1. Introduction
Mold spores and conidia are virtually ubiquitous, spreading though the air all over the world. They are often deposited indoors, and, in the right indoor conditions, mold can flourish and become a serious problem to the building's inhabitants. The most important factor governing mold growth is moisture conditions. Fungal growth begins at a water activity near 0.8 aw, and significant quantities of mycotoxins are produced when aw reaches 0.95. Mold growth is most conducive under a scenario where a water intrusion takes place. Molds flourish under these conditions, and upon dry conditions, spores and conidia are released, dispersed and deposited around the building [
1]. It has been reported that there is an association between human upper respiratory tract symptoms and damp mold infested buildings [
2]. A few common genera of indoor molds are
Penicillium, Aspergillus, Alternaria, Stachybotrys, and
Cladosporium.
Mycotoxins are secondary metabolites produced by molds that act as a defense against plants, bacteria, and other fungi. The World Health Organization International Agency for Research on Cancer studied various mycotoxins for their carcinogenic potentials, and concluded that many were indeed carcinogenic or cancer promoting [
3]. Recent studies in mice exposed to mycotoxin have shown severe neurotoxicity and inflammation within the nose and brain [
4]. Molds also produces MVOCs that are less toxic than mycotoxins. However, studies have shown that children living in homes with elevated MVOC levels have a higher prevalence of asthma, hay fever, and eye irritation [
5]. MVOCs are emitted into the air and can be inhaled. More than 500 different MVOCs have been identified and used to identify hidden sources of fungal contamination [
6,
7]. The MVOCs comprising the odor signatures of a given species of mold can vary based on the substrate on which it is growing [
8,
9]. MVOCs are emitted during all stages of mold growth. Some have very low odor thresholds, where they may be detectable by unaided human olfaction. Different MVOCs vary in toxicity, and one of the most cytotoxic is 1-octen-3-ol [
10].
Recently in the United States, interest in the mold detection industry has increased due to an increased awareness of the possible harmful effects of human exposure to toxic indoor molds and property damage that occurs as a result of a mold infestation. Preliminary sampling of indoor mold can be done by a variety of methods, such as moisture meters, laser particle counters, IR thermometers, and air sampling. Most methods used today involve taking random samples throughout the building in an attempt to locate the source of the mold spores. These techniques are very expensive and time consuming. Though high concentrations of mold may present in buildings, but because of their oftentimes invisible nature, locating the mold is a major problem. It is important to know the hot spots of mold growth for precise sampling and remediation procedures.
Canines have been widely used by law enforcement and forensic investigators in detecting accelerants, drugs, explosives, and human remains for years. Dogs have an exceptional sense of smell due to their increased number of olfactory receptors, allowing them to be specially trained to smell compounds that are virtually undetectable by human smell. They are also ideal since they will work solely for a reward or praise from their handler.
The specialized mammalian behavior “sniffing” improves the canine's olfactory abilities by causing turbulence in the nasal passages, effectively increasing transport of the odor molecules to the olfactory receptors. It is known that detector dogs are capable of detecting parts per million to parts per billion of volatile organic compounds and recent studies have begun to definitively identify the odor signature chemicals and thresholds of canine detection for a variety of specimens [
11]. This is significant since detector dogs must be able to detect small amounts of the volatile organic compounds and move to areas of greater concentration to find the source. Detector dogs can also accurately discriminate between target odors and similar non-target odors. They can be trained to alert to one or two of the most abundant odor components to detect a substance.
In Europe, canines have been trained to detect mold for years, but scientific evidence on the validity of the method is lacking. In the United States, interest in the mold detection industry has increased greatly in recent years due to an increase in awareness of the possible harmful effects of human exposure to toxic indoor molds and property damage that occurs as a result of a mold infestation. It has been determined that elevated MVOC levels can be a preliminary hint of indoor mold growth, so canines that are trained to detect these compounds should be able to discover indoor molds that are non-visible [
12]. Pending scientific validation, using canines as a mold detection method would cut down on cost, time, and labor for the mold detection industry.
2. Methods
Plates of Potato Dextrose Agar (PDA) were prepared for both Penicillium chrysogenum and Aspergillus versicolor. Plates of Corn Meal Agar (CMA) were prepared for Stachybotrys chartarum. Growth media was autoclaved at 121° C for 15 minutes and plated under sterile conditions in a Biological Safety Cabinet. To further ensure the media was not contaminated, plates were incubated at room temperature for a week before samples were cultured. Samples were subsequently cultured in triplicate and were incubated at 30° C for one week. The plates were then examined and purified onto other uncontaminated PDA and CMA plates and incubated for another week at 30° C. A small 1 cm × 1 cm piece of mold and agar was then excised from each plate and aseptically transferred to ½ liter Mason jars. These jars were pre-lined with PDA or CMA, and served to allow headspace samples to be taken over successive weeks while offering the mold a constant and sufficient supply of a nutrient source.
Solid-phase microextraction (SPME) parameters were adjusted through a series of optimization studies, where SPME fiber type, length of exposure, and volume of headspace were varied. Initially, the volatile organic components were extracted from the headspace of the vials using three different SPME fiber types: PDMS/DVB (65 μm StableFlex), CAR/PDMS (75 μm), and CW/DVB (70 μm StableFlex). Blanks of all SPME fibers were run before exposure to the sample to eliminate carryover from previous exposures. Fibers were exposed to the sample for 1, 10, and 18 hours. It was ultimately determined that CW/DVB StableFlex fibers extracted the most compounds from the headspace at an exposure time of 18 hours, using ½ L Mason jars to house the mold samples. After exposing the fibers to the headspace of the Aspergillus versicolor, Penicillium chrysogenum, and Stachybotrys chartarum mold samples, as well as mold-free PDA and CMA for 18 hours, they were introduced to the GC/MS for thermal desorption and analysis. To test the effect of time on odor signatures of mold species, the Stachybotrys chartarum sample was analyzed using SPME-GC/MS for three consecutive weeks.
A splitless injection was used on an Agilent Technologies HP5-MS capillary column with a length of 30 m, an outer diameter of 0.25 mm, and a film thickness of 0.25 μm. Helium was used as a carrier gas, and had a flow rate of 1 milliter/minute. The initial temperature was 40°C, and that temperature was held for 5 minutes. Next, there was a 10°C/min increase to 280°C, and then that temperature was held for 1 minute. The mass spectral data from the resulting chromatograms was compared to the NIST 1998 library for identification.
Canine field tests were run with four pre-trained dogs at the Florida Canine Academy in Safety Harbor, Florida. The Aspergillus versicolor, Penicillium chrysogenum, and Stachybotrys chartarum mold samples were placed in separate round plastic containers with punctured lids to allow the volatiles to escape. Negative controls were also set up which contained no toxic molds. The field tests were then executed in a double-blind fashion with an impartial evaluator to record all alerts and nonalerts for each dog, according to specifications set forth to increase reliability of canine field tests.
3. Results
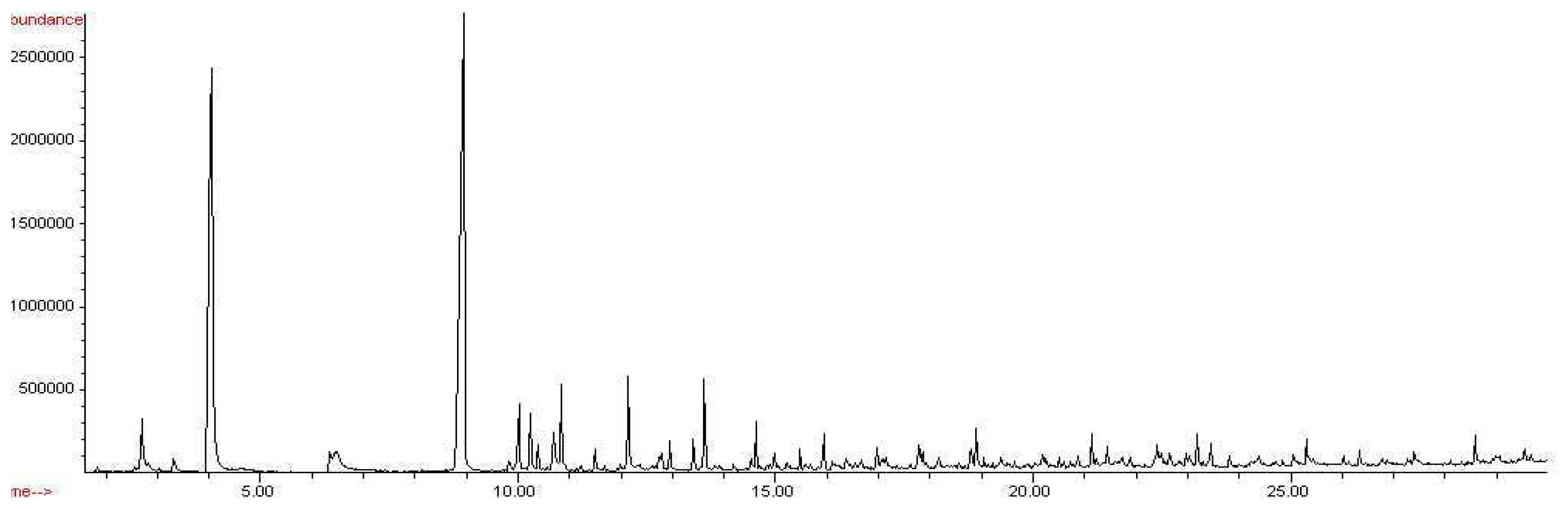
From the chromatographic data obtained, the results indicate that the odor signatures for Aspergillus versicolor, Penicillium chrysogenum, and Stachybotrys chartarum are very different. The CW/DVB fiber successfully extracted 22 known compounds that matched the NIST 98 library from the headspace of Aspergillus versicolor, 19 known compounds from the headspace of Penicillium chrysogenum, and 29 known compounds from the headspace of Stachybotrys chartarum.
Twenty two collective microbial volatile organic compounds were extracted from the headspace of
Aspergillus versicolor and detected by SPME-GC/MS analysis: arsenous acid, 1-octen-3-ol, 3-octanone, 3-octanol, 1-hexanol, hexanoic acid, pentanedinitrile, benzene, 2,3-dimethoxytoluene, 2-undecanone, benzamide, 1-butanol, 2-propenoic acid, 1,4,7,10,13,16,19-heptaoxa-2-cycloheneicosanone, 1-tridecanol, 2-tridecanone, dodecanoic acid, 2,6-bis(1,1-dimethylethyl)-4-(1-oxypropyl)phenol, 9H-fluoren-3-ol, tetradecanoic acid, n-butyl laurate, and n-hexadecanoic acid. Compounds detected in the headspace of PDA were subtracted from the list of compounds detected in the headspace of
Aspergillus versicolor, as they cannot be proven to be microbial volatile organic compounds at this time.
Table 1 below list the compounds detected, as well as their retention times and % peak areas.
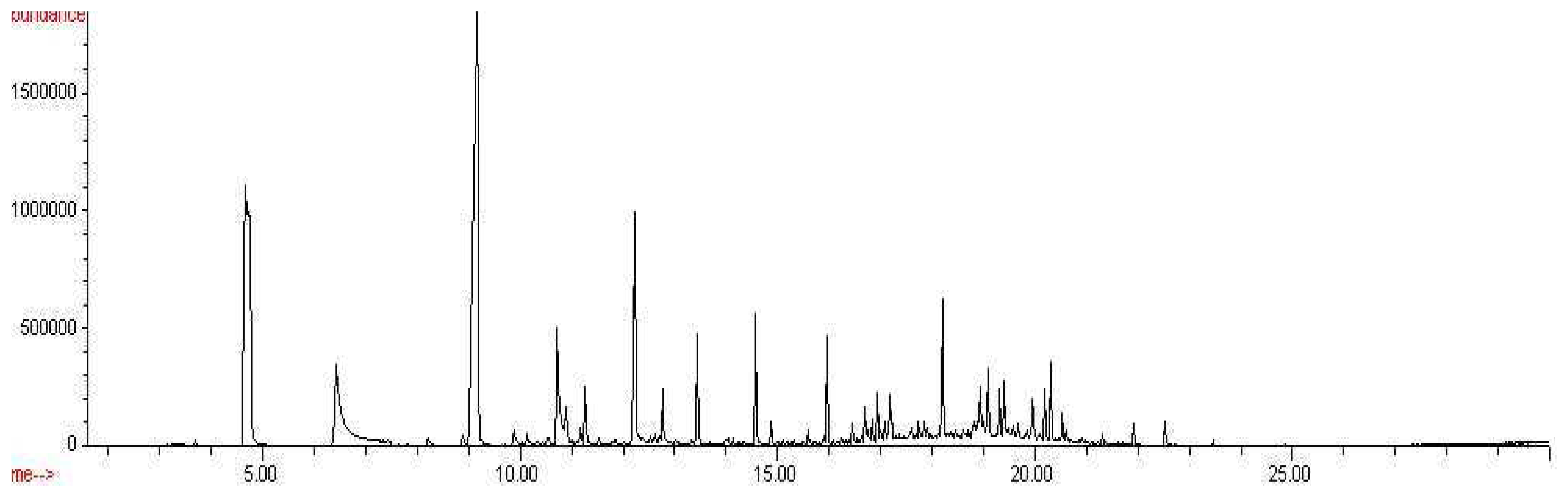
Similarly, a variety of compounds were observed from the headspace of the
Penicillium chrysogenum, as shown in
Figure 4. Nineteen collective microbial volatile organic compounds were extracted from the headspace of
Penicillium chrysogenum and detected by SPME-GC/MS analysis: arsenous acid, 1-methoxycyclohexane, furan, 1-hexanol, nonanal, hexanoic acid, benzene, naphthalene, 1-undecanol, oxirane, cyclododecane, dodecanoic acid, butyl caprate, 1-docosene, tetradecanoic acid, Z,E-2-methyl-3,13-octadecadien-1-ol, n-butyl laurate, 1-heptadecanol, and 1,4,7,10,13,16,19-heptaoxa-2-cycloheneicosanone. Compounds detected in the headspace of PDA were subtracted from the list of compounds detected in the headspace of
Penicillium chrysogenum, as they cannot be proven to be microbial volatile organic compounds.
Table 2 below list the compounds detected, as well as their retention times and % peak areas.
Twenty nine collective microbial volatile organic compounds were extracted from the headspace of
Stachybotrys chartarum, as shown in
Figure 5: benzyl alcohol, cyclopropane, nonanal, hexanoic acid, benzene, 1,3-pentanediol, acetic acid, nonanol, ethanol, decanal, benzothiazole, nonanoic acid, 1-undecanol, tetradecane, diphenyl ether, oxirane, cyclotetradecane, 2,5-cyclohexadiene-1,4-dione, 1-pentadecene, cyclododecane, benzoic acid, n-nonylcyclohexane, 1-heptadecene, methyl pentadecyl ether, n-butyl laurate, octadecane, cyclohexadecane, hexadecanoic acid, and octadecanoic acid. The compounds found in the headspace of Corn Meal agar alone were subtracted from the composite list of compounds found in the headspace of
Stachybotrys chartarum, as they cannot be credited as metabolites of the molds.
Table 3 below list the compounds detected, as well as their retention times and % peak areas.
The time study experiments analyzing the headspace of Stachybotrys chartarum was run for three consecutive weeks. Twenty-seven known compounds were identified from the headspace of Stachybotrys chartarum samples using CW/DVB SPME fibers and an exposure time of 18 hours.
The field test data show that the canines successfully alert to the
in vitro toxic mold samples, with a slightly lower accuracy than previous tests have shown canines alerting to the mold-inoculated pieces of drywall that were used for their training. From the canine field tests, nine of the twelve positive controls grown in the laboratory were alerted to by the four new canines tested. Canine # 1 alerted to 66.6% of the positive controls, missing one. Canine # 2 alerted to 100% of the positive controls. Canine # 3 alerted to 33.3% of the positive controls, missing two. Canine # 4 alerted to 100 % of the positive controls.
Tables 5 and
6 show accuracy statistics in terms of each positive control and each canine tested.
4. Conclusions
These experiments show that canines can effectively detect active odor components of various indoor mold species. By using SPME-GC/MS analysis, detection of the MVOCs emitted from three toxic indoor molds, Penicillium chrysogenum, Aspergillus versicolor, and Stachybotrys chartarum was established. The distinctiveness of volatile secondary metabolite production of these species could be estimated by this technique as well. This mold detection research is important, as canine trainers can use the compounds isolated found in this study to ensure the canines are being trained on the correct odors being emitted from these toxic molds.
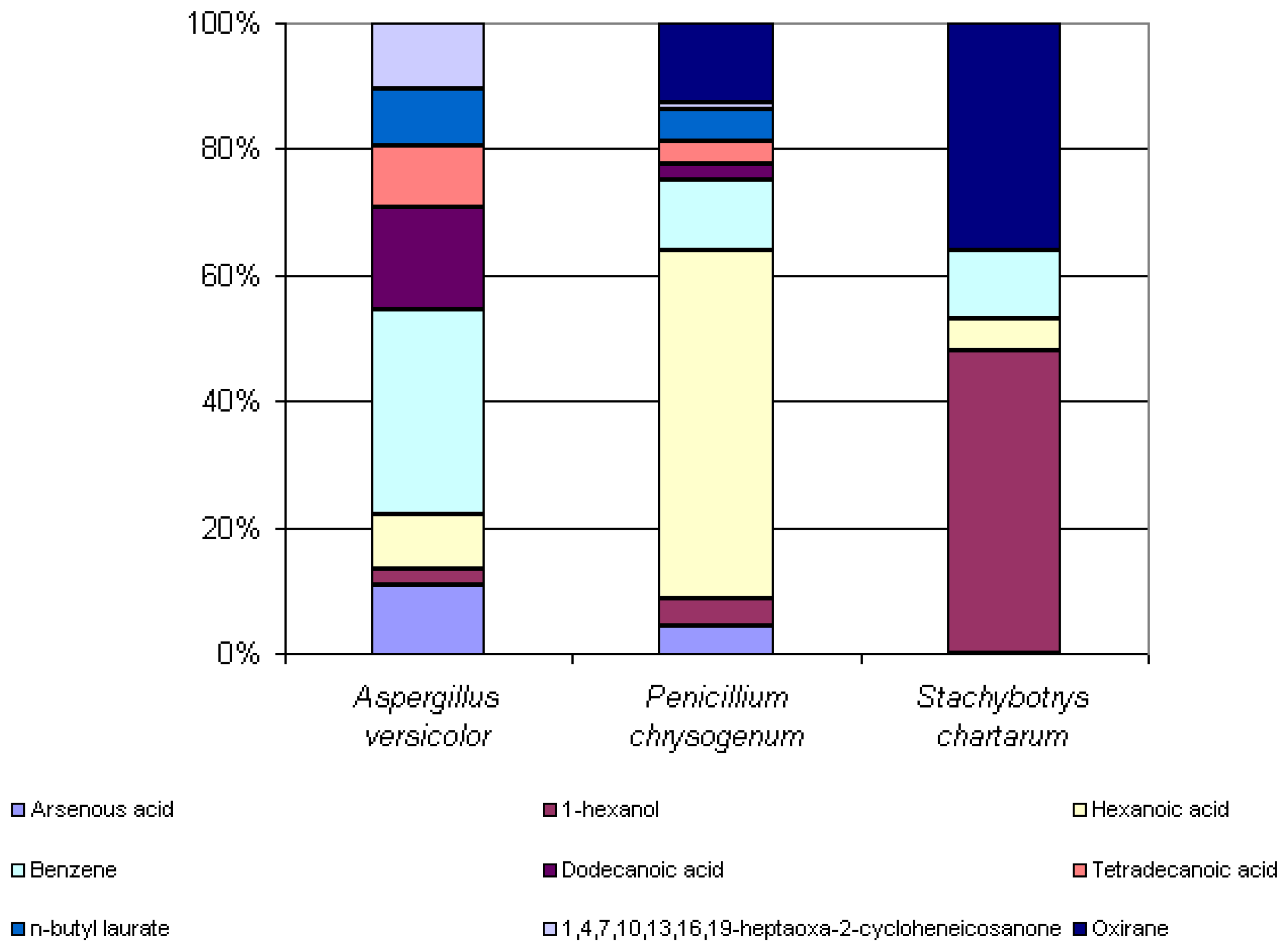
Some microbial volatile organic compounds were common amongst the three species. 1-hexanol, hexanoic acid, and benzene were extracted from the headspaces of all three species analyzed. Arsenous acid, dodecanoic acid, tetradecanoic acid, n-butyl laurate and 1,4,7,10,13,16,19-heptaoxa-2-cycloheneicosanone were extracted from the headspaces of both
Aspergillus versicolor and
Penicillium chrysogenum. Oxirane was extracted from the headspaces of both
Penicillium chrysogenum and
Stachybotrys chartarum. Even amongst these commonly seen compounds, there is a great difference in the percent peak areas detected between these three species. Knowledge of the similarities and differences of the odor signatures of various toxic indoor mold species is very significant and useful in the improvement of existing canine training aids.
Figure 6 below illustrates the differences in the percent peak areas of these common compounds.
The detection of changes in MVOCs composition from the headspace of a toxic mold species over time is a significant finding. If the odor signatures of the molds change significantly over time, training methods may need to be modified to account for changes in composition. The results from the experiments of the effect of time on the Stachybotrys chartarum MVOC composition are conclusive. One compound, 1-H-2-Benzopyran-1-one, remained in the headspace for the three successive weeks. Seven compounds remained in the headspace for the second and third weeks of the experiments: 1-H-2-Benzopyran-1-one, 1-hexanol, benzene, cyclododecane, heptadecane, oxirane, and phenol. With the exception of 1-H-2-Benzopyran-1-one, all other common compounds observed in the second and third weeks of headspace analysis increased in abundance from the second to the third week of growth. The effect of time on Aspergillus versicolor and Penicillium chrysogenum MVOC compositions are less conclusive, as analyses did not yield any identifiable compounds from the headspace for both species at some point in the three weeks.
This study demonstrates that canines can detect laboratory cultured toxic indoor molds at a 75% accuracy. This is significant, as these canines are not trained on molds grown in vitro on agar media, but rather pieces of mold-inoculated drywall. Previous studies have concluded that canines trained at the Florida Canine Academy can detect mold-inoculated drywall training aids at a 94% accuracy [
13]. Although they did not detect molds grown
in vitro on agar media with the same percent accuracy as the results of mold-inoculated drywall training aids studies have shown, they detected the majority of the laboratory cultured toxic mold positive controls in the course despite any differences in metabolites produced by the molds digesting the alternate substrate with which they were trained. Findings of this research are being used to validate the canine training methods used for their detection and may be used to improve the detection of toxic indoor molds.