Using Electromyography to Detect the Weightings of the Local Muscle Factors to the Increase of Perceived Exertion During Stepping Exercise
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Data Analysis
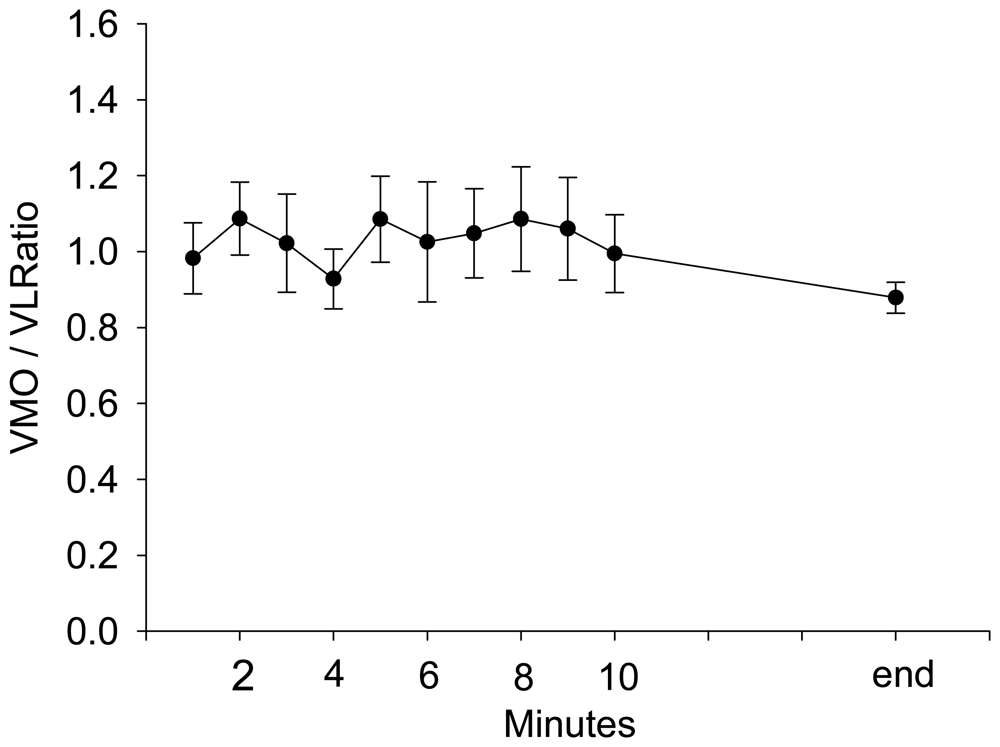
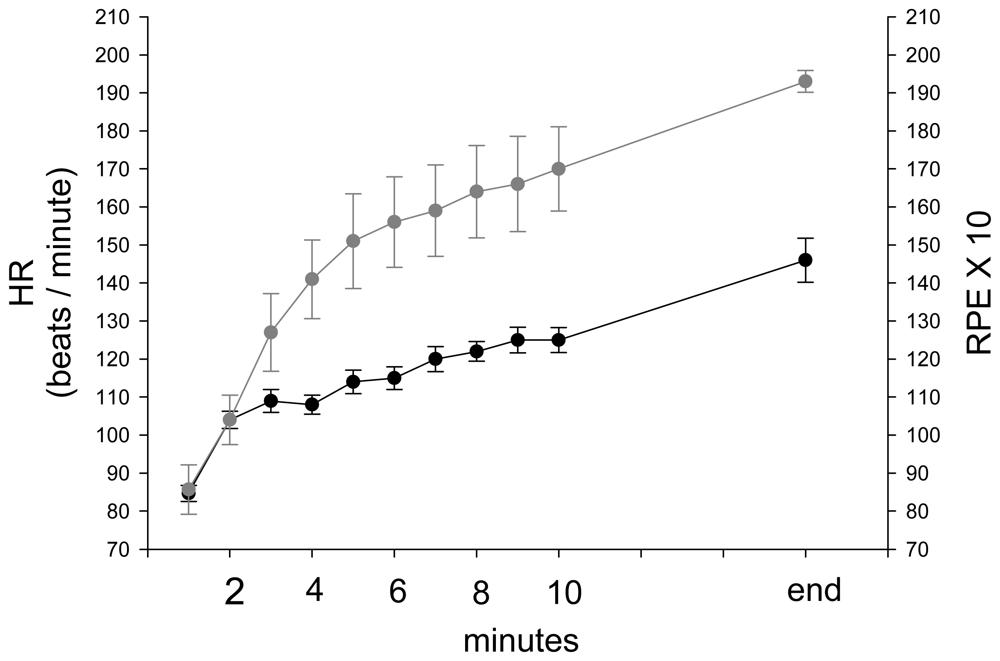
3. Results and Discussion Experimental Section
4. Discussion
5. Conclusion and Clinical Application
Acknowledgments
References
- Arendt-Nielsen, L.; Zwarts, M. Measurement of muscle fiber conduction velocity in humans: techniques and applications. Clinical Neurophysiology 1989, 6, 173–190. [Google Scholar]
- Basmajian, J.; De Luca, C. Muscles Alive: Their Functions Revealed by Electromyography, 5th ed.; Baltimore; Williams/Wilkins, 1985. [Google Scholar]
- Biedermann, H.J. A method for asserting the equivalence of repeated measures of muscle fatigue rates estimated from EMG power spectrum analysis. Journal of Electromyography and Kinesiology 1990, 1, 228–92. [Google Scholar]
- Bigland-Ritchie, B.; Donovan, E.F.; Roussos, C.S. Conduction velocity and EMG power spectrum changes in fatigue of sustained maximal efforts. Journal of Applied Physiology and Exercise Physiology 1981, 51, 1300–1305. [Google Scholar]
- Bigland-Ritchie, B.; Furbush, F.; Woods, J.J. Fatigue of intermittent submaximal voluntary contractions: central and peripheral factors. Journal of Applied Physiology 1986, 61, 421–429. [Google Scholar]
- Bigland-Ritchie, B.; Thomas, C.K.; et al. Muscle temperature, contractile speed, and motoneuron firing rates during human voluntary contractions. Journal of Applied Physiology 1992, 73, 2457–2461. [Google Scholar]
- Bilodeau, M.; Arsenault, A.; et al. EMG power spectra of elbow extensor during ramp and step isometric contraction. European Journal of Applied Physiology 1991, 63, 24–8. [Google Scholar]
- Bilodeau, M.; Schindler-Ivens, S.; et al. EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. Journal of Electromyography and Kinesiology 2003, 13, 83–92. [Google Scholar]
- Boreham, C.A.G.; Wallace, W.F.M.; Nevill, A. Training effects of accumulated daily-stair-climbing exercise in previously sedentary young women. Preventive Medicine 2000, 4, 277–81. [Google Scholar]
- Boucher, J.; King, M.; et al. Quadriceps femoris muscle activity in patellofemoral pain syndrome. American Journal of Sports Medicine 1992, 20, 527–32. [Google Scholar]
- Brunet, M. Patellofemoral rehabilitation. Clinical Sports Medicine 1989, 8, 319–29. [Google Scholar]
- Carton, R.; Rhodes, E. A critical review of the literature on ratings scales for perceived exertion. Sports Medicine 1985, 2, 198–222. [Google Scholar]
- Cerny, K. Vastus medialis oblique/vastus lateralis muscle activity ratios for selected exercises in persons with and without patellofemoral pain syndrome. Physical Therapy 1995, 75, 672–83. [Google Scholar]
- Ciccotti, M.; Kerlan, R.; et al. An electromyographic analysis of the knee during functional activities. American Journal of Sports Medicine 1994, 22, 645–50. [Google Scholar]
- Darques, J.; Jammes, Y. Fatigue-induced changes in group IV muscle afferent activity: differences between high- and low- frequency electrically induced fatigues. Brain Research 1997, 750, 147–54. [Google Scholar]
- De Luca, C. The use of the surface EMG signal for performance evaluation of back muscle. Muscle Nerve 1993, 16, 210–6. [Google Scholar]
- De Luca, C. The use of surface electromyography in biomechanics. Journal of Applied Biomechanics 2000, 13, 135–63. [Google Scholar]
- Doucette, S. The effect of exercise on patellar tracking in lateral patellar compression syndrome. American Journal of Sports Medicine 1992, 21, 434–40. [Google Scholar]
- Earl, J.; Schmitz, R.; Arnold, B. Activation of the VMO and VL during dynamic mini-squat exercises with and without isometric hip adduction. Journal of Electromyography and Kinesiology 2001, 11, 381–86. [Google Scholar]
- Edgerton, V.; Smith, J.; Simpson, D. Muscle fibre type populations of human leg muscles. The Histochemical Journal 1975, 7, 259–66. [Google Scholar]
- Eston, R.; Davies, B.; Williams, J. Use of perceived effort ratings to control exercise intensity in young healthy adults. European Journal of Applied Physiology and Occupational Physiology 1987, 56, 222–4. [Google Scholar]
- Fardy, P.S.; Ilmarinen, J. Evaluating the effects and feasibility of an at work stairclimbing intervention program for men. Medicine and Science in Sports and Exercise 1975, 7, 91–3. [Google Scholar]
- Fuglevand, A.; Macefield, V.; Bigland-Ritchie, B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. Journal of Neurophysiology 1999, 81, 1718–29. [Google Scholar]
- Fuglevand, A.J. The role of the sarcolemma action potential in fatigue. Fatigue: Neural and muscular mechanisms; Gandevia, S.C., Enoka, R.M., McComas, A.J., Stuart, D.G., Thomas, C.K., Eds.; New York/London; Plenum Press, 1995; pp. 101–108. [Google Scholar]
- Garcin, M.; Vautier, J.; et al. Ratings of perceived exertion (RPE) as an index of aerobic endurance during local and general exercises. Ergonomics 1998, 41, 1105–14. [Google Scholar]
- Garland, S. Role of small diameter afferents in reflex inhibition during human muscle fatigue. Journal of Physiology 1991, 435, 547–58. [Google Scholar]
- Garland, S.J.; McComas, A. Reflex inhibition of human soleus muscle during fatigue. Journal of Physiology 1990, 429, 17–27. [Google Scholar]
- Glenn, T. The anatomy of the extensor mechanism. Clinics in Sports Medicine 1989, 8, 163–77. [Google Scholar]
- Grabiner, M.; Koh, T. Neuromechanics of the patellofemoral joint. Medicine and Science in Sports and Exercise 1994, 26, 10–21. [Google Scholar]
- Green, J.; Crews, T.; et al. Heart rate and ratings of perceived exertion during treadmill and elliptical exercise training. Perceptual Motor Skills 2004, 98, 340–8. [Google Scholar]
- Grimes, K. Heart Disease, in Physical Rehabilitation: Assessment and Treatment; O'Sullivan, S., Schmitz, T., Eds.; F. A. Davis Co.: Philadelphia, 2001; pp. 471–518. [Google Scholar]
- Hanten, W.; Schulthies, S. Exercise effect on electromyographic activity of the vastus medialis oblique and vastus lateralis muscles. Physical Therapy 1990, 70, 561–5. [Google Scholar]
- Hertel, J.; Earl, J.; et al. Combining isometric knee extension exercises with hip adduction or abduction does not increase quadriceps EMG activity. Journal of Sports Medicine 2004, 38, 210–13. [Google Scholar]
- Ilmarinen, J.; Ilmarinen, R.; Koskela, A. Effects of stairclimbing during office hours on female employees. Ergonomics 1979, 22, 507–16. [Google Scholar]
- Johnson, M.A.; Polgar, J.; et al. Data on the distribution of fiber types in thirty six human muscles. An autopsy study. Journal of Neurological Science 1973, 18, 111–129. [Google Scholar]
- Juel, C. The effect of p2-adrenoceptor activation on ion-shifts and faituge in mouse soleus muscle stimulated in vitro. Acta Physiologica Scandinavica 1988, 134, 209–216. [Google Scholar]
- Liang, K.; Zeger, S.L. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika 1986, 73, 13–22. [Google Scholar]
- Loy, S.F.; Conley, L.M. Effects of stairclimbing on VO2max and quadriceps strength in middle-aged females. Medicine and Science in Sports and Exercise 1994, 26, 241–7. [Google Scholar]
- Malone, T.; Davies, G.; Walsh, M. Muscular control of the patella. Clinics in Sports Medicine 2002, 21, 349–62. [Google Scholar]
- Mariani, PP.; Caruso, I. An electromyographic investigation of subluxation of the patella. Journal of Bone and Joint Surgery 1979, 61, 169–71. [Google Scholar]
- Merletti, R.; Lo Conte, L.R.; et al. Effect of ankle joint position on electrically evoked surface myoelectric signals of the tibialis anterior muscle. Archives of Physical Medicine and Rehabilitation 1993, 74, 501–506. [Google Scholar]
- Miller, J.; Sedory, D.; Croce, R. Vastus medialis obliquus and vastus lateralis activity in patients with and without patellofemoral pain syndrome. Journal of Sports Rehabilitation 1997, 6, 1–10. [Google Scholar]
- Pandolf, K. Differentiated ratings of perceived exertion during physical exercise. Medicine and Science in Sports and Exercise 1982, 14, 397–405. [Google Scholar]
- Polgar, J.; Johnson, M.; et al. Data on fibre size in thirty-six human muscles-an autopsy study. Journal of the Neurological Sciences 1973, 19, 307–18. [Google Scholar]
- Powers, C.; Landel, R.; Perry, J. Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain syndrome. Physical Therapy 1996, 76, 946–55. [Google Scholar]
- Reynolds, L.; Levin, T.; et al. EMG activity of the vastus medialis oblique and the vastus lateralis in their role in patellar alignment. American Journal of Physical Medicine 1983, 62, 169–71. [Google Scholar]
- Robertson, R.; Falkel, J.; et al. Effect of blood pH on peripheral and central signals of perceived exertion. Medicine and Science in Sports and Exercise 1986, 18, 114–22. [Google Scholar]
- Sagiv, M.; Bar-Eli, M.; et al. Perceptual responses to exercise: the effect of load-awareness on physiological responses during an isometric bout. International Journal of Sports Medicine 1999, 20, 44–7. [Google Scholar]
- Sheehy, P.; Burdett, R.; et al. An electromyographic study of vastus medialis obliquus and vastus lateralis activity while ascending and descending steps. Journal of Orthopedics and. Sports Physical Therapy 1998, 27, 423–9. [Google Scholar]
- Shigematsu, R.; Ueno, L.; et al. Rate of perceived exertion as a tool to monitor cycling exercise intensity in older adults. Journal of Aging and Physical Activity 2004, 12, 3–9. [Google Scholar]
- Souza, D.; Gross, M. Comparison of vastus medialis obliquus: Vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain. Physical Therapy 1991, 71, 310–20. [Google Scholar]
- Stamford, B. Validity and reliability of subjective ratings of perceived exertion during work. Ergonomics 1976, 19, 53–60. [Google Scholar]
- Viitasalo, J.T.; Komi, P.V. Interrelationships of EMG signal characteristics at different levels of muscle tension and during fatigue. Electromyography and Clinical Neurophysiology 1978, 18, 167–178. [Google Scholar]
- Wallman, K.; Goodman, C.; et al. Test-retest reliability of the aerobic power index component of the tri-level fitness profile in a sedentary population. J. Sci. Med. Sport 2003, 6, 443–54. [Google Scholar]
- Zeger, S.L.; Liang, K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–30. [Google Scholar]
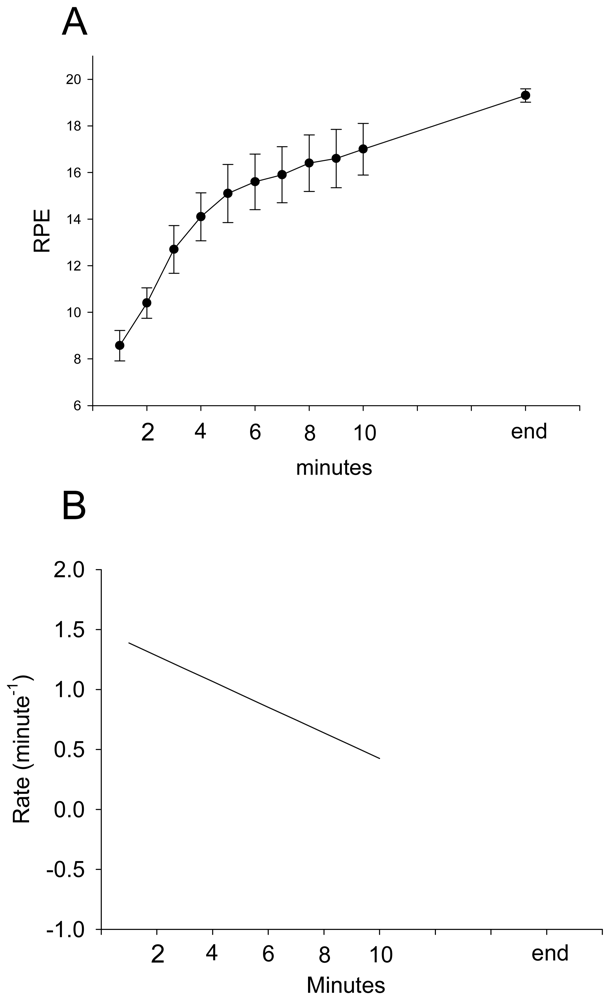
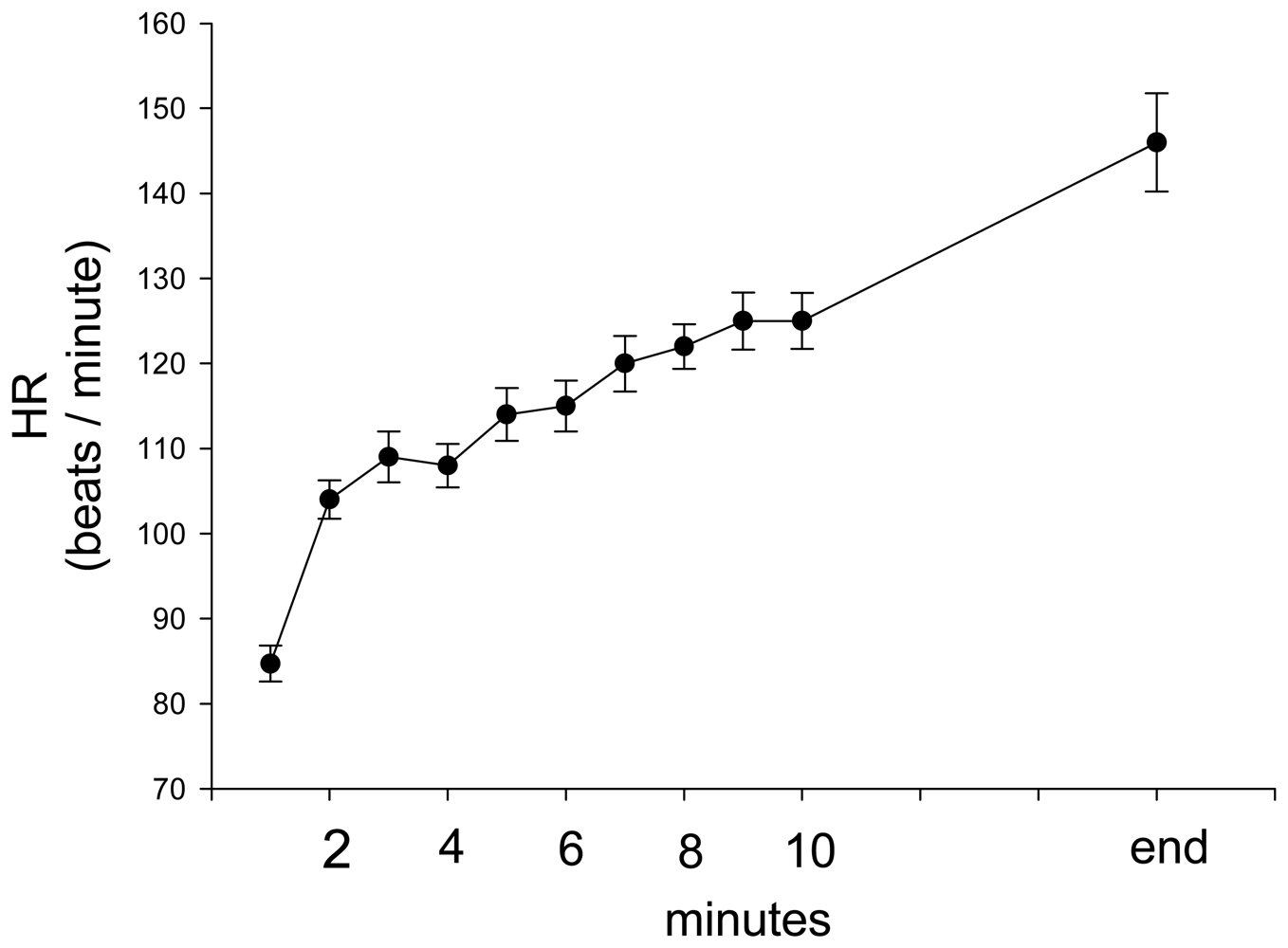
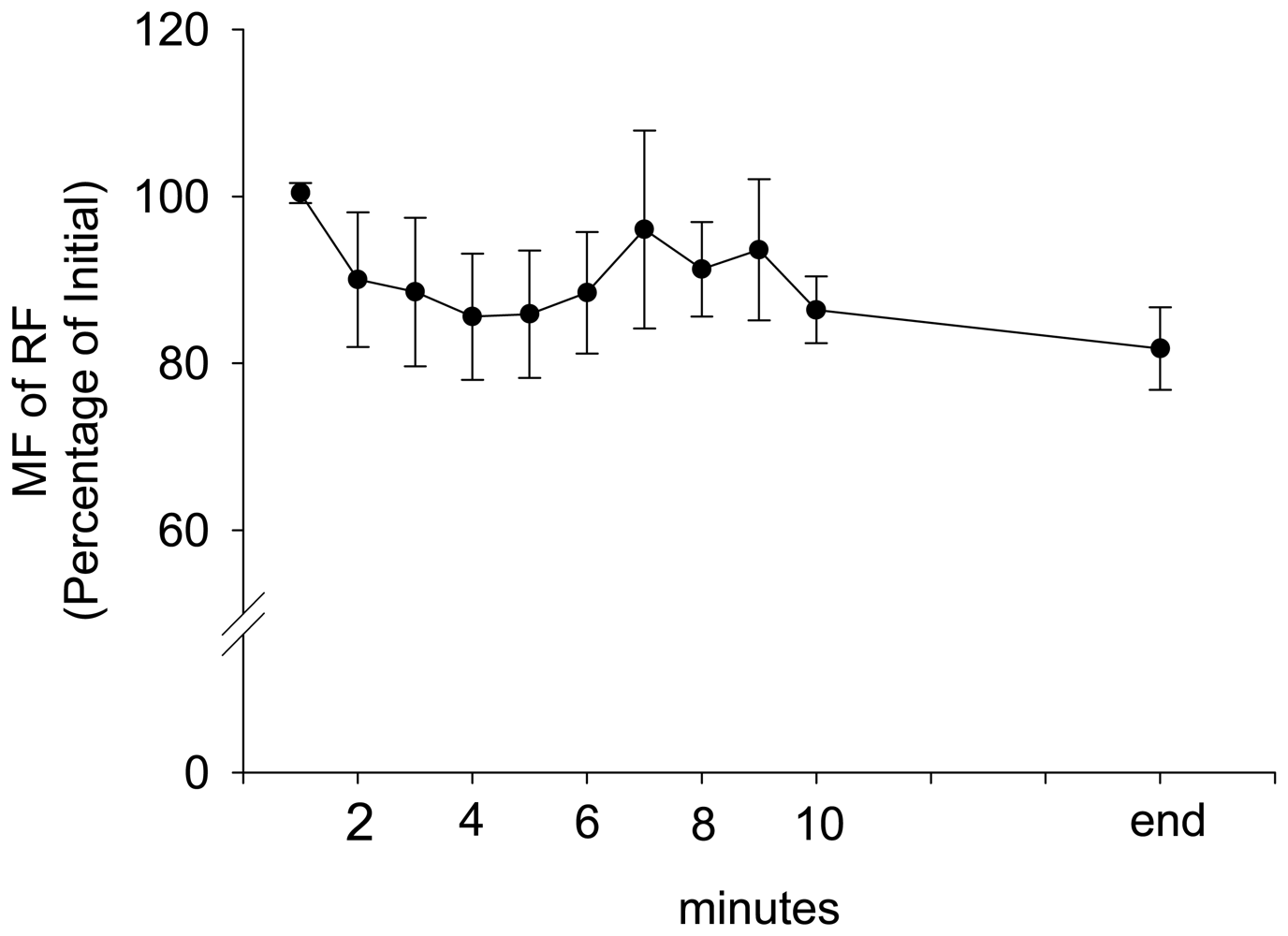
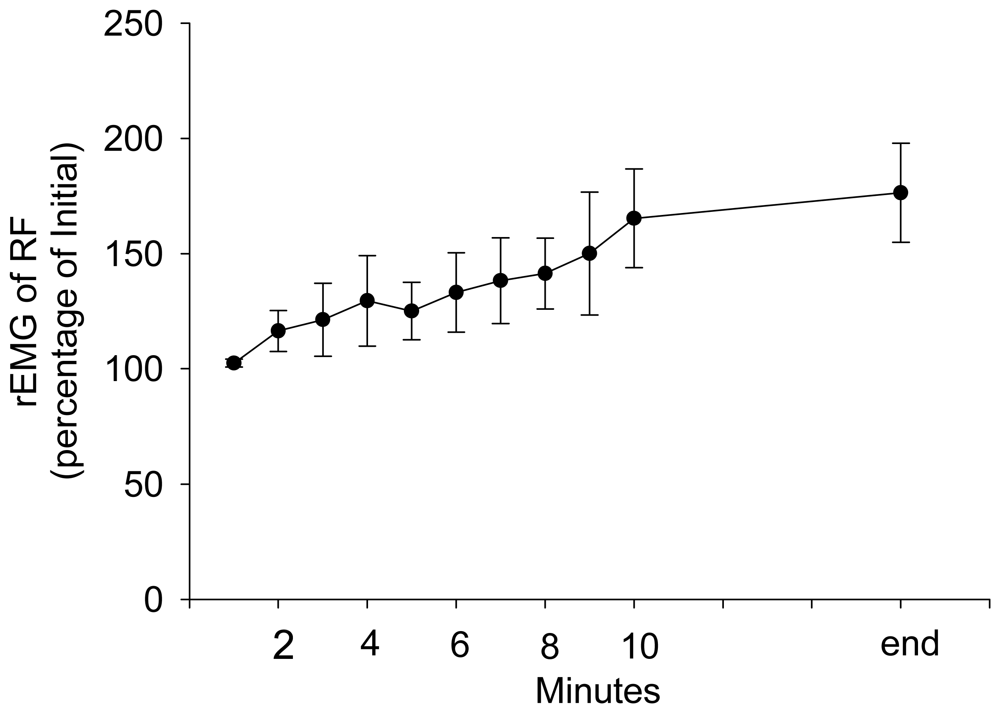
| Parameter | Estimate | Standard Error | 95% Confidence Limits | Z | P | |
|---|---|---|---|---|---|---|
| Intercept | 6.0983 | 2.8901 | 0.4339 | 11.7627 | 2.11 | 0.0349 |
| Time | 1.5629 | 0.2134 | 1.1446 | 1.9813 | 7.32 | <.0001 |
| Time2 | -0.0587 | 0.0132 | -0.0847 | -0.0328 | -4.43 | <.0001 |
| rEMG of RF | -0.7789 | 0.9227 | -2.5873 | 1.0296 | -0.84 | 0.3986 |
| MF of RF | -2.6306 | 1.0238 | -4.6372 | -0.6240 | -2.57 | 0.0102 |
| VM:VL Ratio | 0.5146 | 0.6947 | -0.8471 | 1.8763 | 0.74 | 0.4589 |
| HR | 3.8270 | 1.7014 | 0.4922 | 7.1617 | 2.25 | 0.0245 |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, Y.-J.; Liu, C.-C.; Lin, C.-H.; Tsaih, P.-L.; Hsu, M.-J. Using Electromyography to Detect the Weightings of the Local Muscle Factors to the Increase of Perceived Exertion During Stepping Exercise. Sensors 2008, 8, 3643-3655. https://doi.org/10.3390/s8063643
Chang Y-J, Liu C-C, Lin C-H, Tsaih P-L, Hsu M-J. Using Electromyography to Detect the Weightings of the Local Muscle Factors to the Increase of Perceived Exertion During Stepping Exercise. Sensors. 2008; 8(6):3643-3655. https://doi.org/10.3390/s8063643
Chicago/Turabian StyleChang, Ya-Ju, Chin-Chih Liu, Cheng-Hsiang Lin, Peih-Ling Tsaih, and Miao-Ju Hsu. 2008. "Using Electromyography to Detect the Weightings of the Local Muscle Factors to the Increase of Perceived Exertion During Stepping Exercise" Sensors 8, no. 6: 3643-3655. https://doi.org/10.3390/s8063643




