Electrochemical Determination of Low Molecular Mass Thiols Content in Potatoes (Solanum tuberosum) Cultivated in the Presence of Various Sulphur Forms and Infected by Late Blight (Phytophora infestans)
Abstract
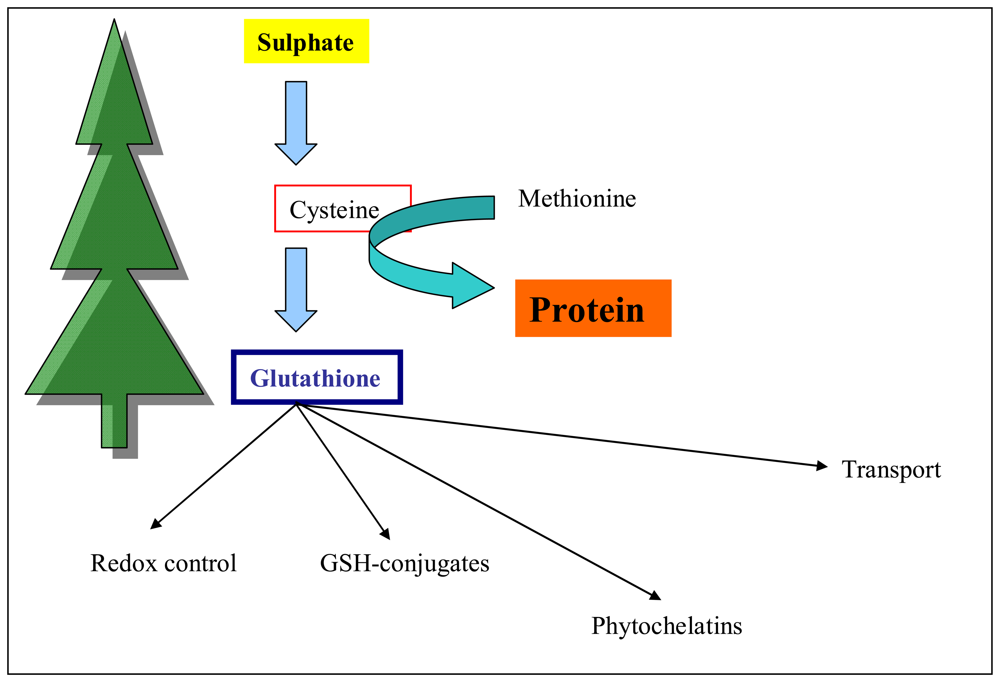
:1. Introduction
2. Material and Methods
2.1 Plant material
2.2 Experimental scheme of pot experiment
2.2.1 Preparation of Phytophora infestans inoculum and evaluation of Phytophora infection
2.2.2 Cultivation of plants in soil supplemented by cadmium(II) ions
2.3 Preparation of samples
2.4 Determination of nutrients according to Mehlich III
2.5 pH of the soil
2.6 Sulphur content in plants
2.7 Determination of cadmium content in plants
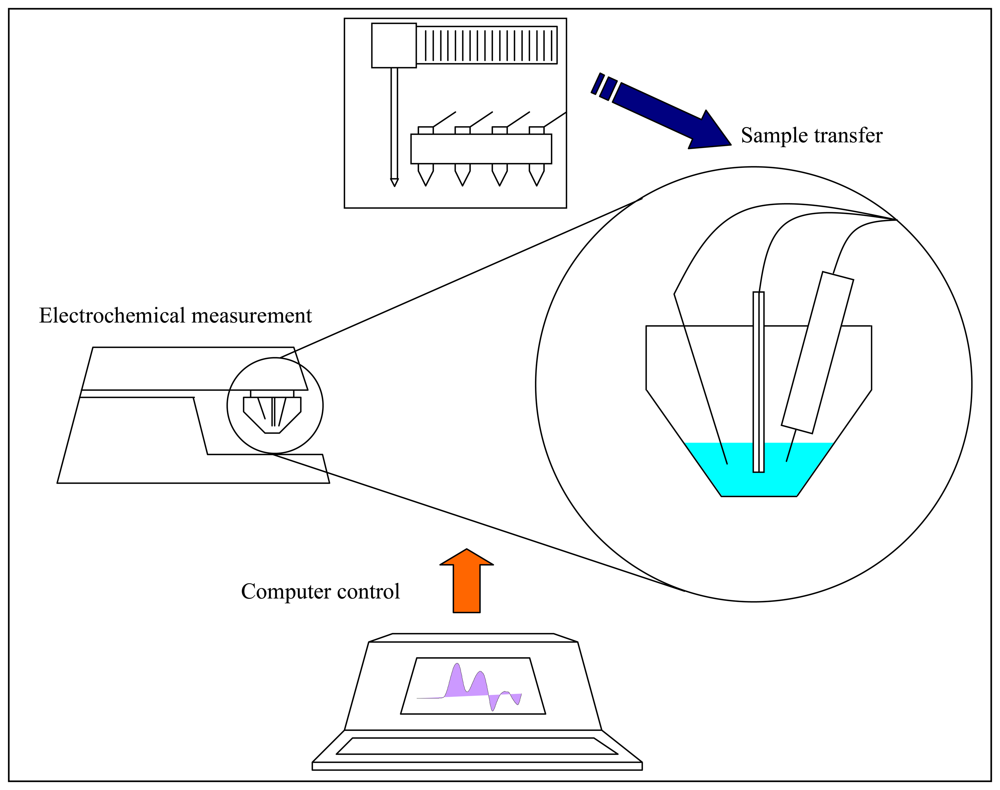
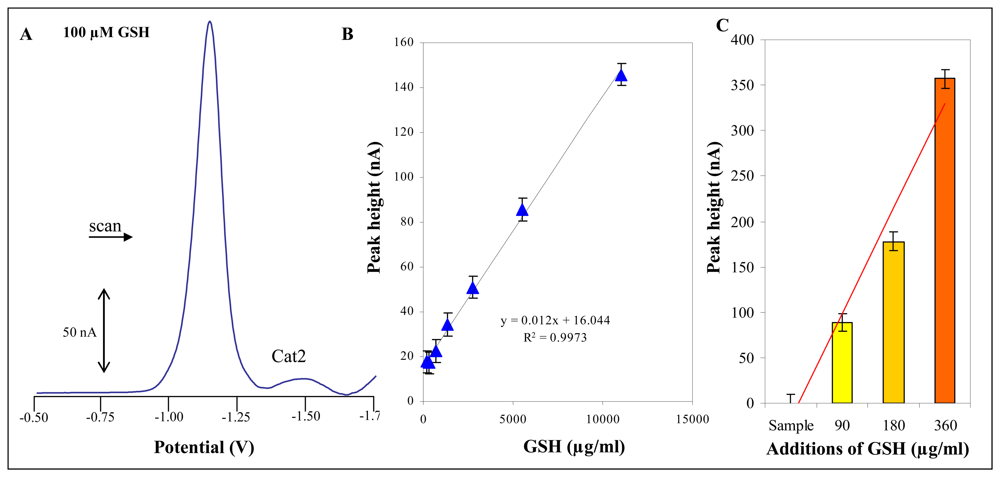
2.8 Electrochemical measurement – Differential pulse voltammetry (DPV) Brdicka reaction
2.8 Statistical analysis
3. Results and Discussion
3.1 Influence of different sulphur forms on potato plants growth, yield of tuber and element content
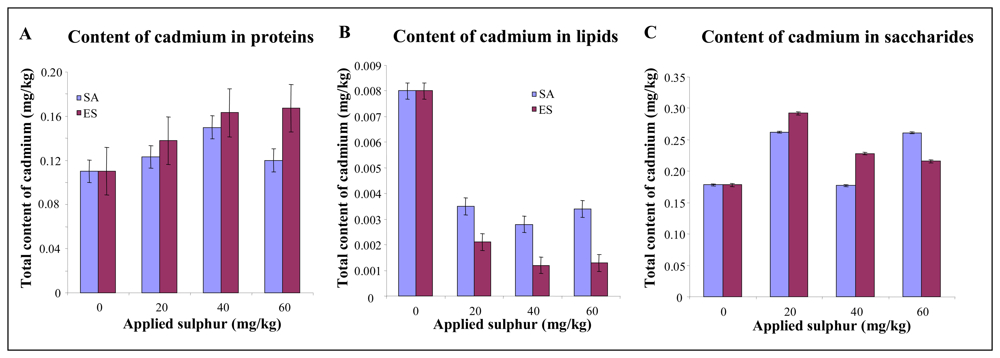
3.2 Effect of sulphur application on growth characteristics and cadmium uptake
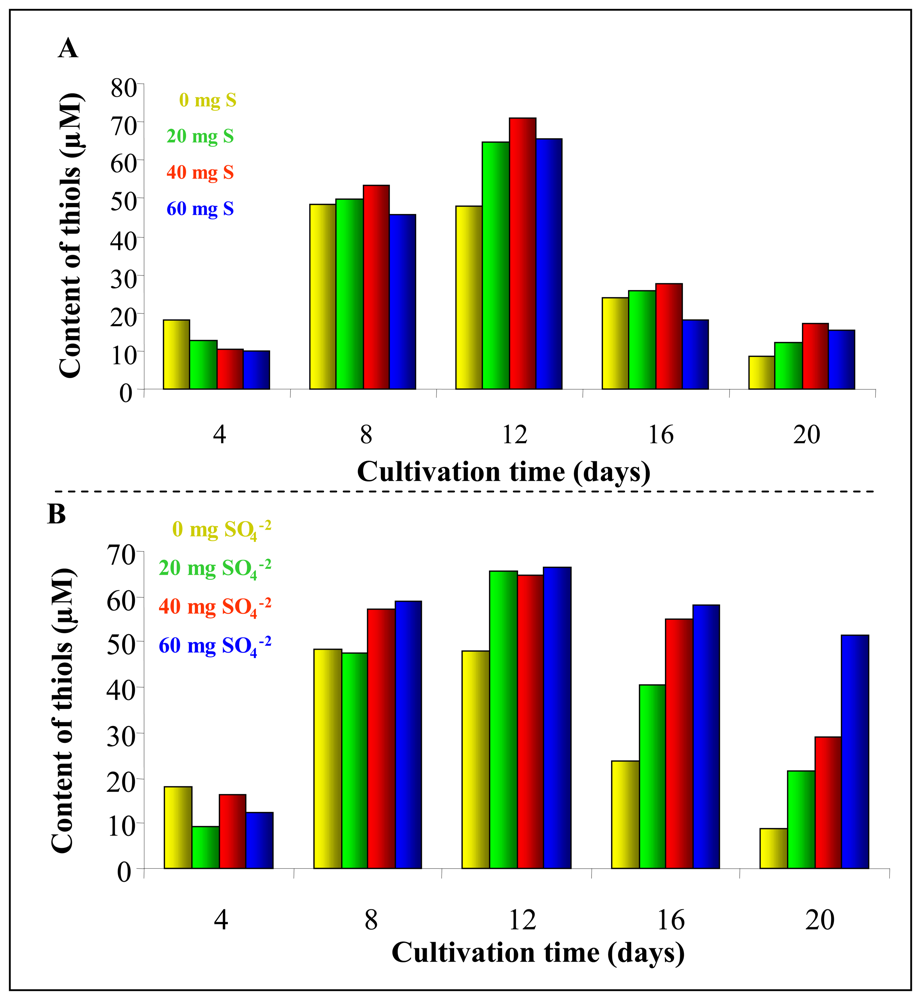
3.3 Total thiols content in plants
3.4 Sulphur supplementation influence on Phytophora infestans infection
4. Conclusion
Acknowledgments
References
- Vestreng, V.; Myhre, G.; Fagerli, H.; Reis, S.; Tarrason, L. Twenty-five years of continuous sulphur dioxide emission reduction in Europe. Atmos. Chem. Phys. 2007, 7, 3663–3681. [Google Scholar]
- Bailey, P.D.; Gough, C.A.; Millock, K.; Chadwick, M.J. Prospects for the joint implementation of sulphur emission reductions in Europe. Energy Policy 1996, 24, 507–516. [Google Scholar]
- Altman, A.; Amann, M.; Klaassen, G.; Ruszczynski, A.; Schopp, W. Cost-effective sulphur emission reduction under uncertainty. Eur. J. Oper. Res. 1996, 90, 395–412. [Google Scholar]
- Beveridg, G.S.; Macarthu, C.A. Sulphur Emission to Atmosphere. Transactions of the Institution of Chemical Engineers and the Chemical Engineer 1969, 47, C304–C305. [Google Scholar]
- Odowd, C.D.; Smith, M.H.; Consterdine, I.E.; Lowe, J.A. Marine aerosol, sea-salt, and the marine sulphur cycle: A short review. Atmos. Environ. 1997, 31, 73–80. [Google Scholar]
- Hrivna, L.; Richter, R.; Losak, T.; Hlusek, J. Effect of increasing doses of nitrogen and sulphur on chemical composition of plants, yields and seed quality in winter rape. Rostl. Vyroba 2002, 48, 1–6. [Google Scholar]
- Smatanova, M.; Richter, R.; Hlusek, J. Spinach and pepper response to nitrogen and sulphur fertilization. Plant Soil Environ. 2004, 50, 303–308. [Google Scholar]
- Ruiz, J.M.; Lopez-Cantarero, I.; Rivero, R.M.; Romero, L. Sulphur phytoaccumulation in plant species characteristic of gypsiferous soils. Int. J. Phytoremediat. 2003, 5, 203–210. [Google Scholar]
- Goh, K.M.; Pamidi, J. Plant uptake of sulphur as related to changes in the HI-reducible and total sulphur fractions in soil. Plant Soil 2003, 250, 1–13. [Google Scholar]
- Hawkesford, M.J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J. Exp. Bot. 2000, 51, 131–138. [Google Scholar]
- Nesheim, L.; Gautneb, H.; Myhr, K. Plant uptake of sulphur and trace elements from pyrite applied on grassland. Acta Agric. Scand. Sect. B-Soil Plant Sci. 1997, 47, 135–141. [Google Scholar]
- Zhao, F.J.; Withers, P.J.A.; Evans, E.J.; Monaghan, J.; Salmon, S.E.; Shewry, P.R.; McGrath, S.P. Sulphur nutrition: An important factor for the quality of wheat and rapeseed (Reprinted from Plant nutrition for sustainable food production and environment, 1997). Soil Sci. Plant Nutr. 1997, 43, 1137–1142. [Google Scholar]
- Ceccotti, S.P. Plant nutrient sulphur - A review of nutrient balance, environmental impact and fertilizers. Fertil. Res. 1996, 43, 117–125. [Google Scholar]
- Fitzgerald, M.A.; Ugalde, T.D.; Anderson, J.W. Sulphur nutrition affects delivery and metabolism of S in developing endosperms of wheat. J. Exp. Bot. 2001, 52, 1519–1526. [Google Scholar]
- Anderson, J.W.; Fitzgerald, M.A. Physiological and metabolic origin of sulphur for the synthesis of seed storage proteins. J. Plant Physiol. 2001, 158, 447–456. [Google Scholar]
- Fitzgerald, M.A.; Ugalde, T.D.; Anderson, J.W. S nutrition affects the pools of S available to developing grains of wheat. J. Exp. Bot. 1999, 50, 1587–1592. [Google Scholar]
- Fitzgerald, M.A.; Ugalde, T.D.; Anderson, J.W. Sulphur nutrition changes the sources of S in vegetative tissues of wheat during generative growth. J. Exp. Bot. 1999, 50, 499–508. [Google Scholar]
- McMahon, P.J.; Anderson, J.W. Preferential allocation of sulphur into gamma-glutamylcysteinyl peptides in wheat plants grown at low sulphur nutrition in the presence of cadmium. Physiol. Plant. 1998, 104, 440–448. [Google Scholar]
- Sunarpi; Anderson, J.W. Effect of nitrogen nutrition on the export of sulphur from leaves in soybean. Plant Soil 1997, 188, 177–187. [Google Scholar]
- Sunarpi; Anderson, J.W. Inhibition of sulphur redistribution into new leaves of vegetative soybean by excision of the maturing leaf. Physiol. Plant. 1997, 99, 538–545. [Google Scholar]
- Adiputra, I.G.K.; Anderson, J.W. Effect of sulphur nutrition on redistribution of sulphur in vegetative barley. Physiol. Plant. 1995, 95, 643–650. [Google Scholar]
- Sunarpi; Anderson, J.W. Mobilization of Sulfur in Soybean Cotyledons During Germination. Physiol. Plant. 1995, 94, 143–150. [Google Scholar]
- Adiputra, I.G.K.; Anderson, J.W. Distribution and Redistribution of Sulfur Taken up from Nutrient Solution During Vegetative Growth in Barley. Physiol. Plant. 1992, 85, 453–460. [Google Scholar]
- Boswell, C.C. Dryland lucerne responses to elemental sulphur of different particle sizes applied at different rates and frequencies in North Otago, New Zealand. N. Z. J. Agric. Res. 1997, 40, 283–295. [Google Scholar]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2004, 27, 1827–1839. [Google Scholar]
- Bloem, E.; Riemenschneider, A.; Volker, J.; Papenbrock, J.; Schmidt, A.; Salac, I.; Haneklaus, S.; Schnug, E. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 2004, 55, 2305–2312. [Google Scholar]
- Zhao, Z.Q.; Zhu, Y.G.; Li, H.Y.; Smith, S.E.; Smith, F.A. Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum, L.). Environ. Int. 2004, 29, 973–978. [Google Scholar]
- Cui, Y.S.; Wang, Q.R.; Christie, P. Effect of elemental sulphur on uptake of cadmium, zinc, and sulphur by oilseed rape growing in soil contaminated with zinc and cadmium. Commun. Soil Sci. Plant Anal. 2004, 35, 2905–2916. [Google Scholar]
- Cui, Y.J.; Zhu, Y.G.; Smith, F.A.; Smith, S.E. Cadmium uptake by different rice genotypes that produce white or dark grains. J. Environ. Sci. 2004, 16, 962–967. [Google Scholar]
- Cui, Y.J.; Zhu, Y.G.; Zhai, R.H.; Chen, D.Y.; Huang, Y.Z.; Qiu, Y.; Liang, J.Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar]
- Supalkova, V.; Beklova, M.; Baloun, J.; Singer, C.; Sures, B.; Adam, V.; Huska, D.; Pikula, J.; Rauscherova, L.; Havel, L.; Zehnalek, J.; Kizek, R. Affecting of aquatic vascular plant Lemna minor by cisplatin revealed by voltammetry. Bioelectrochemistry 2008, 72, 59–65. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Zitka, O.; Stejskal, K.; Kleckerova, A.; Adam, V.; Beklova, M.; Horna, A.; Supalkova, V.; Havel, L.; Kizek, R. Utilizing electrochemical techniques for detection of biological samples. Chem. Listy 2007, 101, 225–231. [Google Scholar]
- Adam, V.; Krizkova, S.; Zitka, O.; Trnkova, L.; Petrlova, J.; Beklova, M.; Kizek, R. Determination of apo-metallothionein using adsorptive transfer stripping technique in connection with differential pulse voltammetry. Electroanalysis 2007, 19, 339–347. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Petrek, J.; Adam, V.; Potesil, D.; Havel, L.; Mikelova, R.; Trnkova, L.; Kizek, R. Electrochemical study of S-nitrosoglutathione and nitric oxide by carbon fibre NO sensor and cyclic voltammetry - possible way of monitoring of nitric oxide. Electrochim. Acta 2006, 51, 5087–5094. [Google Scholar]
- Petrlova, J.; Mikelova, R.; Stejskal, K.; Kleckerova, A.; Zitka, O.; Petrek, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Kizek, R. Simultaneous determination of eight biologically active thiol compounds using gradient elution-liquid chromatography with Coul-Array detection. J. Sep. Sci. 2006, 29, 1166–1173. [Google Scholar]
- Potesil, D.; Petrlova, J.; Adam, V.; Vacek, J.; Klejdus, B.; Zehnalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 2005, 1084, 134–144. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Jelen, F. Cyclic voltammetric study of the redox system of glutathione using the disulfide bond reductant tris(2-carboxyethyl)phosphine. Bioelectrochemistry 2004, 63, 19–24. [Google Scholar]
- Huska, D.; Krizkova, S.; Beklova, M.; Havel, L.; Zehnalek, J.; Diopan, V.; Adam, V.; Zeman, L.; Babula, P.; Kizek, R. Influence of cadmium(II) ions and brewery sludge on metallothionein level in earthworms (Eisenia fetida) - Biotransforming of toxic wastes. Sensors 2008, 8, 1039–1047. [Google Scholar]
- Adam, V.; Beklova, M.; Pikula, J.; Hubalek, J.; Trnkova, L.; Kizek, R. Shapes of differential pulse voltammograms and level of metallothionein at different animal species. Sensors 2007, 7, 2419–2429. [Google Scholar]
- Petrek, J.; Baloun, J.; Vlasinova, H.; Havel, L.; Adam, V.; Vitecek, J.; Babula, P.; Kizek, R. Image analysis and activity of intracellular esterases as new analytical tools for determination of growth and viability of embryonic cultures of spruce (Picea sp.) treated with cadmium. Chem. Listy 2007, 101, 569–577. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Mikelova, R.; Baloun, J.; Petrlova, J.; Adam, V.; Havel, L.; Petrek, H.; Horna, A.; Kizek, R. Electrochemical determination of Ag-ions in environment waters and their action on plant embryos. Bioelectrochemistry 2007, 70, 508–518. [Google Scholar]
- Kizek, R.; Vacek, J.; Trnkova, L.; Klejdus, B.; Havel, L. Application of catalytic reactions on a mercury electrode for electrochemical detection of metallothioneins. Chem. Listy 2004, 98, 166–173. [Google Scholar]
- Vacek, J.; Petrek, J.; Kizek, R.; Havel, L.; Klejdus, B.; Trnkova, L.; Jelen, F. Electrochemical determination of lead and glutathione in a plant cell culture. Bioelectrochemistry 2004, 63, 347–351. [Google Scholar]
- Mizuno, N.; Yoshida, H.; Tadano, T. Efficacy of single application ammonium sulfate in suppressing potato common scab. Soil Sci. Plant Nutr. 2000, 46, 611–616. [Google Scholar]



| Experimental group | Sulphur form used | Sulphur dosage (mg S/kg soil) | Way of application |
|---|---|---|---|
| Control | Control | 0 | Without application |
| 20 SA | (NH4)2SO4 | 20 | Into soil |
| 40 SA | (NH4)2SO4 | 40 | Into soil |
| 60 SA | (NH4)2SO4 | 60 | Into soil |
| 20 ES | Elemental | 20 | Into soil |
| 40 ES | Elemental | 40 | Into soil |
| 60 ES | Elemental | 60 | Into soil |
| Element | Statistical parameter | Control | Form of sulphur application | |||||
|---|---|---|---|---|---|---|---|---|
| Ammonium sulphate | Elemental sulphur | |||||||
| 20 SA | 40 SA | 60 SA | 20 ES | 40 ES | 60 ES | |||
| Nitrogen | mean | 1.2 | 1.1 | 1.1 | 1.0 | 1.0 | 1.1 | 1.1 |
| S.D.* | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | |
| Phosphorus | mean | 0.07 | 0.07 | 0.07 | 0.06 | 0.06 | 0.07 | 0.08 |
| S.D.* | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | |
| Potassium | mean | 5.5 | 5.6 | 5.8 | 5.6 | 5.0 | 4.6 | 4.8 |
| S.D.* | 0.4 | 0.8 | 0.7 | 0.8 | 0.5 | 0.5 | 0.5 | |
| Calcium | mean | 3.8 | 4.0 | 11 | 19 | 3.7 | 3.9 | 3.8 |
| S.D.* | 0.7 | 0.1 | 1 | 15 | 0.5 | 0.1 | 0.4 | |
| Magnesium | mean | 0.59 | 0.56 | 0.52 | 0.46 | 0.57 | 0.58 | 0.60 |
| S.D.* | 0.08 | 0.05 | 0.09 | 0.06 | 0.06 | 0.03 | 0.04 | |
| Sulphur | mean | 410 | 550 | 624 | 692 | 613 | 614 | 627 |
| S.D.* | 1 | 1 | 3 | 1 | 1 | 1 | 1 | |
| Element | Statistical parameter | Control | Form of sulphur application | |||||
|---|---|---|---|---|---|---|---|---|
| Ammonium sulphate | Elemental sulphur | |||||||
| 20 SA | 40 SA | 60 SA | 20 ES | 40 ES | 60 ES | |||
| Nitrogen | mean | 1.9 | 1.8 | 1.7 | 1.6 | 1.7 | 1.6 | 1.8 |
| S.D.* | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | |
| Phosphorus | mean | 0.25 | 0.26 | 0.27 | 0.26 | 0.24 | 0.30 | 0.27 |
| S.D.* | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.06 | 0.01 | |
| Potassium | mean | 2.9 | 2.8 | 2.9 | 2.9 | 2.8 | 2.8 | 2.9 |
| S.D.* | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| Calcium | mean | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.06 |
| S.D.* | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | |
| Magnesium | mean | 0.13 | 0.14 | 0.14 | 0.14 | 0.15 | 0.15 | 0.14 |
| S.D.* | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | |
| Sulphur | mean | 168 | 179 | 174 | 168 | 169 | 164 | 183 |
| S.D.* | 1 | 1 | 1 | 2 | 1 | 1 | 1 | |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the CreativeCommons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ryant, P.; Dolezelova, E.; Fabrik, I.; Baloun, J.; Adam, V.; Babula, P.; Kizek, R. Electrochemical Determination of Low Molecular Mass Thiols Content in Potatoes (Solanum tuberosum) Cultivated in the Presence of Various Sulphur Forms and Infected by Late Blight (Phytophora infestans). Sensors 2008, 8, 3165-3182. https://doi.org/10.3390/s8053165
Ryant P, Dolezelova E, Fabrik I, Baloun J, Adam V, Babula P, Kizek R. Electrochemical Determination of Low Molecular Mass Thiols Content in Potatoes (Solanum tuberosum) Cultivated in the Presence of Various Sulphur Forms and Infected by Late Blight (Phytophora infestans). Sensors. 2008; 8(5):3165-3182. https://doi.org/10.3390/s8053165
Chicago/Turabian StyleRyant, Pavel, Eva Dolezelova, Ivo Fabrik, Jiri Baloun, Vojtech Adam, Petr Babula, and Rene Kizek. 2008. "Electrochemical Determination of Low Molecular Mass Thiols Content in Potatoes (Solanum tuberosum) Cultivated in the Presence of Various Sulphur Forms and Infected by Late Blight (Phytophora infestans)" Sensors 8, no. 5: 3165-3182. https://doi.org/10.3390/s8053165




