Signature Optical Cues: Emerging Technologies for Monitoring Plant Health
Abstract
:1. Introduction
2. Stress-Associated Leaf Spectral Properties
2.1 Fundamentals of plant stress sensing
2.2 Techniques for measuring plant stress
2.2.1 Thermography and Fluorescence
2.2.2 Leaf Reflectance
- blue region (400 – 499 nm) which is strongly influenced by absorption of chlorophylls and carotenoids.
- blue-green edge (500 – 549 nm) leading to the green peak at 550 nm.
- red edge (650 – 699 nm) associated with strong chlorophyll absorption.
2.2.3 Biochemical basis for leaf reflectance properties
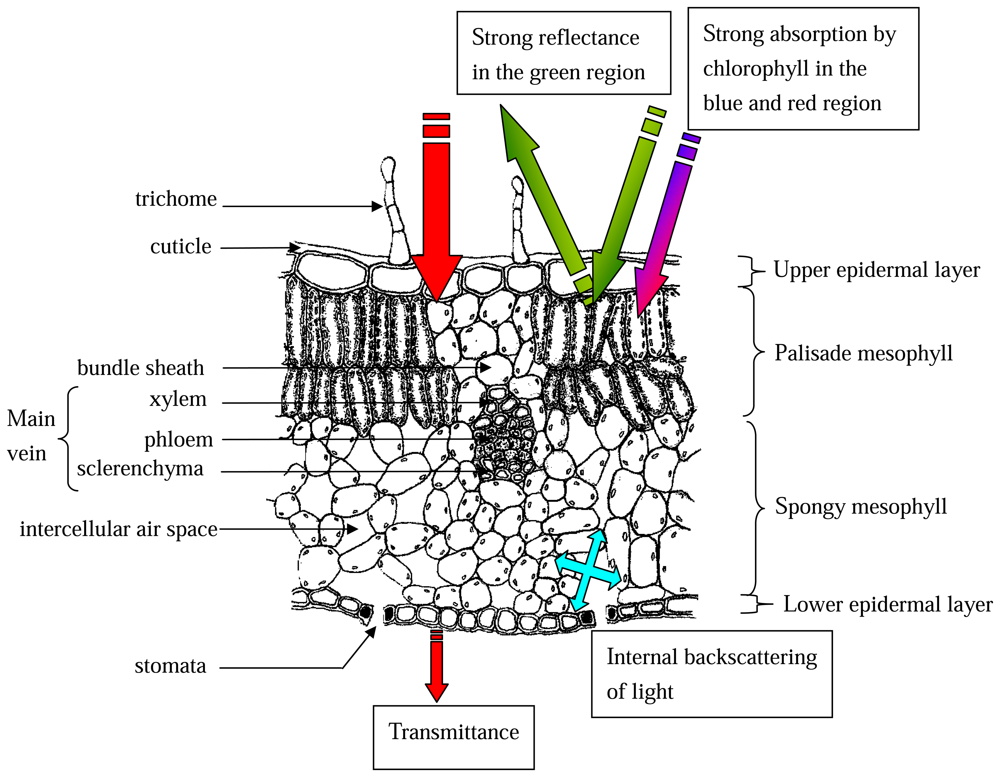
2.2.4 Anatomical basis for leaf reflectance properties
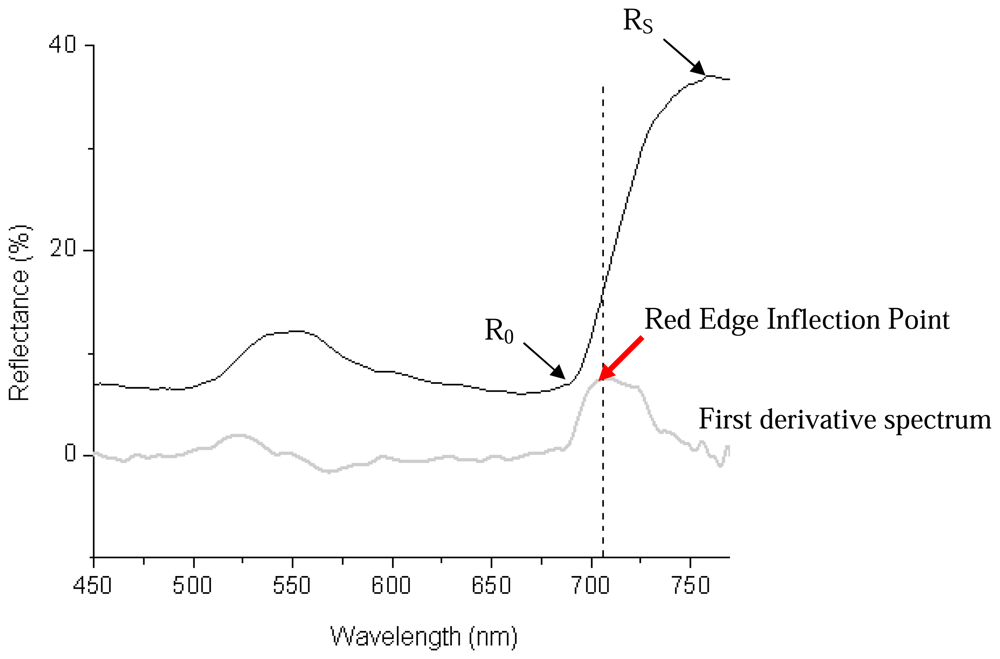
2.2.5 Vegetative indices derived from leaf reflectance spectra
2.3 Challenges to teasing out signature optical stress cues
2.3.1 Confounding factors and limitations in obtaining signature cues for specific stresses
- 1)
- Within-leaf: Multiple measurements per individual leaf at various positions; basal, middle or leaf tip and margins, leaf blade versus main vein. Castro-Esau et al. (2006) [87] reported greater variation in spectral measurements taken over the leaf blade particularly over leaf veins and took precautions to avoid the main vein. This is not surprising since chlorophyll is not distributed evenly across the leaf blade [87-88].
- 2)
- Within-plant: Multiple leaves sampled per plant at terminal young, middle or older basal positions. In many plant species, the terminal young leaves are generally of a lighter green shade compared with the more mature regions.
- 3)
- Between plants within species and same growing conditions: Measurements from multiple plants growing in the same site using the same spectrometer and collection parameters.
2.3.2 Strategies in teasing out signature stress cues
2.4 Opportunities for developing non-contact / remote sensing
3. Transgenic approaches for sensing plant stresses
3.1 Biosensing organisms
- There should be no or at most weak basal (uninduced, background or leaky) reporter gene expression.
- The fusion gene should be highly inducible.
- The range of compounds or conditions that elicit gene expression may be broad or narrow; in other words, specificity is according to user defined objectives.
- The intensity of the signal should be well correlated with the concentration of the inducer compound or the severity of the physical/biological condition.
- The signal response should be easily measurable with a high dynamic range of inducer concentration or conditions.
- The spatial distribution of the signal should be uniform throughout the plant or a specific tissue depending on the specific application.
- The temporal response should be appropriate to user objectives; for example, for sensing water-deficit conditions in plants, a measurable response should be elicited at early onset of water stress before plant entry into permanent wilting point.
- “Switch-off” of the signal should be possible once the inducer compound, triggering event or condition is removed.
- The optical characteristic of the signal should be distinct from any interfering background noise within the plant environment to give high signal-to-noise ratio.
3.2 Inducible promoters for stress detection
3.3 Reporters
3.4 Transgenic phytosensors
4. Space Applications
5. Concluding remarks
Acknowledgments
References
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Nat. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar]
- Chaerle, L.; Van der Straeten, D. Imaging techniques and the early detection of plant stress. Trends Plant Sci. 2000, 5, 495–501. [Google Scholar]
- Ferl, R.; Wheeler, R.; Levine, H.G.; Paul, A.L. Plants in space. Curr. Opin.Plant Biol. 2002, 5, 258–263. [Google Scholar]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll content. Am. J. Bot. 2001, 88, 677–684. [Google Scholar]
- Carter, G.A. Responses of leaf spectral reflectance to plant stress. Am. J. Bot. 1993, 80, 239–243. [Google Scholar]
- Thomas, S.C. Increased leaf reflectance in tropical trees under elevated CO2. Global Change Biol. 2005, 11, 197–202. [Google Scholar]
- Smith, K.L.; Steven, M.D.; Colls, J.J. Use of hyperspectral derivative ratios in the red-edge region to identify plant stress responses to gas leaks. Remote Sens. Environ. 2004, 92, 207–217. [Google Scholar]
- Dobrowski, S.Z.; Pushnik, J.C.; Zarco-Tejada, P.J.; Ustin, S.L. Simple reflectance indices track heat and water-stress induced changes in steady-state chlorophyll fluorescence at the canopy level. Remote Sens. Environ. 2005, 97, 403–414. [Google Scholar]
- Carter, G.A.; Bahadur, R.; Norby, R.J. Effects of elevated atmospheric CO2 and temperature on leaf optical properties in Acer saccharum. Environ. Exp. Bot. 2000, 43, 267–273. [Google Scholar]
- Rosso, P.H.; Pushnik, J.C.; Lay, M.; Ustin, S.L. Reflectance properties and physiological responses of Salicornia virginica to heavy metal and petroleum contamination. Environ. Pollut. 2005, 137, 241–252. [Google Scholar]
- Bornman, J.F.; Vogelmann, T.C. Effect of UV-B radiation on leaf optical properties measured with fibre optics. J. Exp. Bot. 1991, 42, 547–554. [Google Scholar]
- Graeff, S.; Claupein, W. Identification and discrimination of water stress in wheat leaves (Triticum aestivum L.) by means of reflectance measurements. Irrigation Sci. 2007, 26, 61–70. [Google Scholar]
- Riedell, W.E.; Blackmer, T.M. Leaf reflectance spectra of cereal aphid-damaged wheat. Crop Sci. 1999, 39, 1835–1840. [Google Scholar]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar]
- Peñuelas, J.; Isla, R.; Filella, I.; Araus, J.L. Visible and near-infrared reflectance assessment of salinity effects on barley. Crop Sci. 1997, 37, 198–202. [Google Scholar]
- Masoni, A.; Ercoli, L.; Mariotti, M. Spectral properties of leaves deficient in iron, sulphur, magnesium and manganese. Agron. J. 1996, 88, 937–943. [Google Scholar]
- Baltzer, J.L.; Thomas, S.C. Leaf optical responses to light and soil nutrient availability in temperate deciduous trees. Am. J. Bot. 2005, 92, 214–223. [Google Scholar]
- Vogelmann, T.C. Plant tissue optics. Annu. Rev. Plant Phys. 1993, 44, 231–251. [Google Scholar]
- Slaton, M.R.; Hunt, E.R., Jr.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar]
- Buschmann, C.; Lichtenthaler, H.K. Reflectance and chlorophyll fluorescence signatures of leaves. In Applications of chlorophyll fluorescence in photosynthesis research, stress physiology, hydrobiology and remote sensing; Lichtenthaler, H.K., Ed.; Kluwer Academic Press, 1988; pp. 325–332. [Google Scholar]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of non-invasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar]
- Carter, G.A.; Spiering, B.A. Optical properties of intact leaves for estimating chlorophyll concentration. J. Environ. Qual. 2002, 31, 1424–1432. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar]
- Tarpley, L.; Reddy, K.R.; Sassenrath-Cole, G.F. Reflectance indices with precision and accuracy in predicting cotton leaf nitrogen concentration. Crop Sci. 2000, 40, 1814–1819. [Google Scholar]
- Yoder, B.J.; Pettigrew-Crosby, R.E. Predicting nitrogen and chlorophyll content and concentrations from reflectance spectra (400-2500 nm) at leaf and canopy scales. Remote Sens. Environ. 1995, 53, 199–211. [Google Scholar]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzylak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol 2002, 75, 272–281. [Google Scholar]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar]
- Grant, O.M.; Tronina, L.; Jones, H.G.; Chaves, M.M. Exploring thermal imaging variables for the detection of stress responses in grapevine under different irrigation regimes. J. Exp. Bot. 2007, 58, 815–825. [Google Scholar]
- Jones, H.G. Use of thermography for quantitative studies of spatial and temporal variation of stomatal conductance over leaf surfaces. Plant Cell Environ. 1999, 22, 1043–1055. [Google Scholar]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and ecophysiology. Adv. Bot. Res. 2004, 41, 107–163. [Google Scholar]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar]
- Leinonen, I.; Jones, H.G. Combining thermal and visible imagery for estimating canopy temperature and identifying plant stress. J. Exp. Bot. 2004, 55, 1423–1431. [Google Scholar]
- Chaerle, L.; Van Caeneghem, W.; Messens, E.; Lambers, H.; Van Montagu, M.; Van der Straeten, D. Presymptomatic visualization of plant-virus interactions by thermography. Nat. Biotechnol. 1999, 17, 813–816. [Google Scholar]
- Oerke, E.C.; Steiner, U.; Dehne, H.W.; Lindenthal, M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006, 57, 2121–2132. [Google Scholar]
- Subhash, N.; Mohanan, C.N. Curve-fit analysis of chlorophyll fluorescence spectra: Application to nutrient stress detection in sunflower. Remote Sens. Environ. 1997, 60, 347–356. [Google Scholar]
- Lichtenthaler, H.K.; Miehé, J.A. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997, 2, 316–320. [Google Scholar]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit. Rev. Anal. Chem. 1988, 19(suppl. 1), S29–S85. [Google Scholar]
- Buschmann, C.; Lichtenthaler, H.K. Principles and characteristics of multi-color fluorescence imaging of plants. J. Plant Physiol. 1998, 152, 297–314. [Google Scholar]
- Valentini, R.; Cecchi, G.; Mazzinghi, P.; Mugnozza, G.S.; Agati, G.; Bazzani, M.; De Angelis, P.; Fusi, F.; Matteucci, G.; Raimondi, V. Remote sensing of chlorophyll a fluorescence of vegetation canopies: 2. Physiological significance of fluorescence signal in response to environmental stresses. Remote Sens. Environ. 1994, 47, 29–35. [Google Scholar]
- Chaerle, L.; Hagenbeek, D.; De Bruyne, E.; Valcke, R.; Van der Straeten, D. Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol. 2004, 45, 887–896. [Google Scholar]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; Van der Straeten, D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot. 2007, 58, 773–784. [Google Scholar]
- Fourty, Th.; Baret, F.; Jacquemoud, S.; Schmuck, G.; Verdebout, J. Leaf optical properties with explicit description of its biochemical composition: direct and inverse problems. Remote Sens. Environ. 1996, 56, 104–117. [Google Scholar]
- Marquez, U.M.L.; Sinnecker, P. Chlorophylls: Properties, biosynthesis, degradation and functions. In Food Colorants Chemical and Functional Properties; Socaciu, C., Ed.; Taylor and Francis: Boca Raton, 2007; pp. 25–49. [Google Scholar]
- Merritt, J.E.; Loening, K.L. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN): Nomenclature of tetrapyrroles: Recommendations 1978. Eur. J. Biochem. 1980, 108, 1–30. [Google Scholar]
- Peñuelas, J.; Filella, I. Visible and near infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar]
- Li, B.; Liew, O.W.; Asundi, A.K. Pre-visual detection of iron and phosphorus deficiency by transformed reflectance spectra. J. Photoch. Photobio. B. 2006, 85, 131–139. [Google Scholar]
- Ayala-Silva, T.; Beyl, C.A. Changes in spectral reflectance of wheat leaves in response to specific macronutrient deficiency. Adv. Space Res. 2005, 35, 305–317. [Google Scholar]
- Adams, M.L.; Philpot, W.D.; Norvell, W.A. Yellowness index: An application of spectral second derivatives to estimate chlorosis of leaves in stressed vegetation. Int. J. Remote Sens. 1999, 20, 3663–3675. [Google Scholar]
- Myers, D.A.; Vogelmann, T.C.; Bornman, J.F. Epidermal focussing and effects on light utilization in Oxalis acetosella. Physiol. Plantarum 1994, 91, 651–656. [Google Scholar]
- Bone, R.A.; Lee, D.W.; Norman, J.M. Epidermal cells functioning as lenses in leaves of tropical rain-forest shade plants. Appl. Optics 1985, 24, 1408–1412. [Google Scholar]
- Barnes, J.D.; Cardosa-Vilhena, J. Interactions between electromagnetic radiation and the plant cuticle. In Plant cuticles: an integrated functional approach; Kirstens, G., Ed.; BIOS Scientific Publishers: Oxford, UK, 1996; pp. 157–170. [Google Scholar]
- Grant, L.; Daughtry, C.S.T.; Vanderbilt, V.C. Polarized and specular reflectance variation with leaf surface features. Physiol. Plantarum 1993, 88, 1–9. [Google Scholar]
- Grant, L. Diffuse and specular characteristics of leaf reflectance. Remote Sens. Environ. 1987, 22, 309–322. [Google Scholar]
- Ehleringer, J.; Björkman, O.; Mooney, H.A. Leaf pubescence: effects on absorptance and photosynthesis in a desert shrub. Science 1976, 192, 376–377. [Google Scholar]
- DeLucia, E.H.; Nelson, K.; Vogelmann, T.C.; Smith, W.K. Contribution of intercellular reflectance to photosynthesis in shade leaves. Plant Cell Environ. 1996, 19, 159–170. [Google Scholar]
- Vogelmann, T.C.; Martin, G. The functional significance of palisade tissue: penetration of directional versus diffuse light. Plant Cell Environ. 1993, 16, 65–72. [Google Scholar]
- Vogelmann, T.C.; Knapp, A.K.; McClean, T.M.; Smith, W.K. Measurement of light within thin plant tissues with fibre optic microprobes. Physiol. Plantarum 1988, 72, 623–630. [Google Scholar]
- Knapp, A.K.; Carter, G.A. Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 1998, 85, 940–946. [Google Scholar]
- Woolley, J.T. Refractive index of soybean leaf cell walls. Plant Physiol. 1975, 55, 172–174. [Google Scholar]
- Allen, W.A.; Gausman, H.G.; Richardson, A.J.; Thomas, J.R. Interaction of isotropic light with a compact plant leaf. J. Opt. Soc. Am. 1969, 59, 1376–1379. [Google Scholar]
- Jacquemoud, S.; Ustin, S.L.; Verdebout, J.; Schmuck, G.; Andreoli, G.; Hosgood, B. Estimating leaf biochemistry using the PROSPECT leaf optical properties model. Remote Sens. Environ. 1996, 56, 194–202. [Google Scholar]
- Jacquemoud, S.; Baret, F. PROSPECT: a model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar]
- Yamada, N.; Fujimura, S. A mathematical model of reflectance and transmittance of plant leaves as a function of chlorophyll pigment content. Proceedings of the International Geoscience and Remote Sensing Symposium, (IGARSS '88) Edinburgh Scotland, 13-16 Sep, 1988; pp. 833–834.
- Gausman, H.W.; Allen, W.A.; Cardenas, R.; Richardson, A.J. Relation of light reflectance to histological and physical evaluations of cotton leaf maturity. Appl. Optics 1970, 9, 545–552. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environment 2002, 81, 337–354. [Google Scholar]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plantarum 1999, 106, 135–141. [Google Scholar]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar]
- le Maire, G.; Francois, C.; Dufrene, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar]
- Bonham-Carter, G.F. Numerical procedures and computer program for fitting an inverted Gaussian model to vegetation reflectance data. Comput. Geosci. 1988, 14, 339–356. [Google Scholar]
- Danson, F.M.; Plummer, S.E. Red-edge response to forest leaf area index. Int. J. Remote Sens. 1995, 16, 183–188. [Google Scholar]
- Cutler, M.E.J.; Curran, P.J. An observation of shifts in the position of the red edge at different spatial resolutions. In RSS96: Remote Sensing Science and Industry; Nottingham; Remote Sensing Society, 1996; pp. 290–297. [Google Scholar]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar]
- Chen, Z.; Elvidge, C.D. Description of derivative-based high-spectral-resolution (AVIRIS) green vegetation index. In Proceedings of the SPIE vol. 1937 Imaging Spectrometry of the Terrestrial Environment; Vare, G., Ed.; 1993; pp. 43–54. [Google Scholar]
- Railyan, V.Y.; Korobov, R.M. Red edge structure of canopy reflectance spectra of Triticale. Remote Sens. Environ. 1993, 46, 172–182. [Google Scholar]
- Dawson, T.P.; Curran, P.J. A new technique for interpolating the reflectance red edge position. Int. J. Remote Sens. 1998, 19, 2133–2139. [Google Scholar]
- Li, B.; Liew, O. W.; Anand, A.K. Early detection of calcium deficiency in plants using red edge position. In Proceedings of SPIE Vol. 5996, Optical Sensors and Sensing Systems for Natural Resources and Food Safety and Quality; Chen, Y.R., Meyer, G. E., Tu, S.I., Eds.; 2005; pp. 609–617. [Google Scholar]
- Levizou, E.; Drilias, P.; Psaras, G.K.; Manetas, Y. Nondestructive assessment of leaf chemistry and physiology through spectral reflectance measurements may be misleading when changes in trichome density co-occur. New Phytol. 2005, 165, 463–472. [Google Scholar]
- Barton, C.V. A theoretical analysis of the influence of heterogeneity in chlorophyll distribution on leaf reflectance. Tree Physiol. 2001, 21, 789–795. [Google Scholar]
- Milton, N.M.; Eiswerth, B.A.; Ager, C.M. Effect of phosphorus deficiency on spectral reflectance and morphology of soybean plants. Remote Sens. Environ. 1991, 36, 121–127. [Google Scholar]
- Horler, D.N.H.; Barber, J.; Barringer, A.R. Effects of heavy metals on the absorbance and reflectance spectra of plants. Int. J. Remote Sens. 1980, 1, 121–136. [Google Scholar]
- Bacci, L.; De Vincenzi, M.; Rapi, B.; Arca, B.; Benincasa, F. Two methods for the analysis of colorimetric components applied to plant stress monitoring. Comput. Electron. Agric. 1998, 19, 167–186. [Google Scholar]
- CIE, 1976. , Colorimetry, 2nd edition; Publication CIE no. 15.2Central Bureau of the Commission Internationale de L'Eclairage: Vienna, 1986. [Google Scholar]
- Wyszecki, G.; Stiles, W.S. Colorimetry. In Color Science: Concepts and Methods, Quantitative Data and Formulae; Wyszecki, G., Stiles, W.S., Eds.; New York; John Wiley and Sons, 2000; pp. 117–248. [Google Scholar]
- Price, J.C. How unique are spectral signatures? Remote Sens. Environ. 1994, 49, 181–186. [Google Scholar]
- Castro-Esau, K.L.; Sanchez-Azofeifa, G.A.; Rivard, B.; Wright, S.J.; Quesada, M. Variability in leaf optical properties of Mesoamerican trees and the potential for species classification. Am. J. Bot. 2006, 93, 517–530. [Google Scholar]
- Fukshansky, L.; Remisowsky, A.M.V.; McClendon, J.; Ritterbusch, A.; Richter, T.; Mohr, H. Absorption spectra of leaves corrected for scattering and distributional error: a radiative transfer and absorption statistics treatment. Photochem. Photobiol. 1993, 57, 538–555. [Google Scholar]
- Ponzoni, F.J.; Goncalves, J.L.; de, M. Spectral features associated with nitrogen, phosphorus and potassium deficiencies in Eucaliptus saligna seedling leaves. Int. J. Remote Sens. 1999, 20, 2249–2264. [Google Scholar]
- Liu, L.; Wang, J.; Huang, W.; Zhao, C.; Zhang, B.; Tong, Q. Estimating winter wheat plant water content using red edge parameters. Int. J. Remote Sens. 2004, 25, 3331–3342. [Google Scholar]
- Graeff, S.; Claupein, W. Quantifying nitrogen status of corn (Zea mays L.) in the field by reflectance measurements. Eur. J. Agron. 2003, 19, 611–618. [Google Scholar]
- Mariotti, M.; Ercoli, L.; Masoni, A. Spectral properties of iron-deficient corn and sunflower leaves. Remote Sens. Environ. 1996, 58, 282–288. [Google Scholar]
- Adams, M.L.; Norvell, W.A.; Philpot, W.D.; Peverly, J.H. Spectral detection of micronutrient deficiency in ‘Bragg’ soybean. Agron. J. 2000, 92, 261–268. [Google Scholar]
- Adams, M.L.; Norvell, W.A.; Philpot, W.D.; Peverly, J.H. Toward the discrimination of manganese, zinc, copper and iron deficiency in ‘Bragg’ soybean using spectral detection methods. Agron. J. 2000, 92, 268–274. [Google Scholar]
- Maccioni, A.; Agati, G.; Mazzinghi, P. New vegetation indices for remote measurement of chlorophylls based on leaf directional reflectance spectra. J. Photoch. Photobio. B 2001, 61, 52–61. [Google Scholar]
- Li, B.; Liew, O. W.; Anand, A.K. Use of reflectance spectroscopy for early detection of calcium deficiency in plants. Proceeding of SPIE 5882 Third International Conference on Experimental Mechanics and Third Conference of the Asian Committee on Experimental Mechanics, SPIE, Bellingham, WA; Quan, C., Chou, F.S., Asundi, A., Wong, B.S., Lim, C.T., Eds.; 2005; pp. 693–697. [Google Scholar]
- Graeff, S.; Steffens, D.; Schubert, S. Use of reflectance measurements for the early detection of N, P., Mg, and Fe deficiencies in Zea mays L. J. Plant Nutr. Soil Sc. 2001, 164, 445–450. [Google Scholar]
- Morales, F.; Abadía, A.; Abadía, J. Characterization of the xanthophyll cycle and other photosynthetic pigment changes induced by iron deficiency in sugar beet (Beta vulgaris L.). Plant Physiol. 1990, 94, 607–613. [Google Scholar]
- Morales, F.; Abadía, A.; Belkhodja, R.; Abadía, J. Iron deficiency-induced changes in the photosynthetic pigment composition of field-grown pear (Pyrus communis L.) leaves. Plant Cell Environ. 1994, 17, 1153–1160. [Google Scholar]
- Wulder, M. Optical remote-sensing techniques for the assessment of forest inventory and biophysical parameters. Prog. Phys. Geog. 1998, 22, 449–476. [Google Scholar]
- Wulder, M.; Niemann, K.O.; Goodenough, D.G. Local maximum filtering for the extraction of tree locations and basal area from high spatial resolution imagery. Remote Sens. Environ. 2000, 73, 103–114. [Google Scholar]
- Filella, I.; Peñuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar]
- Asner, G.P.; Vitousek, P.M. Remote analysis of biological invasion and biogeochemical change. Proc. Natl. Acad. Sci. USA 2005, 102, 4383–4386. [Google Scholar]
- Ustin, S.L.; Roberts, D.A.; Gamon, J.A.; Asner, G.P.; Green, R.O. Using imaging spectroscopy to study ecosystem processes and properties. Bioscience 2004, 54, 523–534. [Google Scholar]
- Curran, P.J. Imaging Spectrometry. Prog. Phys. Geog. 1994, 18, 247–266. [Google Scholar]
- Myneni, R.B.; Maggion, S.; Iaquinta, J.; Privette, J.L.; Gobron, N.; Pinty, B.; Kimes, D.S.; Verstraete, M.M.; Williams, D.L. Optical remote sensing of vegetation: Modeling, caveats and algorithms. Remote Sens. Environ. 1995, 51, 169–188. [Google Scholar]
- Woodhouse, R.; Heeb, M.; Berry, W.; Hoshizaki, T.; Wood, M. Analysis of remote reflection spectroscopy to monitor plant health. Adv. Space Res 1994, 14, 199–202. [Google Scholar]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar]
- Broge, N.H.; Mortensen, J.V. Deriving green crop area index and canopy chlorophyll density of winter wheat from spectral reflectance data. Remote Sens. Environ. 2002, 81, 45–57. [Google Scholar]
- Boochs, F.; Kupfer, G.; Dockter, K.; Kühbauch, W. Shape of red edge as vitality indicator of plants. Int. J. Remote Sens. 1990, 11, 1741–1753. [Google Scholar]
- Capelle, G.; Jones, S. Plant stress measurements using laser-induced fluorescence excitation: Poland Experiment. U.S. Department of Energy Technical report no. DOE/ENV 11718—351. , 1999. http://www.osti.gov/bridge/servlets/purl/10608-izdl32/webviewable/10608.pdf.
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar]
- Omasa, K.; Hosoi, F.; Konishi, A. 3D lidar imaging for detecting and understanding plant responses and canopy structure. J. Exp. Bot. 2007, 58, 881–898. [Google Scholar]
- Baret, F.; Houlès, V.; Guérif, M. Quantification of plant stress using remote sensing observations and crop models: the case of nitrogen management. J. Exp. Bot. 2007, 58, 869–880. [Google Scholar]
- Asner, G.P.; Nepstad, D.; Cardinot, G.; Ray, D. Drought stress and carbon uptake in an Amazon forest measured with spaceborne imaging spectroscopy. Proc. Nat. Acad. Sci. USA 2004, 101, 6039–6044. [Google Scholar]
- Chaerle, L.; Van der Straeten, D. Seeing is believing: imaging techniques that monitor plant health. Biochim. Biophys. Acta 2001, 1519, 153–166. [Google Scholar]
- Belkin, S. Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar]
- Cheng Vollmer, A.; Van Dyk, T.K. Stress responsive bacteria: biosensors as environmental monitors. Adv. Microb. Physiol. 2004, 49, 131–174. [Google Scholar]
- Gu, M.B.; Mitchell, R.J.; Kim, B.C. Whole-cell-based biosensors for environmental biomonitoring and application. Adv. Biochem. Eng. Biotechnol. 2004, 87, 269–305. [Google Scholar]
- Sorensen, S.J.; Burmolle, M.; Hansen, L.H. Making bio-sense of toxicity: New developments in whole-cell biosensors. Curr. Opin. Biotechnol. 2006, 17, 11–16. [Google Scholar]
- Yagi, K. Applications of whole-cell bacterial sensors in biotechnology and environmental science. Appl. Microbiol. Biot. 2007, 73, 1251–1258. [Google Scholar]
- Tecon, R.; van der Meer, J.R. Information from single-cell bacterial biosensors: What is it good for? Curr. Opin. Biotechnol. 2006, 17, 4–10. [Google Scholar]
- Sharpe, M. It's a Bug's Life: Biosensors for Environmental Monitoring. J. Environ. Monitor. 2003, 6, 109N–113N. [Google Scholar]
- Ron, E.Z. Biosensing environmental pollution. Curr. Opin. Biotechnol. 2007, 18, 252–256. [Google Scholar]
- Krizek, B.A.; Prost, V.; Joshi, R.M.; Stoming, T.; Glen, T.C. Developing transgenic Arabidopsis plants to be metal-specific bioindicators. Environ. Toxicol. Chem. 2003, 22, 175–181. [Google Scholar]
- Padidam, M. Chemically regulated gene expression in plants. Curr. Opin. Plant Biol. 2003, 6, 169–177. [Google Scholar]
- Reynolds, P.H.S. Inducible gene expression in plants; New York; CABI Publishing, 1999. [Google Scholar]
- Aarts, M.G.M.; Fiers, M.W.E.J. What drives plant stress genes. Trends Plant Sci. 2003, 8, 99–102. [Google Scholar]
- Sreenivasulu, N.; Radchuk, V.; Strickert, M.; Miersch, O.; Weschke, W.; Wobus, U. Gene expression patterns reveal tissue-specific signaling networks controlling programmed cell death and ABA-regulated maturation in developing barley seeds. Plant J. 2006, 47, 310–327. [Google Scholar]
- Tamaoki, M.; Matsuyama, T.; Nakajima, N.; Aono, M.; Kubo, A.; Saji, H. A method for diagnosis of plant environmental stresses by gene expression profiling using a cDNA macroarray. Environ. Pollut. 2004, 131, 137–145. [Google Scholar]
- Chen, W.; Provart, N.J.; Glazebrook, J.; Katagiri, F.; Chang, H.S.; Eulgem, T.; Mauch, F.; Luan, S.; Zou, G.; Whitham, S.A.; Budworth, P.R.; Tao, Y.; Xie, Z.; Chen, X.; Lam, S.; Kreps, J.A.; Harper, J.F.; Si-Ammour, A.; Mauch-Mani, B.; Heinlein, M.; Kobayashi, K.; Hohn, T.; Dangl, J.L.; Wang, X.; Zhu, T. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 2002, 14, 559–574. [Google Scholar]
- Matsuyama, T.; Tamaoki, M.; Nakajima, N.; Aono, M.; Kubo, A.; Moriya, S.; Ichihara, T.; Suzuki, O.; Saji, H. cDNA microarray assessment for ozone-stressed Arabidopsis thaliana. Environ. Pollut. 2002, 117, 191–194. [Google Scholar]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; Satou, M.; Akiyama, K.; Taji, T.; Yamaguchi-Shinozaki, K.; Carninci, P.; Kawai, J.; Hayashizaki, Y.; Shinozaki, K. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002, 31, 279–292. [Google Scholar]
- Klok, E.J.; Wilson, I.W.; Wilson, D.; Chapman, S.C.; Ewing, R.M.; Somerville, S.C.; James Peacock, W.; Dolferus, R.; Dennis, E.S. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 2002, 14, 2481–2494. [Google Scholar]
- Bailey, T.L.; Elkans, C. The value of prior knowledge in discovering motifs with MEME. Proc. Int Conf. Intell. Syst. Mol. Biol. 1995, 3, 21–29. [Google Scholar]
- Lawrence, C.E.; Altschul, S.F.; Boguski, M.S.; Liu, J.S.; Neuwald, A.F.; Wootton, J.C. Detecting subtle sequence signals: a Gibbs sampling strategy for multiple alignment. Science 1993, 262, 208–214. [Google Scholar]
- Zhang, M.Q. Computational analyses of eukaryotic promoters. BMC Bioinformatics 2007, 8(suppl. 6), S3. [Google Scholar]
- Wang, T.; Stormo, G.D. Identifying the conserved network of cis-regulatory sites of a eukaryotic genome. Proc. Nat. Acad. Sci. USA 2005, 102, 17400–17405. [Google Scholar]
- Rombauts, S.; Florquin, K.; Lescot, M.; Marchal, K.; Rouzé, P.; Van de Peer, Y. Computational approaches to identify promoters and cis-regulatory elements in plant genomes. Plant Physiol. 2003, 132, 1162–1176. [Google Scholar]
- Blanchette, M.; Tompa, M. Discovery of regulatory elements by a computational method for phyologenetic footprinting. Genome Res. 2002, 12, 739–748. [Google Scholar]
- Naylor, L.H. Reporter gene technology: the future looks bright. Biochem. Pharmacol. 1999, 58, 749–757. [Google Scholar]
- Van der Meer, J.R. Analytics with engineered bacterial bioreporter strains and systems. Curr. Opin. Biotechnol. 2006, 17, 1–3. [Google Scholar]
- Stewart, C.N., Jr. Go with the glow: fluorescent proteins to light up transgenic organisms. Trends Biotechnol. 2006, 24, 155–162. [Google Scholar]
- Van Roessel, P.; Brand, A.H. Imaging into the future: visualizing gene expression and protein interactions with fluorescent proteins. Nat. Cell Biol. 2002, 4, E15–E20. [Google Scholar]
- Chiu, W.L.; Niwa, Y.; Zeng, W.; Hirano, T.; Kobayashi, H.; Sheen, J. Engineered GFP as a vital reporter in plants. Curr. Biol. 1996, 6, 325–330. [Google Scholar]
- Kogure, T.; Karasawa, S.; Araki, T.; Saito, K.; Kinjo, M.; Miyawaki, A. A fluorescent variant of a protein from the stony coral Montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat. Biotechnol. 2006, 24, 577–581. [Google Scholar]
- Ando, R.; Hama, H.; Yamamoto-Hino, M.; Mizuno, H.; Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 2002, 99, 12651–12656. [Google Scholar]
- Ando, R.; Mizuno, H.; Miyawaki, A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science 2004, 306, 1370–1373. [Google Scholar]
- Takeuchi, M.; Ozawa, T. Methods for imaging and analyses of intracellular organelles using fluorescent and luminescent proteins. Anal. Sci. 2007, 23, 25–29. [Google Scholar]
- Li, X.; Zhao, X.; Fang, Y.; Jiang, X.; Duong, T.; Fan, C.; Huang, C.C.; Kain, S.R. Generation of destabilized green fluorescent protein as a transcription reporter. J. Biol. Chem. 1998, 273, 34970–34975. [Google Scholar]
- Clontech. Living Colors® User Manual; Protocol No. PT2040-1 (Version no. PR1Y691); pp. 1–47. 2001. [Google Scholar]
- Greer, L.F., III; Szalay, A.A. Imaging of light emission from the expression of luciferases in living cells and organisms: a review. Luminescence 2002, 17, 43–74. [Google Scholar]
- Koncz, C.; Langridge, W.H.R.; Olsson, O.; Schell, J.; Szalay, A.A. Bacterial and firefly luciferase genes in transgenic plants: Advantages and disadvantages of a reporter gene. Dev. Genet. 1990, 11, 224–232. [Google Scholar]
- Ramonell, K.M.; Somerville, S. The genomics parade of defense responses: to infinity and beyond. Curr. Opin. Plant Biol. 2002, 5, 291–294. [Google Scholar]
- Hammond, J.P.; Bennett, M.J.; Bowen, H.C.; Broadley, M.R.; Eastwood, D.C.; May, S.T.; Rahn, C.; Swarup, R.; Woolaway, K.E.; White, P.J. Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol. 2003, 132, 578–596. [Google Scholar]
- Kovalchuk, I.; Kovalchuk, O.; Arkhipov, A.; Hohn, B. Transgenic plants are sensitive bioindicators of nuclear pollution caused by the Chernobyl accident. Nat. Biotechnol. 1998, 16, 1054–1059. [Google Scholar]
- Kovalchuk, O.; Titov, V.; Hohn, B.; Kovalchuk, I. A sensitive transgenic plant system to detect toxic inorganic compounds in the environment. Nat. Biotechnol. 2001, 19, 568–572. [Google Scholar]
- Kooshki, M.; Mentewab, A.; Neal Stewart, C., Jr. Pathogen inducible reporting in transgenic tobacco using a GFP construct. Plant Sci. 2003, 165, 213–219. [Google Scholar]
- Chong, J-P.C.; Liew, O.W.; Li, B.Q.; Asundi, A.K. Optical fluorescence biosensor for water stress detection. Proceedings of SPIE Vol. 6535, Saratov Fall Meeting: Optical Technologies in Biophysics and Medicine VIII; Tuchin, V. V., Ed.; 2007; pp. 65350S1–S8. [Google Scholar]
- Taji, T.; Seki, M.; Yamaguchi-Shinozaki, K.; Kamada, H.; Giraudat, J.; Shinozaki, K. Mapping of 25 drought-inducible genes, RD and ERD, in Arabidopsis thaliana. Plant Cell Physiol. 1999, 40, 119–123. [Google Scholar]
- Yamaguchi-Shinozaki, K.; Koizumi, M.; Urao, S.; Shinozaki, K. Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: Sequence analysis of one cDNA clone that encodes a putative transmembrane channel protein. Plant Cell Physiol. 1992, 33, 217–224. [Google Scholar]
- Koizumi, M.; Yamaguchi-Shinozaki, K.; Tsuji, H.; Shinozaki, K. Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene 1993, 129, 175–182. [Google Scholar]
- Knight, A.W.; Billinton, N. Distinguishing GFP from cellular autofluorescence. Biophot. Int. 2001, 8, 42–47. [Google Scholar]
- Chappelle, E.W.; Williams, D.L. Laser-induced fluorescence (LIF) from plant foliage. IEEE Transactions on Geoscience and Remote Sensing 1987, 25, 726–736. [Google Scholar]
- Halfhill, M.D.; Millwood, R.J.; Rufty, T.W.; Weissinger, A.K.; Stewart, C.N., Jr. Spatial and temporal patterns of green fluorescent protein (GFP) fluorescence during leaf canopy development in transgenic oilseed rape, Brassica napus L. Plant Cell Rep. 2003, 22, 338–343. [Google Scholar]
- Sunilkumar, G.; Mohr, L.; Lopata-Finch, E.; Emani, C.; Rathore, K.S. Development and tissue-specific expression of CaMV 35S promoter in cotton as revealed by GFP. Plant Mol. Biol. 2002, 50, 463–474. [Google Scholar]
- Harper, B.K.; Stewart, C.N. Patterns of green fluorescent protein expression in transgenic plants. Plant Mol. Biol. Rep. 2000, 18, 141a–141i. [Google Scholar]
- Niwa, Y.; Hirano, T.; Yoshimoto, K.; Shimizu, M.; Kobayashi, H. Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J. 1999, 18, 455–463. [Google Scholar]
- Blumenthal, A.; Kuznetzova, L.; Edelbaum, O.; Raskin, V.; Levy, M.; Sela, I. Measurement of green fluorescence protein in plants: quantification, correlation to expression, rapid screening and differential gene expression. Plant Sci. 1999, 142, 93–99. [Google Scholar]
- Millwood, R.J.; Halfhill, M.D.; Harkins, D.; Russotti, R.; Stewart, C.N., Jr. Instrumentation and methodology for quantifying GFP fluorescence in intact plant organs. Biotechniques 2003, 34, 638–643. [Google Scholar]
- Liew, O.W.; Chean, X.Y.; Chong, J-P.C.; Ho, J-Z.E.; Chen, J.W.; Asundi, A.K.; Aiemwiwattanakul, O. Development of fibre optic spectroscopy for detection of genetically modified plants. Proceedings of SPIE Vol. 5771, Saratov Fall Meeting: Optical Technologies in Biophysics and Medicine VI, SPIE, Bellingham, WA; Tuchin, V. V., Ed.; 2005; pp. 202–208. [Google Scholar]
- Liew, O.W.; Chong, J-P.C.; Asundi, A.K. Spectroscopic detection of fluorescent protein marker gene activity in genetically modified plants. Proceeding of SPIE Vol. 5882, Third International Conference on Experimental Mechanics and Third Conference of the Asian Committee on Experimental Mechanics, SPIE, Bellingham, WA; Quan, C., Chou, F.S., Asundi, A., Wong, B.S., Lim, C.T., Eds.; 2005; pp. 717–723. [Google Scholar]
- Stewart, C.N., Jr.; Millwood, R.J.; Halfhill, M.D.; Ayalew, M.; Cardoza, V.; Kooshki, M.; Capelle, G.A.; Kyle, K.R.; Piaseki, D.; McCrum, G.; Di Benedetto, J. Laser-induced fluorescence imaging and spectroscopy of GFP transgenic plants. J. Fluoresc. 2005, 15, 697–705. [Google Scholar]
- Oberg, J.E.; Oberg, A.R. Pioneering space, Living on the next Frontier; McGraw Hill: New York, 1986. [Google Scholar]
- Giacomelli, G.A.; Ting, K.C.; Ling, P.P. Systems approach to instrumenting and controlling plant growth systems. Adv. Space Res. 1994, 14, 191–197. [Google Scholar]
- Kitaya, Y.; Tani, A.; Goto, E.; Saito, T.; Takahashi, H. Development of a plant growth unit for growing plants over a long-term life cycle under microgravity conditions. Adv. Space Res. 2000, 26, 281–288. [Google Scholar]
- Baratto, C.; Faglia, G.; Pardo, M.; Vezzoli, M.; Boarino, L.; Maffei, M.; Bossi, S.; Sberveglieri, G. Monitoring plants health in greenhouse for space missions. Sensor Actuator B 2005, 108, 278–284. [Google Scholar]
- Tabacco, M.B.; DiGiuseppe, T.G. Optical chemical sensors for environmental control and system management. Adv. Space Res. 1996, 18, 125–134. [Google Scholar]
- Tabacco, M.B.; Zhou, Q.; DiGiuseppe, T.G. Optical sensors for monitoring and control of plant growth systems. Adv. Space Res. 1994, 14, 223–226. [Google Scholar]
- Ehret, D.L.; Lau, A.; Bittman, S.; Lin, W.; Shelford, T. Automated monitoring of greenhouse crops. Agron. Sustain. Dev. 2001, 21, 403–414. [Google Scholar]
- Hetzroni, A.; Miles, G.E.; Engel, B.A.; Hammer, P.A.; Latin, R.X. Machine vision monitoring of plant health. Adv. Space Res. 1994, 14, 203–212. [Google Scholar]



© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liew, O.W.; Chong, P.C.J.; Li, B.; Asundi, A.K. Signature Optical Cues: Emerging Technologies for Monitoring Plant Health. Sensors 2008, 8, 3205-3239. https://doi.org/10.3390/s8053205
Liew OW, Chong PCJ, Li B, Asundi AK. Signature Optical Cues: Emerging Technologies for Monitoring Plant Health. Sensors. 2008; 8(5):3205-3239. https://doi.org/10.3390/s8053205
Chicago/Turabian StyleLiew, Oi Wah, Pek Ching Jenny Chong, Bingqing Li, and Anand K. Asundi. 2008. "Signature Optical Cues: Emerging Technologies for Monitoring Plant Health" Sensors 8, no. 5: 3205-3239. https://doi.org/10.3390/s8053205



