Sensitive Aflatoxin B1 Determination Using a Magnetic Particles-Based Enzyme-Linked Immunosorbent Assay
Abstract
:1. Introduction
2. Results and Discussion
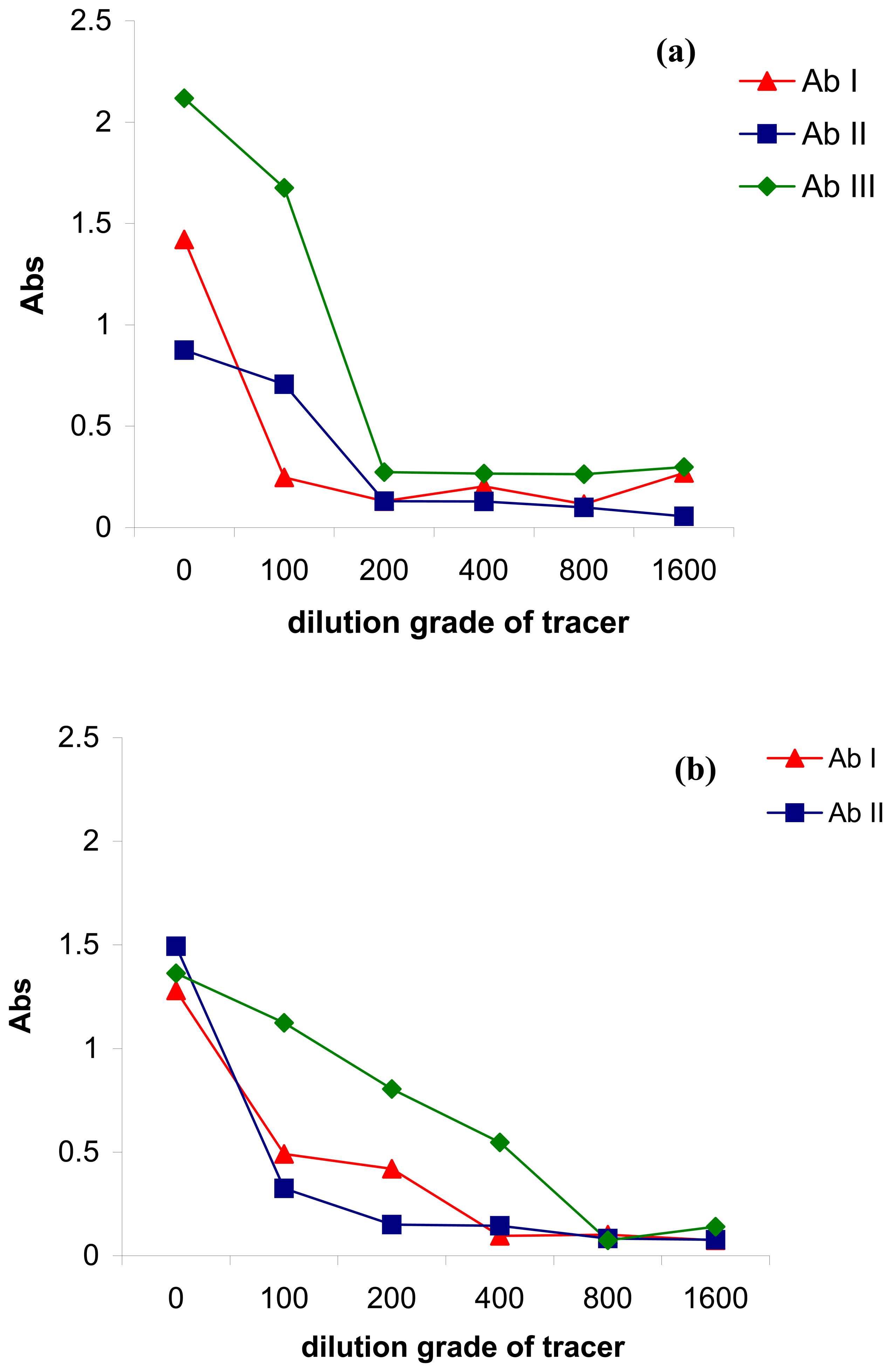
2.1. Antibody immobilization on the magnetic particles
2.2. Calibration of mp-ELISA method for Aflatoxin B1 determination
3. Experimental Section
3.1. Chemicals and instruments
3.2. mp-ELISA Method
4. Conclusions
Acknowledgments
References and Notes
- Hussein, H.S.; Brassel, J.M. Toxicity, metabolism, and impact of mycotoxins on human and animals. Toxicology 2001, 167, 101–134. [Google Scholar]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; MacDonald, S.; Crews, C. Mycotoxin analysis: An update. Food Add. Contam. 2008, 25, 152–163. [Google Scholar]
- EU Directive Commission Directive 2003/100/EC of 31 October 2003 amending Annex I of Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed, L285//233–L285/237; EU Commission: Brussels, Belgium, 2003.
- Commission Regulation (EC) No. 466/2001 of 8 March 8 2001 setting maximum levels for certain contaminants in foodstuffs. Off J. Eur. 2001, 77, 1–6.
- Pestka, J.J. High performance thin layer chromatography ELISAGRAM: Application of a multi-hapten immunoassay to analysis of the zearalenone and aflatoxin mycotoxin families. J. Immunol. Method. 1991, 136, 177–183. [Google Scholar]
- Fernandez, A.; Belio, R.; Ramos, J.J.; Sanz, M.C.; Saez, T. Aflatoxins and their metabolites in the tissues, faeces and urine from lambs feeding on an aflatoxin-contaminated diet. J. Sci. Food Agric. 1997, 74, 161–168. [Google Scholar]
- Jaimez, J.; Fente, C.A.; Vazquez, B.I.; Franco, C.M.; Cepeda, A.; Mahhizier, G.; Profnon, P. Application of the assay of aflatoxins by liquid chromatography with fluorescence detection in food analysis. J. Chromatogr. A 2000, 882, 1–10. [Google Scholar]
- Decastelli, L.; Lai, J.; Gramaglia, M.; Monaco, A.; Nachtmann, C.; Oldano, F.; Ruffier, M.; Sezian, A.; Bandirola, C. Aflatoxins occurrence in milk and feed in Northern Italy during 2004-2005. Food Control 2007, 18, 1263–1266. [Google Scholar]
- Shim, W.B.; Choi, J.G.; Kim, J.Y.; Yang, Z.Y.; Lee, K.H.; Kim, M.G.; Ha, S.D.; Chung, D.H. Enhanced rapidity for qualitative detection of listeria monocytogenes using an enzyme-linked immunosorbent assay strip test combined with immunomagnetic bead separation. J. Food Prot. 2008, 71, 781–789. [Google Scholar]
- Ammida, N.H.S.; Micheli, L.; Palleschi, G. Electrochemical immunosensor for determination of aflatoxin B1 in barley. In First International Symposium on Recent Advances in Food Analysis; Prague, Czech Republic, 2004; pp. 159–164. [Google Scholar]
- Liu, Y.; Qin, Z.; Wu, X.; Jiang, H. Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on micro-comb electrode. Biochem. Eng. J. 2006, 32, 211–217. [Google Scholar]
- Bhattacharya, D.; Bhattacharya, R.; Dhar, T.K. A novel signal amplification technology for ELISA based on catalyzed reporter deposition. Demonstration of its applicability for measuring aflatoxin B1. J. Immunol. Method. 1999, 230, 71–86. [Google Scholar]
- Kolosova, A.Y.; Shim, W.B.; Yang, Z.Y.; Eremin, S.A.; Chung, D.H. Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B1. Stabilization of ELISA kit components and application to grain samples. Anal. Bioanal. Chem. 2006, 384, 286–294. [Google Scholar]
- Giersch, T. A new monclonal antibody for the sensitive detection of atrazine with immunoassay in microtiter plate and dipstick format. j. Agric. Food Chem. 1993, 41, 1006–1011. [Google Scholar]
- Tudorache, M.; Co, M.; Lifgren, H.; Emneus, J. Ultrasensitive magnetic particle-based immuno supported liquid membrane assay (m-ISLMA). Anal. Chem. 2005, 77, 7156–7162. [Google Scholar]
- Tudorache, M.; Zdrojewska, I.A.; Emneus, J. Evaluation of progesterone content in saliva using magnetic particle-based immuno supported liquid membrane assay (m-ISLMA). Biosens. Bioelectron. 2006, 22, 241–246. [Google Scholar]
- Kriz, K. New analytical approaches: magneto immunoassays and SIRE technology based biosensors. Thesis, Lund University, Lund, Sweden, 2003. [Google Scholar]
- Sole, S.; Merkoci, A.; Alegret, S. New material for electrochemical sensing beads (III). Trends Anal. Chem. 2001, 20, 102–110. [Google Scholar]
- Rad, A.Y.; Yavuz, H.; Kocakulak, M.; Denuzli, A. Bilirubin removal from human plasma with albumin immobilised magnetic poly(2-hydroxyethyl methacrylate) beads. Macromol. Biosci. 2003, 3, 471–476. [Google Scholar]
- Zhao, X.; Shippy, S.A. Competitive immunoassay for microliter protein samples with magnetic beads and near-infrared fluorescence detection. Anal. Chem. 2004, 76, 1871–1876. [Google Scholar]
- Hermanson, G.T.; Mallia, A.K.; Smith, P.K. Immobilization Affinity Ligand Techniques, 1rd Ed ed; Academic Press Inc.: San Diego, California, USA, 1992. [Google Scholar]
- Ammida, N.H.S.; Micheli, L.; Piermarini, S.; Palleschi, G. Detection of aflatoxin B1 in barley: Comparative study of immunosensor and HPLC. Anal. Lett. 2006, 39, 1559–1572. [Google Scholar]
- Piermarini, S.; Micheli, L.; Ammida, N.H.S.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar]
- Shim, W.B.; Kim, J.S.; Kim, J.Y.; Choi, J.G.; Je, J.H.; Kuzmina, N.S.; Eremin, S.A.; Chung, D.H. Determination of aflatoxin B1 in rice, barley and feed by non-instrumental immunochromatography strip-test and high sensitive ELISA. Food Sci. Biotech. 2008, 17, 623–630. [Google Scholar]

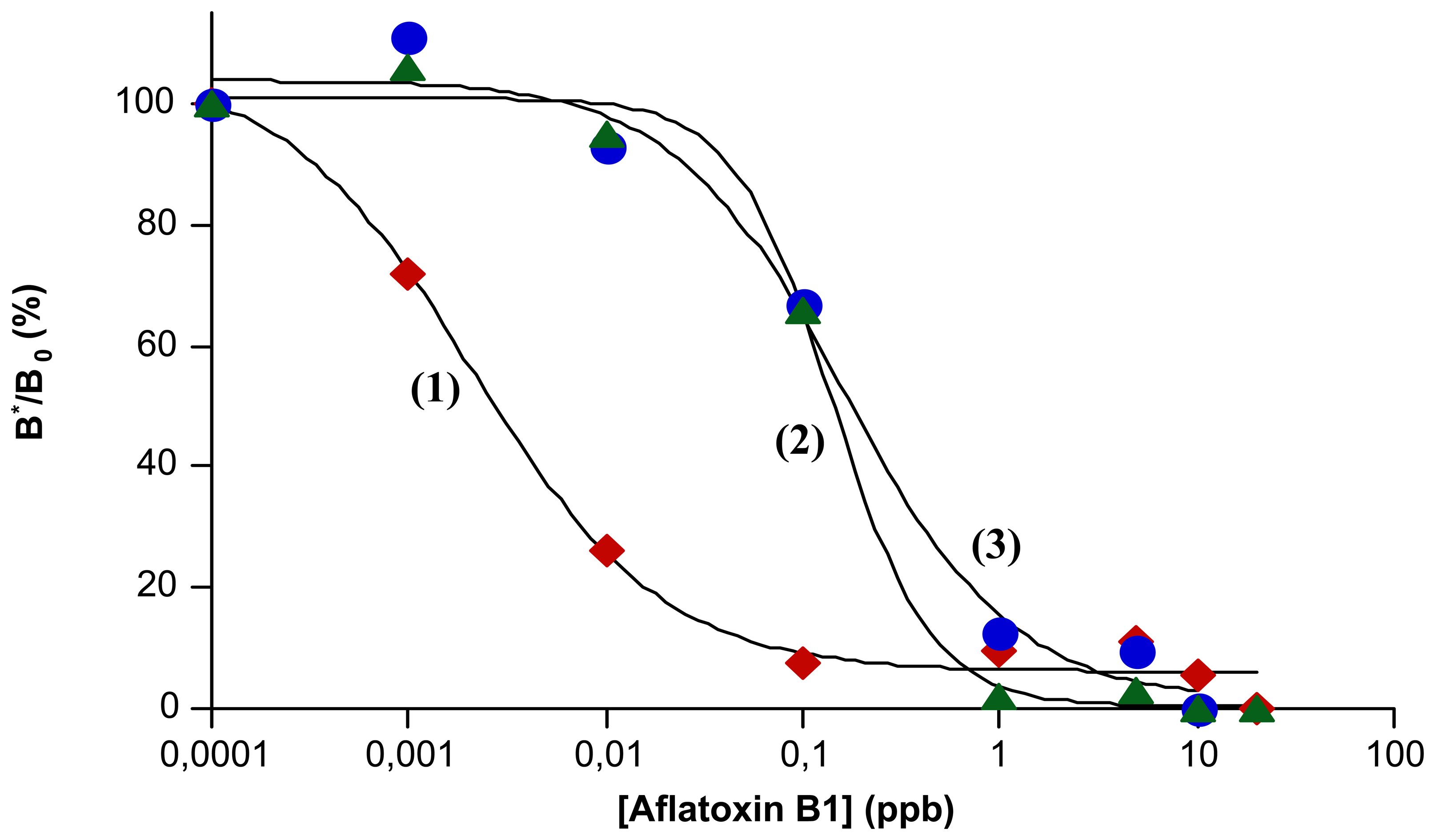
| Antibody immobilization | LOD (ppt) | IC50% (ppt) | DR (ppt) | |
|---|---|---|---|---|
| Covalent interaction Ab-bead | 1 | 2 | 1 - 10 | |
| Affinity interaction | Ab-protein G-bead | 50 | 150 | 80 - 500 |
| Ab-protein A-bead | 100 | 200 | 50 - 500 | |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the CreativeCommons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tudorache, M.; Bala, C. Sensitive Aflatoxin B1 Determination Using a Magnetic Particles-Based Enzyme-Linked Immunosorbent Assay. Sensors 2008, 8, 7571-7580. https://doi.org/10.3390/s8127571
Tudorache M, Bala C. Sensitive Aflatoxin B1 Determination Using a Magnetic Particles-Based Enzyme-Linked Immunosorbent Assay. Sensors. 2008; 8(12):7571-7580. https://doi.org/10.3390/s8127571
Chicago/Turabian StyleTudorache, Madalina, and Camelia Bala. 2008. "Sensitive Aflatoxin B1 Determination Using a Magnetic Particles-Based Enzyme-Linked Immunosorbent Assay" Sensors 8, no. 12: 7571-7580. https://doi.org/10.3390/s8127571





