weimar, U.; Meixner, H. Detection of volatile compounds correlated to human diseases through breath analysis with chemical sensors. Sens. Actuat. B 2002, 83, 245–249. [Google Scholar]
- Sigrist, M.W.; Bartlome, R.; Marinov, D.; Rey, J.M.; Vogler, D.E.; Wachter, H. Trace gas monitoring with infrared laser-based detection schemes. Appl. Phys. B: Lasers Opt. 2008, 90, 289–300. [Google Scholar]
- McCurdy, M.R.; Bakhirkin, Y.; Wysocki, G.; Lewicki, R.; Tittel, F.K. Recent advances of laser-spectroscopy-based techniques for applications in breath analysis. J. Breath Res. 2007, 1, 014001/1–12. [Google Scholar]
- Risby, T.H.; Amann, A.; Smith, D. Current status of clinical breath analysis. In Breath gas analysis for clinical diagnosis and therapeutic monitoring; Amman, A., Smith, D., Eds.; World Scientific Publishing: Singapore, 2005; pp. 251–266. [Google Scholar]
- Mürtz, M. Breath diagnostics using laser spectroscopy. Opt. Photon. News 2005, 16, 30–35. [Google Scholar]
- Miekisch, W.; Schubert, J. From highly sophisticated analytical techniques to life-saving diagnostics: Technical developments in breath analysis. Trends Anal. Chem. 2006, 25, 665–673. [Google Scholar]
- Smith, D.; Spanel, P. The challenge of breath analysis for clinical diagnosis and therapeutic monitoring. Analyst 2007, 132, 390–396. [Google Scholar]
- Cao, W.; Duan, Y. Current status of methods and techniques for breath analysis. Critical Rev. Anal. Chem. 2007, 37, 3–13. [Google Scholar]
- Mihalcea, R.M.; Baer, D.S.; Hanson, R.K. Tunable diode-laser absorption measurements of NO2 near 670 and 395 nm. Appl. Opt. 1996, 35, 4059–4064. [Google Scholar]
- Baer, D.S.; Hanson, R.K.; Newfield, M.E.; Gopaul, N.K.L.M. Multiplexed diode-laser sensor system for simultaneous H2O, O2, and temperature measurements. Opt. Lett. 1994, 19, 1900–1902. [Google Scholar]
- O'Keefe, A.; Deacon, D.A.G. Cavity ring-down optical spectrometer for absorption measurements using pulsed laser sources. Rev. Sci. Instrum. 1988, 59, 2544–2551. [Google Scholar]
- Busch, K.W.; Busch, M.A. Cavity Ringdown Spectroscopy: An Ultra-Trace Absorption Measurement Technique; ACS symposium series; Oxford University Press: New York, NY, USA, 1999; pp. 1–720. [Google Scholar]
- Berden, G.; Peeters, R.; Meijer, G. Cavity ring-down spectroscopy: Experimental schemes and applications. Int. Rev. Phys. Chem. 2000, 19, 565–607. [Google Scholar]
- Mazurenka, M.; Orr-Ewing, A.J.; Peverall, R.; Ritchie, G.A.D. Cavity ring-down and cavity enhanced spectroscopy using diode lasers. Annu. Rep. Prog. Chem. Sect. C: Phys. Chem. 2005, 101, 100–142. [Google Scholar]
- Scherer, J.J.; Paul, J.B.; Jiao, H.; O'Keefe, A. Broadband ringdown spectral photography. Appl. Opt. 2001, 40, 6725–6732. [Google Scholar]
- Baer, D.S.; Paul, J.B.; Gupta, J.B.; O'Keefe, A. Sensitive absorption measurements in the near-infrared region using off-axis integrated-cavity-output spectroscopy. Appl. Phys. B: Lasers Opt. 2002, 75, 261–265. [Google Scholar]
- Bakhirkin, Y.A.; Kosterev, A.A.; Roller, C.; Curl, R.F.; Tittel, F.K. Mid-infrared quantum cascade laser based off-axis integrated cavity output spectroscopy for biogenic nitric oxide detection. Appl. Opt. 2004, 43, 2257–2266. [Google Scholar]
- Bakhirkin, Y.A.; Kosterev, A.A.; Curl, R.; Tittel, F.K.; Yarekha, D.A.; Hvozdara, L.; Giovannini, M.; Faist, J. Sub ppbv nitric oxide concentration measurements using cw thermoelectrically cooled quantum cascade laser-based integrated cavity output spectroscopy. Appl. Phys. B: Lasers Opt. 2006, 82, 149–154. [Google Scholar]
- Peeters, R.; Berden, G.; Apituley, A.; Meijer, G. Open-path trace gas detection of ammonia based on cavity-enhanced absorption spectroscopy. Appl. Phys. B: Lasers Opt. 2000, 71, 231–236. [Google Scholar]
- Dahnke, H.; Kleine, D.; Hering, P.; Mürtz, M. Real-time monitoring of ethane in human breath using mid-infrared cavity leak-out spectroscopy. Appl. Phys. B: Lasers Opt. 2001, 72, 971–975. [Google Scholar]
- Dahnke, H.; Kleine, D.; Urban, C.; Hering, P.; Murtz, M. Isotopic ratio measurement of methane in ambient air using mid-infrared cavity leak-out spectroscopy. Appl. Phys. B: Lasers Opt. 2001, 72, 121–125. [Google Scholar]
- von Basum, G.; Halmer, D.; Hering, P.; Murtz, M.; Schiller, S.; Mueller, F.; Popp, A.; Kuehnemann, F. Parts per trillion sensitivity for ethane in air with an optical parametric oscillator cavity leak-out spectrometer. Opt. Lett. 2004, 29, 797–799. [Google Scholar]
- Halmer, D.; Thelen, S.; Hering, P.; Mürtz, M. Online monitoring of ethane traces in exhaled breath with a difference frequency generation spectrometer. Appl. Phys. B: Lasers Opt. 2006, 85, 437–443. [Google Scholar]
- Halmer, D.; von Basum, G.; Hering, P.; Murtz, M. Mid-infrared cavity leak-out spectroscopy for ultrasensitive detection of carbonyl sulfide. Opt. Lett. 2005, 30, 2314–2316. [Google Scholar]
- Hofstetter, D.; Beck, M.; Faist, J.; Nagele, M.; Sigrist, M.W. Photoacoustic spectroscopy with quantum cascade distributed-feedback lasers. Opt. Lett. 2001, 26, 887–889. [Google Scholar]
- Kosterev, A.A.; Bakhirkin, Y.A.; Curl, R.F.; Tittel, F.K. Quartz-enhanced photoacoustic spectroscopy. Opt. Lett. 2002, 27, 1902–1904. [Google Scholar]
- Thorpe, M.J.; Moll, K.D.; Jones, J.R.; Safdi, B.; Ye, J. Broadband cavity ringdown spectroscopy for sensitive and rapid molecular detection. Science 2006, 311, 1595–1599. [Google Scholar]
- Thorpe, M.J.; Balslev-Clausen, D.; Kirchner, M.S.; Ye, J. Cavity-enhanced optical frequency comb spectroscopy: application to human breath analysis. Opt. Express 2008, 16, 2387–2397. [Google Scholar]
- Kharitonov, S.A.; Barnes, P.J. Nitric oxide, nitrotyrosine, and nitric oxide modulators in asthma and chronic obstructive pulmonary disease. Curr. Allergy Asthma Rep. 2003, 3, 121–129. [Google Scholar]
- Kharitonov, S.A.; Wells, A.U.; O'Connor, B.J.; Cole, P.J.; Hansell, D.M.; Logan-Sinclair, R.B.; Barnes, P.J. Elevated levels of exhaled nitric oxide in bronchiectasis. Am. J. Respir. Crit. Care Med. 1995, 151, 1889–1893. [Google Scholar]
- Schilling, J.; Holzer, P.; Guggenbach, M.; Gyurech, D.; Marathia, K.; Geroulanos, S. Reduced endogenous nitric oxide in the exhaled air of smokers and hypertensives. Eur. Respir. J. 1994, 7, 467–471. [Google Scholar]
- Martin, U.; Bryden, K.; Devoy, M.; Howarth, P. Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J. Allergy Clin. Immunol. 1996, 97, 768–772. [Google Scholar]
- Refat, M.; Moore, T.J.; Kazui, M.; Risby, T.H.; Perman, J.A.; Schwarz, K.B. Utility of breath ethane as a noninvasive biomarker of vitamin E status in children. Pediatr. Res. 1991, 30, 396–403. [Google Scholar]
- Riely, C.A.; Cohen, G.; Lieberman, M. Ethane evolution: a new index of lipid peroxidation. Science 1974, 183, 208–210. [Google Scholar]
- Lawrence, G.D.; Cohen, G. Ethane exhalation as an index of in vivo lipid peroxidation: concentrating ethane from a breath collection chamber. Anal. Biochem. 1982, 122, 283–290. [Google Scholar]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Elevation of exhaled ethane concentration in asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 1450–1454. [Google Scholar]
- Jobsis, Q.; Raatgeep, H.C.; Hermans, P.W.; de Jongste, J.C. Hydrogen peroxide in exhaled air is increased in stable asthmatic children. Eur. Respir. J. 1997, 10, 519–521. [Google Scholar]
- MacGregor, G.; Ellis, S.; Andrews, J.; Imrie, M.; Innes, A.; Greening, A.P.; Cunningham, S. Breath condensate ammonium is lower in children with chronic asthma. Eur. Respir. J. 2005, 26, 271–276. [Google Scholar]
- Kneepkens, C.M.; Lepage, G.; Roy, C.C. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic. Biol. Med. 1994, 17, 127–160. [Google Scholar]
- Li, P.; Xu, G.; Wang, C.; Gong, Y.; He, Y. Breath pentane: an indicator for early and continuous monitoring of lipid peroxidation in hepatic ischaemia-reperfusion injury. Eur. J. Anaesthesiol. 2009, 26, 513–519. [Google Scholar]
- Phillips, M.; Sabas, M.; Greenberg, J. Increased pentane and carbon disulfide in the breath of patients with schizophrenia. J. Clin. Pathol. 1993, 46, 861–864. [Google Scholar]
- Rothman, L.S.; Barbe, A.; Benner, D.C. The HITRAN molecular spectroscopic database: edition of 2000 including updates through 2001. J. Quant. Spectrosc. Radiat. Transfer. 2003, 82, 5–44. [Google Scholar]
- Liu, J.T.C.; Jeffries, J.B.; Hanson, R.K. Large-modulation-depth 2f spectroscopy with diode lasers for rapid temperature and species measurements in gases with blended and broadened spectra. Appl. Opt. 2004, 43, 6500–6509. [Google Scholar]
- Kluczynski, P.; Gustafsson, J.; Lindberg, A.M.; Axner, O. Wavelength modulation absorption spectrometry — an extensive scrutiny of the generation of signals. Spectrochim. Acta B 2001, 56, 1277–1354. [Google Scholar]
- Miklos, A.; Hess, P.; Bozoki, Z. Application of acoustic resonators in photoacoustic trace gas analysis and metrology. Rev. Sci. Instru. 2001, 72, 1937–1955. [Google Scholar]
- Tranchart, S.; Bachir, I.H.; Destomber, J.L. Sensitive trace gas detection with near-infrared laser diode and an integrating sphere. Appl. Opt. 1996, 35, 7070–7074. [Google Scholar]
- Sauer, C.G.; Pisano, J.T.; Fitz, D.R. Tunable diode laser absorption spectrometer measurements of ambient nitrogen dioxide, nitric acid, formaldehyde, and hydrogen peroxide in Parlier, California. Atmos. Environ. 2003, 37, 1583–1591. [Google Scholar]
- Romanini, D.; Lehmann, K.K. Ring-down cavity absorption spectroscopy of the very weak HCN overtone bands with six, seven, and eight stretching quanta. J. Chem. Phys. 1993, 99, 6287–6301. [Google Scholar]
- Kosterev, A.A.; Mosely, T.S.; Tittel, F.K. Impact of humidity on quartz-enhanced photoacoustic spectroscopy based detection of HCN. Appl. Phys. B: Lasers Opt. 2006, 85, 295–300. [Google Scholar]
- Chen, W.; Kosterev, A.A.; Tittel, F.K.; Gao, X.; Zhao, W. H2S trace concentration measurements using off-axis integrated cavity output spectroscopy in the near-infrared. Appl. Phys. B: Lasers Opt. 2008, 90, 311–315. [Google Scholar]
- Frish, M.B.; White, M.A.; Allen, M.G. Handheld laser-based sensor for remote detection of toxic and hazardous gases. SPIE 2005. No. 4199-05. [Google Scholar]
- Varga, A.; Bozoki, Z.; Szakall, M.; Szabo, G. Photoacoustic system for on-line process monitoring of hydrogen sulfide (H2S) concentration in natural gas streams. Appl. Phys. B: Lasers Opt. 2006, 85, 315–321. [Google Scholar]
- Kamat, P.C.; Roller, C.B.; Namjou, K.; Jeffers, J.D.; Faramarzalian, A.; Salas, R.; McCann, P.J. Measurement of acetaldehyde in exhaled breath using a laser absorption spectrometer. Appl. Opt. 2007, 46, 3969–3975. [Google Scholar]
- Wang, C.; Mbi, A. A new acetone detection device using cavity ringdown spectroscopy at 266 nm: evaluation of the instrument performance using acetone sample solutions. Meas. Sci. Technol. 2007, 18, 2731–2741. [Google Scholar]
- Wang, C.; Mbi, A.; Shepherd, M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: Exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens. J. 2009, (in press). [Google Scholar]
- Wang, C.; Surampudi, A.B. An acetone breath analyzer using cavity ringdown spectroscopy: an initial test with human subjects under various situations. Meas. Sci. Technol. 2008, 19, 105604–105614. [Google Scholar]
- Narasimhan, L.R.; Goodman, W.; Patel, C.K.N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. USA 2001, 98, 4617–4621. [Google Scholar]
- Lachish, U.; Rotter, S.; Adler, E.; El-Hanany, U. Tunable diode laser based spectroscopic system for ammonia detection in human respiration. Rev. Sci. Instrum. 1987, 58, 923–927. [Google Scholar]
- Manne, J.; Sukhorukov, O.; Jager, W.; Tulip, J. Pulsed quantum cascade laser-based cavity ring-down spectroscopy for ammonia detection in breath. Appl. Opt. 2006, 45, 9230–9237. [Google Scholar]
- Manne, J.; Jager, W.; Tulip, J. Sensitive detection of ammonia and ethylene with a pulsed quantum cascade laser using intra and interpulse spectroscopic techniques. Appl. Phys. B: Lasers Opt. 2009, 94, 337–344. [Google Scholar]
- Moskalenko, K.L.; Nadezhdinskii, A.I.; Adamovskaya, I.A. Human breath trace gas content study by tunable diode laser spectroscopy technique. Infrared Phys. Tech. 1996, 37, 181–192. [Google Scholar]
- Van Herpen, M.M.J.W.; Ngai, A.K.Y.; Bisson, S.E.; Hackstein, J.H.P.; Woltering, E.J.; Harren, F.J.M. Optical parametric oscillator-based photoacoustic detection of CO2 at 4.23 μm allows real-time monitoring of the respiration of small insects. Appl. Phys. B: Lasers Opt. 2006, 82, 665–669. [Google Scholar]
- Crosson, E.R.; Ricci, K.N.; Richman, B.A.; Chilese, F.C.; Owano, T.G.; Provencal, R.A.; Todd, M.W.; Glasser, J.; Kachanow, A.A.; Paldus, B.A.; Spence, T.G.; Zare, R.N. Stable isotope ratios using cavity ring-down spectroscopy: determination of 13C/12C for carbon dioxide in human breath. Anal. Chem. 2002, 74, 2003–2007. [Google Scholar]
- Weldon, V.; O'Gorman, J.; Phelan, P.; Hegarty, J.; Tanbun-Ek, T. H2S and CO2 gas sensing using DFB laser diodes emitting at 1.57 μm. Sens. Actuat. B. 1995, 29, 101–107. [Google Scholar]
- Wysocki, G.; McCurdy, M.; So, S.; Weidmann, D.; Roller, C.; Curl, R.F.; Tittel, F.K. Pulsed quantum-cascade laser-based sensor for trace-gas detection of carbonyl sulfide. Appl. Opt. 2004, 43, 6040–6046. [Google Scholar]
- McCurdy, M.R.; Bakhirkin, Y.; Wysocki, G.; Tittel, F.K. Performance of an exhaled nitric oxide and carbon dioxide sensor using quantum cascade laser-based integrated cavity output spectroscopy. J. Biomed. Opt. 2007, 12, 034034:1–034034:9. [Google Scholar]
- Lee, P.S.; Majkowski, R.F.; Perry, T.A. Tunable diode laser spectroscopy for isotope analysis-detection of isotopic carbon monoxide in exhaled breath. IEEE Trans. Biomed. Eng. 1991, 38, 966–972. [Google Scholar]
- Roller, C.; Kosterev, A.A.; Tittel, F.K.; Uehara, K.; Gmachl, C.; Sivco, D.L. Carbonyl sulfide detection with a thermoelectrically cooled midinfrared quantum cascade laser. Opt. Lett. 2003, 28, 2052–2054. [Google Scholar]
- Bartlome, R.; Sigrist, M.W. laser based human breath analysis: D/H isotope ratio increases following heavy water intake. Opt. Lett. 2009, 34, 866–868. [Google Scholar]
- Parameswaran, K.R.; Rosen, D.I.; Allen, M.G.; Ganz, A.M.; Risby, T.H. Off-axis integrated cavity output spectroscopy with a mid-infrared interband cascade laser for real-time breath ethane measurements. Appl. Opt. 2009, 48, B73–B79. [Google Scholar]
- Skeldon, K.D.; McMillan, L.C.; Wyse, C.A.; Monk, S.D.; Gibson, G.; Patterson, C.; France, T.; Longbottom, C.; Padgett, M.J. Application of laser spectroscopy for measurement of exhaled ethane in patients with lung cancer. Respir. Med. 2006, 100, 300–306. [Google Scholar]
- Patterson, C.S.; McMillan, L.C.; Stevenson, K.; Radhakrishnan, K.; Shiels, P.G.; Padgett, M.J.; Skeldon, K.D. Dynamic study of oxidative stress in renal dialysis patients based on breath ethane measured by optical spectroscopy. J. Breath Res. 2007, 1, 026005:1–026005:8. [Google Scholar]
- Puiu, A.; Giubileo, G.; Bangrazi, C. Laser sensors for trace gases in human breath. Int. J. Environ. Anal. Chem. 2005, 85, 1001–1012. [Google Scholar]
- Skeldon, K.D.; Patterson, C.; Wyse, C.A.; Gibson, G.M.; Padgett, M.J.; Longbottom, C.; McMillan, L.C. The potential offered by real-time, high-sensitivity monitoring of ethane in breath and some pilot studies using optical spectroscopy. J. Opt. A: Pure Appl. Opt. 2005, 7, S376–S384. [Google Scholar]
- Dumitras, D.C.; Dutu, D.C.; Matei, C.; Magureanu, A.M.; Petrus, M.; Popa, C.; Patachia, V. Measurements of ethylene concentration by laser photoacoustic techniques with applications at breath analysis. Romanian Reports in Physics 2008, 60, 593–602. [Google Scholar]
- Miller, J.H.; Bakhirkin, Y.A.; Ajtai, T.; Tittel, F.K.; Hill, C.J.; Yang, R.Q. Detection of formaldehyde using off-axis integrated cavity output spectroscopy with an interband cascade laser. Appl. Phys. B: Laser Opt. 2006, 85, 391–396. [Google Scholar]
- Rehle, D.; Leleux, D.; Erdelyi, M.; Tittel, F.; Fraser, M.; Friedfeld, S. Ambient formaldehyde detection with a laser spectrometer based on difference-frequency generation in PPLN. Appl. Phys. B: Laser Opt. 2001, 72, 947–952. [Google Scholar]
- Dahnke, H.; von Basum, G.; Kleinermanns, K.; Hering, P.; Murtz, M. Rapid formaldehyde monitoring in ambient air by means of mid-infrared cavity leak-out spectroscopy. Appl. Phys. B: Lasers Opt. 2002, 75, 311–316. [Google Scholar]
- Angelmahr, M.; Miklos, A.; Hess, P. Photoacoustic spectroscopy of formaldehyde with tunable laser radiation at the parts per billion level. Appl. Phys. B: Lasers Opt. 2006, 85, 285–288. [Google Scholar]
- Horstjann, M.; Bakhirkin, Y.A.; Kosterev, A.A.; Curl, R.F.; Tittel, F.K.; Wong, C.M.; Hill, C.J.; Yang, R.Q. Formaldehyde sensor using interband cascade laser based quartz-enhanced photoacoustic spectroscopy. Appl. Phys. B: Lasers Opt. 2004, 79, 799–803. [Google Scholar]
- Richter, D.; Fried, A.; Wert, B.P.; Walega, J.G.; Tittel, F.K. Development of a tunable mid-IR difference frequency laser source for highly sensitive airborne trace gas detection. Appl. Phys. B: Lasers Opt. 2002, 75, 281–288. [Google Scholar]
- Ciaffoni, L.; Grilli, R.; Hancock, G.; Orr-Ewing, A.J.; Peverall, R.; Ritchie, G.A.D. 3.5 μm high-resolution gas sensing employing a LiNbO3 QPM-DFG waveguide module. Appl. Phys. B: Lasers Opt. 2009, 94, 517–525. [Google Scholar]
- Marinov, D.; Rey, J.M.; Muller, M.G.; Sigrist, M.W. Spectroscopic investigation of methylated amines by a cavity-ringdown-based spectrometer. Appl. Opt. 2007, 46, 3981–3986. [Google Scholar]
- Namjou, K.; Roller, C.B.; Reich, T.E.; Jeffers, J.D.; McMillen, G.L.; McCann, P.J.; Camp, M.A. Determination of exhaled nitric oxide distributions in a diverse sample population using tunable diode laser absorption spectroscopy. Appl. Phys. B: Lasers Opt. 2006, 85, 427–435. [Google Scholar]
- Menzel, L.; Kosterev, A.A.; Curl, R.F.; Tittel, F.K.; Gmachl, C.; Capasso, F.; Sivco, D.L.; Baillargeon, J.N.; Hutchinson, A.L.; Cho, A.Y.; Urban, W. Spectroscopic detection of biological NO with a quantum cascade laser. Appl. Phys. B: Lasers Opt. 2001, 72, 859–863. [Google Scholar]
- Kosterev, A.A.; Malinovsky, A.L.; Tittel, F.K.; Gmachl, C.; Capasso, F.; Sivco, D.L.; Baillargeon, J.N.; Hutchinson, A.L.; Cho, A.Y. Cavity ringdown spectroscopic detection of nitric oxide with a continuous-wave quantum-cascade laser. Appl. Opt. 2001, 40, 5522–5529. [Google Scholar]
- Roller, C.; Namjou, K.; Jeffers, J.D.; Camp, M.; Mock, A.; McCann, P.J.; Grego, J. Nitric oxide breath testing by tunable-diode laser absorption spectroscopy: application in monitoring respiratory inflammation. Appl. Opt. 2002, 41, 6018–6029. [Google Scholar]
- Namjou, K.; Roller, C.B.; McMillen, G. Breath analysis using mid infrared tunable laser spectroscopy. Proceedings of the 6th Annual IEEE Conference on Sensors, Atlanta, GA, USA; 2007; pp. 1337–1340. [Google Scholar]
- Heinrich, K.; Fritsch, T.; Hering, P.; Murtz, M. Infrared laser-spectroscopic analysis of 14NO and 15NO in human breath. Appl. Phys. B: Lasers Opt. 2009, 95, 281–286. [Google Scholar]
- Smith, D.; Wang, T.; Sule-Suso, J.; Spanel, P.; Haj, A.E. Quantification of acetaldehyde released by lung cancer cells in vitro using selected-ion flow-tube mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 845–850. [Google Scholar]
- Turner, C.; Spanel, P.; Smith, D. A longitudinal study of ethanol and acetaldehyde in the exhaled breath of healthy volunteers using selected-ion flow-tube mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 61–68. [Google Scholar]
- Omens, J.; Zuckermann, H.; Persijn, S.; Parker, D.H.; Harren, F.J.M. CO-laser-based photoacoustic trace-gas detection: applications in postharvest physiology. Appl. Phys. B: Lasers Opt. 1998, 67, 459–466. [Google Scholar]
- Persijn, S.T.; Parker, D.H.; Harren, F.J.M.; Veltman, R.H. Online laser-based detection of trace gas emission by avocado under changing atmospheric conditions. Acta Hortic. (ISHS) 2001, 553, 499–504. [Google Scholar]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar]
- Musa-Veloso, K.; Likhodii, S.S.; Rarama, E.; Benoit, S.; Liu, Y.M. C.; Chartrand, D.; Curtis, R.; Carmant, L.; Lortie, A.; Comeau, F.J.E.; Cunnane, S.C. Breath acetone predicts plasma ketone bodies in children with epilepsy on a ketogenic diet. Nutrition 2006, 22, 1–8. [Google Scholar]
- Pabst, F.; Miekisch, W.; Fuchs, P.; Kischkel, S.; Schubert, J.K. Monitoring of oxidative and metabolic stress during cardiac surgery by means of breath biomarkers: an observational study. J. Cardiothorac. Surg. 2007, 2, 37. [Google Scholar]
- Kupari, M.; Lommi, J.; Ventila, M.; Karjalainen, U. Breath acetone in congestive heart failure. Am. J. Cardiol. 1995, 76, 1076–1078. [Google Scholar]
- Ferreira da Silva1, F.; Nobrel, M.; Fernandes1, A.; Antunes1, R.; Almeida1, D.; Garcia, G.; Mason, N.J.; Limão-Vieira1, P. Spectroscopic studies of ketones as a marker for patients with diabetes. J. Phys.: Conf. Series 2008, 101, 012011/1–7. [Google Scholar]
- Kearney, D.J.; Hubbard, T.; Putnam, D. Breath ammonia measurement in Helicobacter pylori infection. Dig. Dis. Sci. 2002, 47, 2523–2530. [Google Scholar]
- Smith, D.; Wang, T.; Pysanenko, A.; Spanel, P. A selected ion flow tube mass spectrometry study of ammonia in mouth- and nose-exhaled breath and in the oral cavity. Rapid Commun. Mass Spectrom. 2008, 22, 783–789. [Google Scholar]
- Graham, D.Y.; Klein, P.D.; Evans, D.J., Jr. Campylobacter pylori detected noninvasively by the 13C-urea breath test. Lancet 1987, 11, 1174–1177. [Google Scholar]
- Eggers, R.H.; Kulp, A.; Tegeler, R.; Ludtke, F.E.; Lepsien, G.; Meyer, B.; Bauer, F.E. A methodological analysis of the 13C-urea breath test for detection of Helicobacter pylori infections: high sensitivity and specificity within 30 min using 75 mg of 13C-urea. Eur. J. Gastroenterol. Hepatol. 1990, 2, 437–444. [Google Scholar]
- Klein, P.D.; Malaty, H.M.; Martin, R.F.; Graham, K.S. Pylori infection in clinical practice: the 13C-urea breath test. Am. J. Gastroenterol. 1996, 91, 690–694. [Google Scholar]
- Giannini, E.G.; Testa, R. 13C-breath tests and liver fibrosis. Eur. Rev. Med. Pharmacol. Sci. 2004, 8, 51–54. [Google Scholar]
- Solomons, N.W.; Schoeler, D.A.; Wagonfeld, J.B.; Ott, D.G.; Rosenberg, I.H.; Klein, P.D. Application of a stable isotope 13C-labeled glycocholate breath test to diagnosis of bacterial overgrowth and ileal dysfunction. J. Lab. Clin. Med. 1977, 90, 431–439. [Google Scholar]
- Becker, J.F.; Sauke, T.B.; Loewenstein, M. Stable isotope analysis using tunable diode laser spectroscopy. Appl. Opt. 1992, 31, 1921–1927. [Google Scholar]
- Erdélyi, M.; Richter, D.; Tittel, F.K. 13CO2/13CO2 isotopic ratio measurements using a difference frequency-based sensor operating at 4.35 μm. Appl. Phys. B: Lasers Opt. 2002, 75, 289–295. [Google Scholar]
- Castrillo, A.; Casa, G.; van Burgel, M.; Tedesco, D.; Gianfrani, L. First field determination of the 13C/12C isotope ratio in volcanic CO2 by diode-laser spectrometry. Opt. Express. 2004, 12, 6515–6523. [Google Scholar]
- Castrillo, A.; Casa, G.; Kerstel, E.; Gianfrani, L. Diode laser absorption spectrometry for 13CO2/12CO2 isotope ratio analysis: Investigation on precision and accuracy levels. Appl. Phys. B: Lasers Opt. 2005, 81, 863–869. [Google Scholar]
- Wahl, E.H.; Fidric, B.; Rella, C.W.; Koulikov, S.; Kharlamov, B.; Tan, S.; Kachanov, A.A.; Richman, B.A.; Crosson, E.R.; Paldus, B.A.; Kalaskar, S.; Bowling, D.R. Application of cavity-ringdown spectroscopy to high precision isotope ratio measurement of 13C/12C in carbon dioxide. Isotopes Environ. Health Stud. 2006, 42, 21–35. [Google Scholar]
- Horner, G.; Lau, S.; Lohmannsroben, H-G. NIR diode laser spectroscopy for isotope selective sensing of soil-respired carbon dioxide. SPIE Proc. Ser. 2004, 5544, 47–54. [Google Scholar]
- Nelson, D.D.; McManus, J.B.; Herndon, S.C.; Zahniser, M.S.; Tuzson, B.; Emmenegger, L. New method for isotopic ratio measurements of atmospheric carbon dioxide using a 4.3 μm pulsed quantum cascade laser. Appl. Phys. B: Lasers Opt. 2008, 90, 301–309. [Google Scholar]
- Dirk, R.; Wert, B.P.; Fried, A.; Weibring, P.; Walega, J.G.; White, J.W.C.; Vaughn, B.H.; Tittel, F.K. High-precision CO2 isotopologue spectrometer with a difference-frequency-generation laser source. Opt. Lett. 2009, 34, 172–174. [Google Scholar]
- Vreman, H.J.; Mahoney, J.J.; Stevenson, D.K. Carbon monoxide and carboxyhemoglobin. Adv. Pediatr. 1995, 42, 330–334. [Google Scholar]
- Stevenson, D.K.; Vreman, H.J. Carbon monoxide and bilirubin production in neonates. Pediatr. Rev. 1997, 100, 252–259. [Google Scholar]
- Applegate, L.A.; Luscher, P.; Tyrrell, R.M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991, 51, 974–978. [Google Scholar]
- Yamaya, M.; Sekizawa, K.; Ishizuka, S.; Monma, M.; Mizuta, K.; Sasaki, H. Increased carbon monoxide in exhaled air of subjects with upper respiratory tract infections. Am. J. Respir. Crit. Care Med. 1998, 158, 311–314. [Google Scholar]
- Zayasu, K.; Sekizawa, K.; Okinaga, S.; Yamaya, M.; Ohrui, T.; Sasaki, H. Increased carbon monoxide in exhaled air of asthmatic patients. Am. J. Respir. Crit. Care Med. 1997, 156, 1140–1143. [Google Scholar]
- Moeskops, B.W.; Cristescu, S.M.; Harren, F.J. Sub-part-per-billion monitoring of nitric oxide by use of wavelength modulation spectroscopy in combination with a thermoelectrically cooled, continuous-wave quantum cascade laser. Opt. Lett. 2006, 31, 823–825. [Google Scholar]
- Sehnert, S.S.; Jiang, L.; Burdick, J.F.; Risby, T.H. Breath biomarkers for detection of human liver diseases: preliminary study. Biomarkers 2002, 7, 174–187. [Google Scholar]
- Studer, S.M.; Orens, J.B.; Rosas, I.; Krishnan, J.A.; Cope, K.A.; Yang, S.; Conte, J.V.; Becker, P.B.; Risby, T.H. Patterns and significance of exhaled-breath biomarkers in lung transplant recipients with acute allograft rejection. J. Heart Lung Transplant. 2001, 20, 1158–1166. [Google Scholar]
- Fried, A.; Drummound, J.R.; Henry, B.; Fox, J. Versatile integrated tunable diode laser system for high precsion: application for ambient measurement of OCS. Appl. Opt. 1991, 30, 1916–1932. [Google Scholar]
- Fischer, C.; Sigrist, M.W. Trace gas sensing in the 3.3 μm region using a diode based difference frequency laser photoaocustic system. Appl. Phys. B: Lasers Opt. 2002, 75, 305–310. [Google Scholar]
- Cope, K.A.; Solga, S.F.; Hummers, L.K.; Wigley, F.M.; Diehl, A.M.; Risby, T.H. Abnormal exhaled ethane concentrations in scleroderma. Biomarkers 2006, 11, 70–84. [Google Scholar]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Shah, P.L.; Cramer, D.; Hodson, M.E.; Barnes, P.J. Exhaled ethane is elevated in cystic fibrosis and correlates with carbon monoxide levels and airway obstruction. Am. J. Respir. Crit. Care Med. 2000, 161, 1247–1251. [Google Scholar]
- Harren, F.J.M.; Berkelmans, R.; Kuiper, K.; te Lintel Hekkert, S.; Scheepers, P.; Dekhuijzen, R.; Hollander, P.; Parker, D.H. On-line laser photoacoustic detection of ethene in exhaled air as biomarker of ultraviolet radiation damage of the human skin. Appl. Phys. Lett. 1999, 74, 1761–1763. [Google Scholar]
- Stolik, S.; Ramon-Gallegos, E.; Pacheco, M.; Tomas, S.A.; Cruz-Orea, A.; Perez-Zapata, A.J.; Gaebler, R.; Sanchez-Sinencio, F. Photoacoustic measurement of ethylene as a real time biomarker of lipid peroxidation processes in mice. Anal. Sci. 2001, 17, s365–s367. [Google Scholar]
- Wehinger, A.; Schmid, A.; Mechtcheriakov, S.; Ledochowski, M.; Grabmer, C.; Guenther, A.; Gastl, G.A.; Amann, A. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 2007, 265, 49–59. [Google Scholar]
- Koletzko, B.; Sauerwald, T.; Demmelmair, H. Safety of stable isotope use. Eur. J. Pediatr. 1997, 156, S12–S17. [Google Scholar]
- Davies, S.; Spanel, P.; Smith, D. Rapid measurement of deuterium content of breath following oral ingestion to determine body water. Physiol. Meas. 2001, 22, 651–659. [Google Scholar]
- Le Marchand, L.; Wilkens, L.R.; Harwood, P.; Cooney, R.V. Use of breath hydrogen and methane as markers of colonic fermentation in epidemiologic studies: circadian patterns of excretion. Environ. Health Perspect. 1992, 98, 199–202. [Google Scholar]
- Scotoni, M.; Rossi, A.; Bassi, D.; Buffa, R.; Iannotta, S.; Boschetti, A. Simultaneous detection of ammonia, methane and ethylene at 1.63 μm with diode laser photoacoustic spectroscopy. Appl. Phys. B: Lasers Opt. 2006, 82, 495–500. [Google Scholar]
- Grossel, A.; Zeninari, V.; Joly, L.; Parvitte, B.; Courtois, D.; Durry, G. New improvements in methane detection using a Helmholtz resonant photoacoustic laser sensor: A comparison between near-IR diode lasers and mid-IR quantum cascade lasers. Spectrochim. Acta, Part A 2006, 63, 1021–1028. [Google Scholar]
- Stry, S.; Hering, P.; Murtz, M. Portable difference-frequency laser-based cavity leak-out spectrometer for trace-gas analysis. Appl. Phys. B: Lasers Opt. 2002, 75, 297–303. [Google Scholar]
- Hennig, O.; Strzoda, R.; Magori, E.; Chemisky, E.; Tump, C.; Fleischer, M.; Meixner, H.; Eisele, I. Hand-held unit for simultaneous detection of methane and ethane based on NIR-absorption spectroscopy. Sens. Actuators, B 2003, 95, 151–156. [Google Scholar]
- Cristescu, S.M.; Persijn, S.T.; Hekkert, S.T.L.; Harren, F.J.M. Laser-based systems for trace gas detection in life sciences. Appl. Phys. B: Lasers Opt. 2008, 92, 343–349. [Google Scholar]
- Welzel, S.; Lombardi, G.; Davies, P.B.; Engeln, R.; Schram, D.C.; Ropcke, J. Trace gas measurements using optically resonant cavities and quantum cascade lasers operating at room temperature. J. Appl. Phys. 2008, 104, 093115/1–15. [Google Scholar]
- Wang, C.; Srivastava, N.; Jones, B.A.; Reese, R.B. A novel multiple species ringdown spectrometer for in situ measurements of methane, carbon dioxide, and carbon isotope. Appl. Phys. B: Lasers Opt. 2008, 92, 259–270. [Google Scholar]
- Kharitonov, S.A.; Barnes, P.J. Nitric oxide in exhaled air is a new marker of airway inflammation. Monaldi. Arch. Chest Dis. 1996, 51, 533–537. [Google Scholar]
- McCluskie, K.; Birrell, M.A.; Wong, S.; Belvisi, M.G. Nitric oxide as a noninvasive biomarker of lipopolysaccharide-induced airway inflammation: possible role in lung neutrophilia. J. Pharmacol. Exp. Ther. 2004, 311, 625–633. [Google Scholar]
- Birrell, M.A.; McCluskie, K.; Hardaker, E.; Knowles, R.; Belvisi, M.G. Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model. Eur. Respir. J. 2006, 28, 1236–1244. [Google Scholar]
- Breathmeter. Available online: http://www.ekipstech.com/pages/homepage/breathmeter/webpagecategory.xml (accessed October 16, 2009).
- Gas Sensors. Available online: http://www.pranalytica.com/technology-gas-sensors.html (accessed October 16, 2009).
- Lewicki, R.; Wysocki, G.; Kosterev, A.A.; Tittel, F.K. QEPAS based detection of broadband absorbing molecules using a widely tunable, cw quantum cascade laser at 8.4 μm. Opt. Express. 2007, 15, 7357–7366. [Google Scholar]
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930, (This Joint Statement of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) was adopted by the ATS Board of Directors, December 2004, and by the ERS Executive Committee, June 2004).
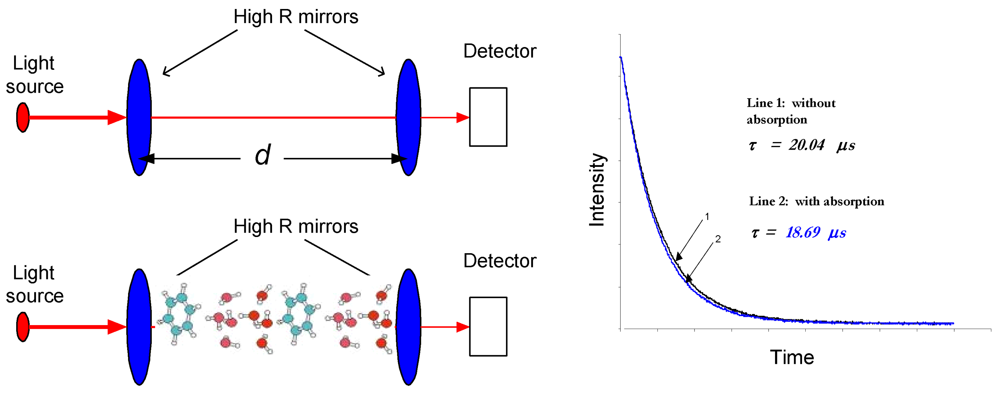
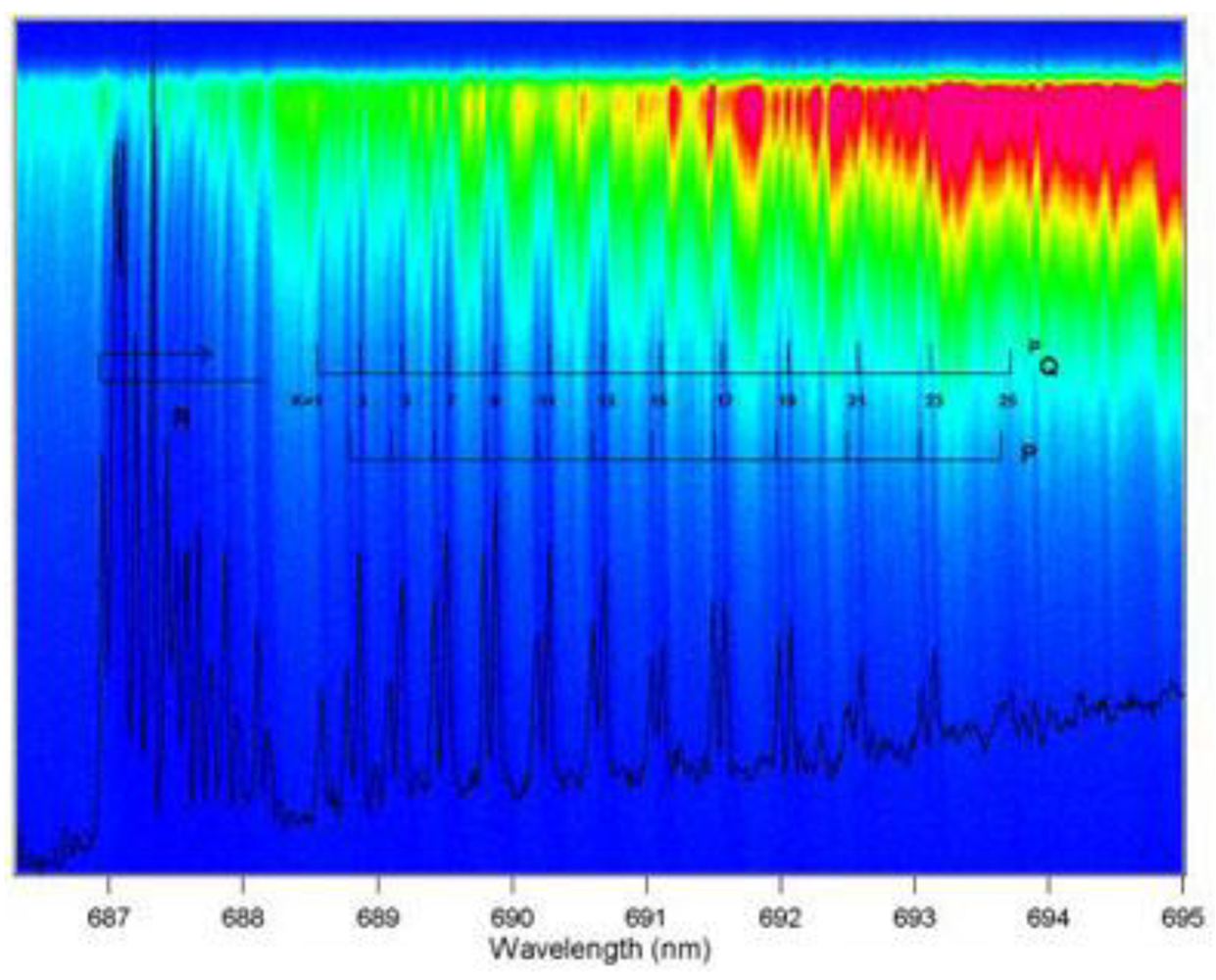
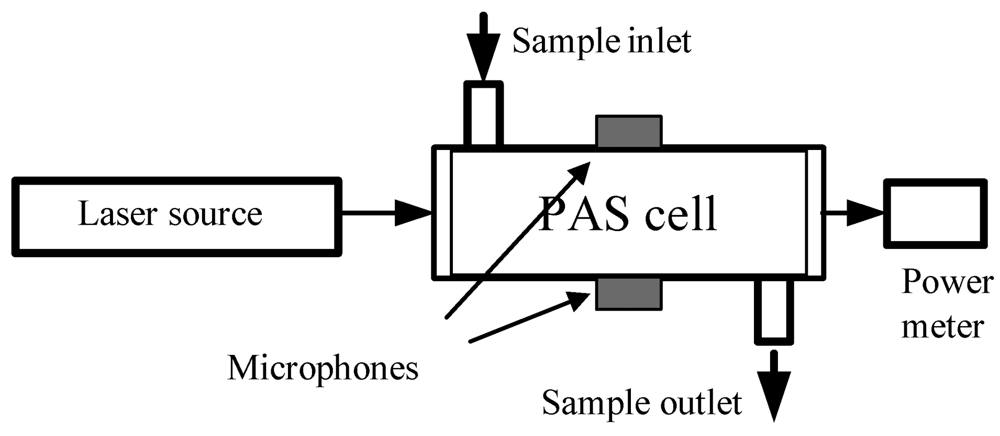
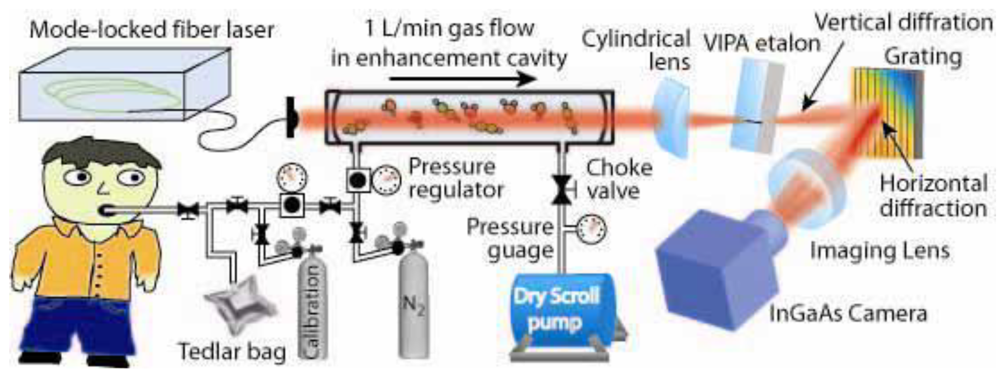

| Biomarkers | Metabolic Disorders / Diseases |
|---|---|
| Acetone (OC(CH3)2) | Lung cancer, diabetes, dietary fat losses, congestive heart failure, brain seizure |
| Acetaldehyde (CH3CHO) | Alcoholism, liver related diseases, lung cancer |
| Ammonia (NH3) | Renal diseases, asthma |
| Butane (C4H10) | Tumor marker in lung cancer |
| Carbon monoxide (CO) | Oxidative stress, respiratory infection, anaemias |
| Carbon disulphide (CS2) | Schizophrenia, coronary, and artery diseases |
| Carbon dioxide (CO2) (13C-Isotopes) | Oxidative stress |
| Carbonyl sulfide (OCS) | Liver related diseases |
| Ethane (C2H6) | Vitamin E deficiency in children, lipid peroxidation, oxidative stress |
| Ethanol (C2H5OH) | Production of gut bacteria |
| Ethylene (C2H4) | Lipid peroxidation, ultra violet radiation damage of skin |
| Hydrogen (H2) | Indigestion in infants, intestinal upset, colonic fermentation |
| H/D isotope | Body water |
| Hydrogen peroxide (H2O2) | Asthma |
| Hydrogen cyanide (HCN) | Pseudomonas aeruginosa in children affected with cystic fibrosis |
| 8-Isoprostane | Oxidative stress |
| Isoprene | Blood cholesterol |
| Methane (CH4) | Intestinal problems, colonic fermentation |
| Methanethiol (CH3SH) | Halitosis |
| Methanol (CH3OH) | Nervous system disorder |
| Methylated amines | Protein metabolism in body |
| Methyl nitrate (CH3NO3) | Hyperglycemia in Type 1 diabetes |
| Nitrogen monoxide (NO) | Asthma, bronchiectasis, hypertension, rhinitis, lung diseases |
| Nitrotyrosine (C9H10N2O5) | Asthma |
| Oxygen (O2) | Respiration |
| Pentane (C5H12) | Peroxidation of lipids, liver diseases, schizophrenia, breast cancer, rheumatoid arthritis |
| Pyridine (C5H5N) | Periodontal disease |
| Sulfur compounds | Hepatic diseases and malordor, lung cancer |
| Hydrocarbons (Toulene (C6H5CH3), Benzene (C6H6), Heptane (C7H16), Decane (C10H22), Styrene (C8H8), Octane (C8H18), Pentamethylheptane (C12H26)) | Lipid peroxidation, lung cancer, oxidative stress, airway inflammation |
| Breath bio- markers (chemical formula) | Spectral fingerprints (UV-MIR) (μm) | Laser spectroscopic technique | Detection limits/Measured concentrations | References |
|---|---|---|---|---|
| Acetaldehyde (CH3COH) | 5.79 | TDLAS | 80 ppb (5 sec) | Kamat et al. [75] |
| Acetone (OC(CH3)2) | 0.266 | CRDS | 0.2 ppm | Wang et al. [76-78] |
| Ammonia (NH3) | 9–10.7 | PAS | 100 ppm (3 sec) | Narasimhan et al. [79] |
| NH3 | 11.0 | TDLAS | 1 ppm (10 sec) | Lachish et al. [80] |
| NH3 | 10.0 | TDLAS | 50 ppb (20 sec) | Manne et al. [81] |
| NH3 | 10.0 | TDLAS | 3 ppb (10 sec) 4 ppb (5 sec) | Manne et al. [82] |
| NH3 | 10.3 | TDLAS | 5 ppb (30 sec) | Moskalenko et al. [83] |
| NH3 | 1.5 | OFC-CEAS | 4 ppm | Thorpe et al. [50] |
| Carbon dioxide and C-isotope [CO2 & 13CO2/12CO2] | 4.23 | PAS | 7 ppb | Herpen et al. (CO2 from insects in atmosphere) [84] |
| CO2 | 1.6 | CRDS | 3 ppm | Crosson et al. [85] |
| CO2 | 1.59 | TDLAS/WM | 100 ppm | Weldon et al. [86] |
| CO2 | 4.9 | TDLAS | 0.5 ppm (50 μs−1 ms) | Moskalenko et al. [83] |
| CO2 | 4.8 | TDLAS | ∼5.1% as compared with a spectrum of 5% CO2 in air. | Wysocki et al. [87] |
| CO2 | 4.9 | CALOS | (3.778 ± 0.004)% | Halmer et al. [46] |
| CO2 | 5.2 | ICOS | - | McCurdy et al. [88] |
| 13CO2/12CO2 | 1.6 | CRDS | Precision, 0.2‰ | Crosson et al. [85] |
| 13CO2/12CO2 | 1.6 | OFC-CEAS | Precision, 4.1‰ | Thorpe et al. [50] |
| Carbon monoxide (CO) | 1.6 | OFC-CEAS | 900 ppb | Thorpe et al. [50] |
| CO | 4.6 | TDLAS | 0.5 ppm | Moskalenko et al. [83] |
| CO | 4.88 | TDLAS | - | Lee et al. [89] |
| Carbonyl sulphide (OCS) | 4.86 | TDLAS | 1.2 ppb | Wysocki et al. [87] |
| OCS | 4.86 | TDLAS with Thermo- electrically QC laser | 30 ppb | Roller et al. [90] |
| OCS | 4.9 | CALOS | 438 ppt | Halmer et al. [88] |
| D/H isotopic ratio (D2O/H2O) | 3.50-3.65 | TDLAS | 55.2% ± 1.8% body water | Bartlome et al. [91] |
| Ethane (C2H6) | 3.4 | OA-ICOS | 0.12 ppb | Parameswaran et al. [92] |
| C2H6 | 3.4 | TDLAS | 0.1 ppb | Skeldon et al. [93] |
| C2H6 | 3.3 | CALOS | 270 ppt | Halmer et al. [45] |
| C2H6 | 3.0 | CALOS | 100 ppt | Dahnke et al. [43] |
| C2H6 | 2.6–4.0 | CALOS | 500 ppt (<800 ms) | von Basum et al. [44] |
| C2H6 | 3.4 | TDLAS | 0–12 ppb | Patterson et al. [94] |
| C2H6 | 3.3 | PAS | - | Puiu et al. [95] |
| C2H6 | 3.4 | TDLAS/WM | 70 ppt | Skeldon et al. [96] |
| Ethylene (C2H4) | 10.5 | PAS | - | Puiu et al. [95] |
| C2H4 | 9.2–10.8 | PAS | - | Dumitras et al. [97] |
| Formaldehyde (CH2O) | 3.53 | ICOS | 150 ppb | Miller et al. [98] |
| CH2O | 3.53 | TDLAS | 320 ppt | Rehle et al. [99] |
| CH2O | 3.53 | CALOS | 2 ppb | Dahnke et al. [100] |
| CH2O | 3.53 | PAS | 3 ppb | Angelmahr et al. [101] |
| CH2O | 3.53 | QEPAS | 0.6 ppm (10 sec) | Horastjann et al. [102] |
| CH2O | 3.53 | TDLAS | 77 ppt (1 min) | Richter et al. [103] |
| CH2O | 3.53 | TDLAS | 1.2 ppm | Ciaffoni et al. [104] |
| Methane (CH4) | 3.35 | TDLAS | 0.5 ppm (50 μs−1 ms) | Moskalenko et al. [83] |
| Methylamine (CH3NH2) | 1.51–1.53 | CRDS | 2.3 ppm | Marinov et al. [105] |
| Dimethylamine (CH3)2NH3) | 1.51–1.53 | CRDS | 10 ppm | Marinov et al. [105] |
| Nitric Oxide (NO) | 5.2 | ICOS | 1 ppb (4 sec) | Silva et al. [39] |
| NO | 5.2 | TDLAS | 2 ppb | Namjou et al. [106] |
| NO | 5.2 | TDLAS | 3 ppb (200 sec) | Menzel et al. [107] |
| NO | 5.2 | CEAS | 16 ppb | Menzel et al. [107] |
| NO | 5.2 | CRDS | 0.7 ppb | Kosterev et al. [108] |
| NO | 5.2 | TDLAS | 1.5 ppb (4 sec) | Roller et al. [109] |
| NO | 5.2 | TDLAS | 2 ppb | Namjou et al. [110] |
| NO | 5.2 | ICOS | 400 ppt (<1 sec) | McCurdy et al. [88] |
| 14NO and 15NO | 5.0 | CALOS | 7 ppt (70 sec) | Heinrich et al. [111] |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, C.; Sahay, P. Breath Analysis Using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits. Sensors 2009, 9, 8230-8262. https://doi.org/10.3390/s91008230
Wang C, Sahay P. Breath Analysis Using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits. Sensors. 2009; 9(10):8230-8262. https://doi.org/10.3390/s91008230
Chicago/Turabian StyleWang, Chuji, and Peeyush Sahay. 2009. "Breath Analysis Using Laser Spectroscopic Techniques: Breath Biomarkers, Spectral Fingerprints, and Detection Limits" Sensors 9, no. 10: 8230-8262. https://doi.org/10.3390/s91008230




