Effects of Palladium Loading on the Response of Thick Film Flame-made ZnO Gas Sensor for Detection of Ethanol Vapor
Abstract
:1. Introduction
2. Experimental
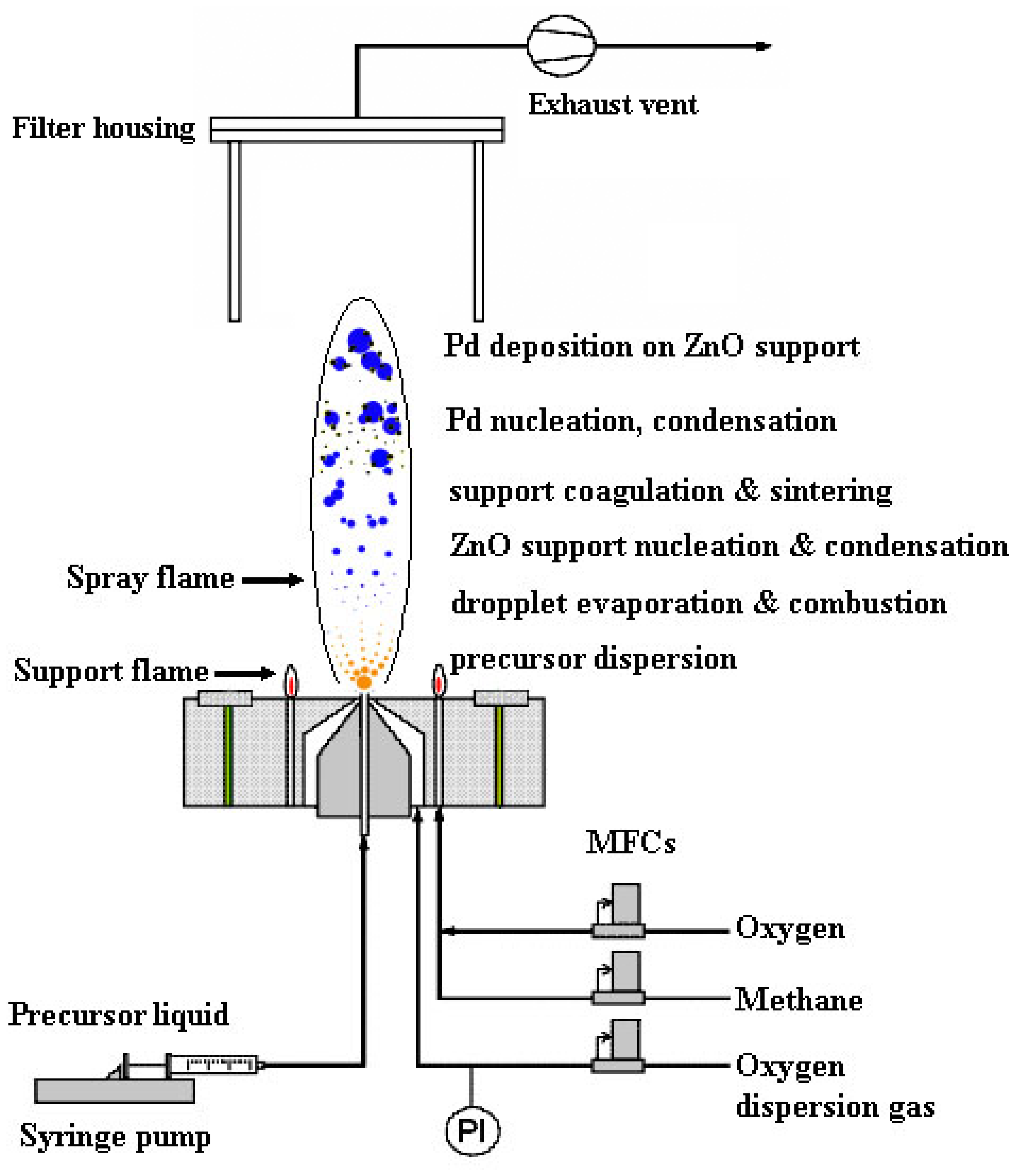
2.1. Flame-synthesis of Pd-ZnO Nanoparticles
2.2. Particle and Doping Characterizations
2.3. Sensor Preparation
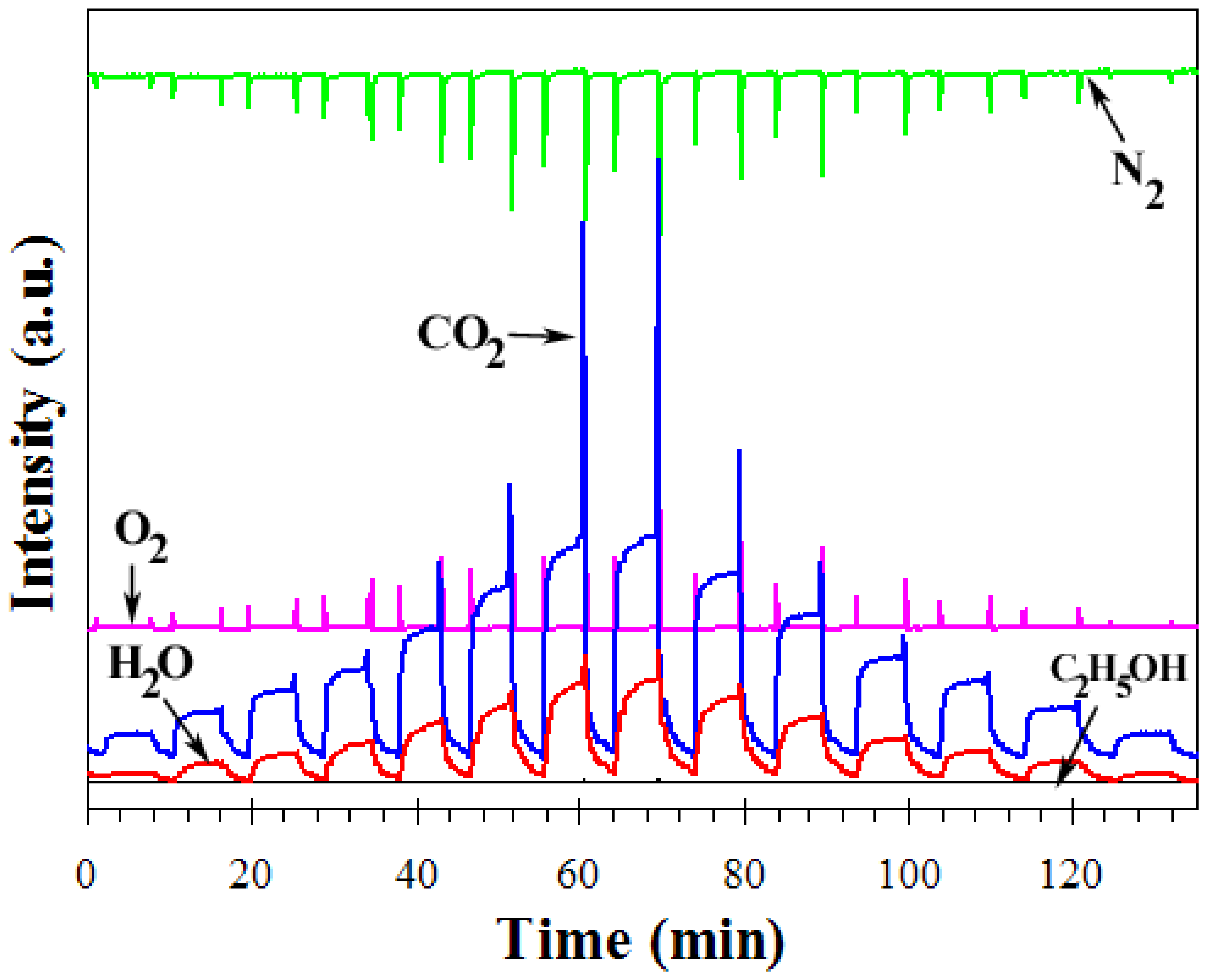
2.4. Characterization of Gas Sensing Properties and Mass Spectrometry Measurements
3. Results and Discussion
3.1. Particle Properties
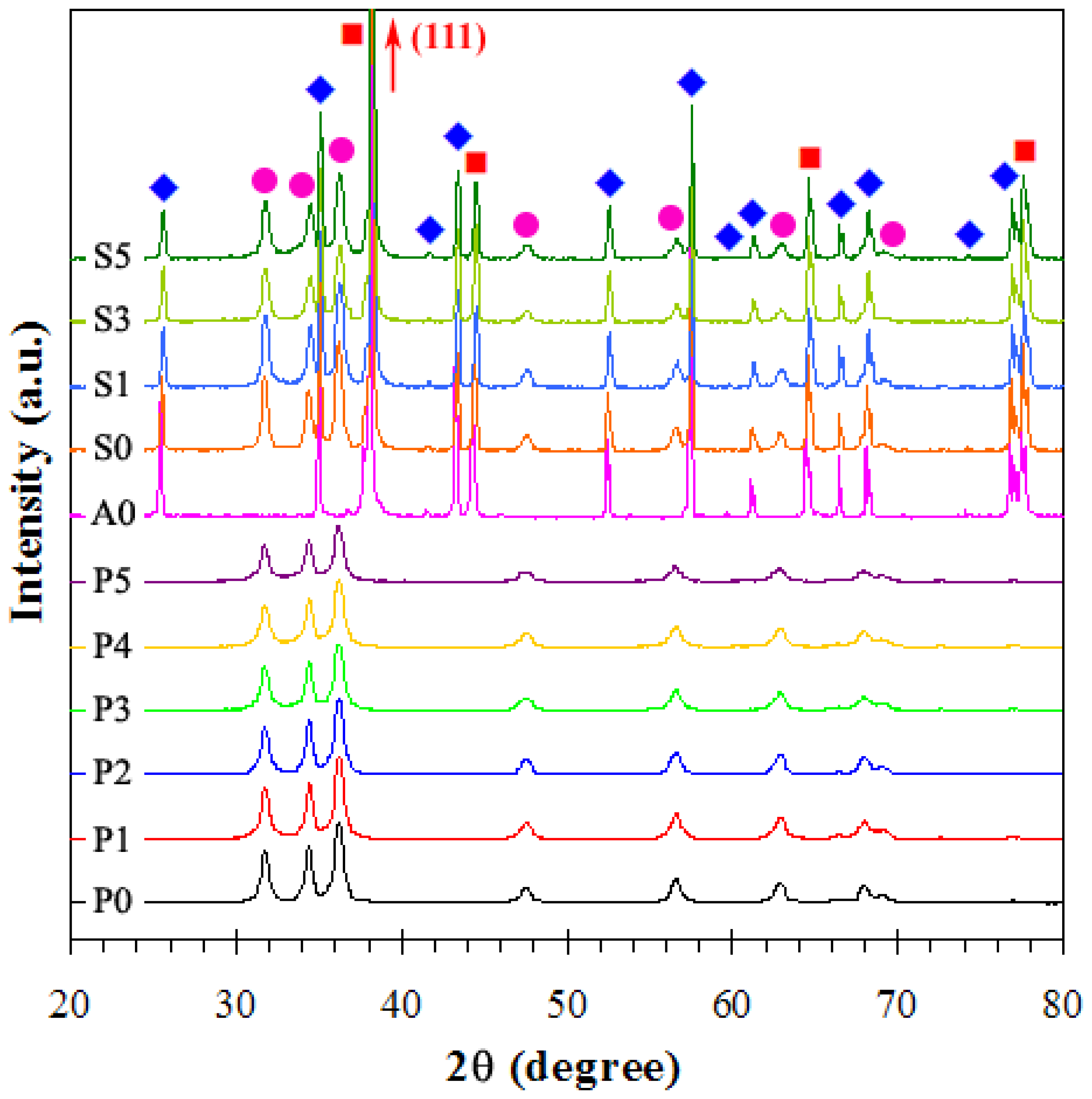
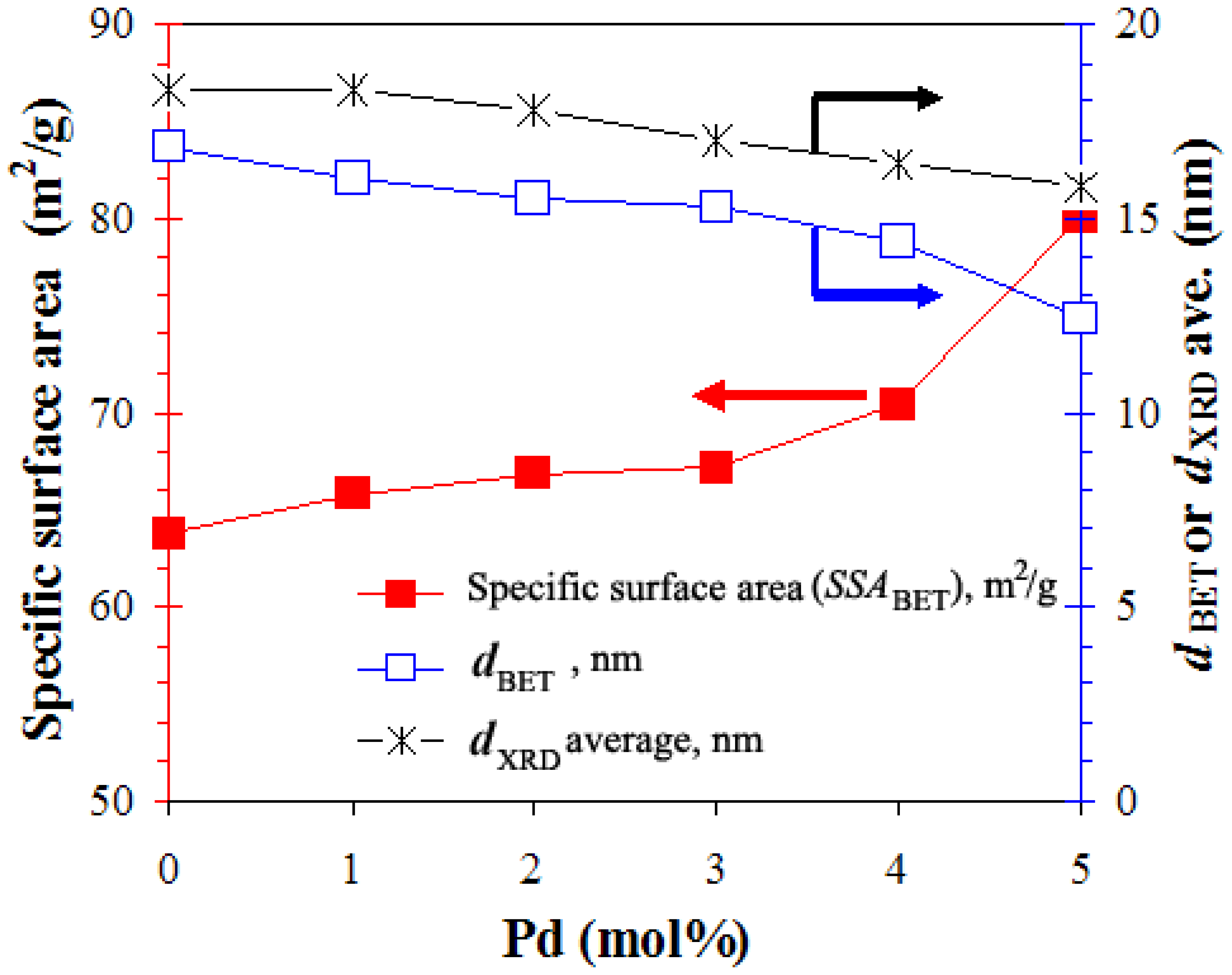
3.1.1. X-ray Diffraction and BET Analyses
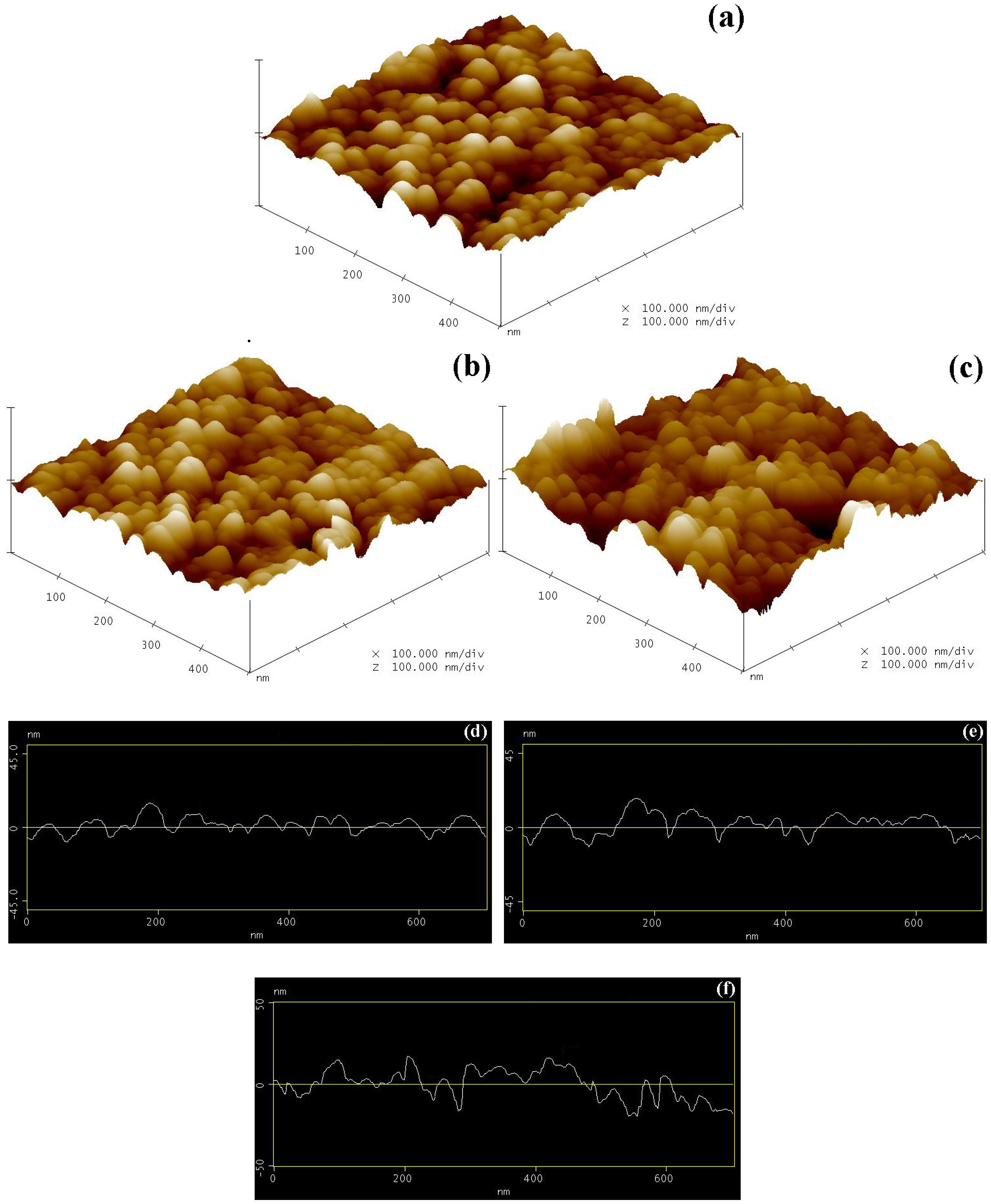
3.1.2. Atomic Force Microscopy (AFM) Analysis
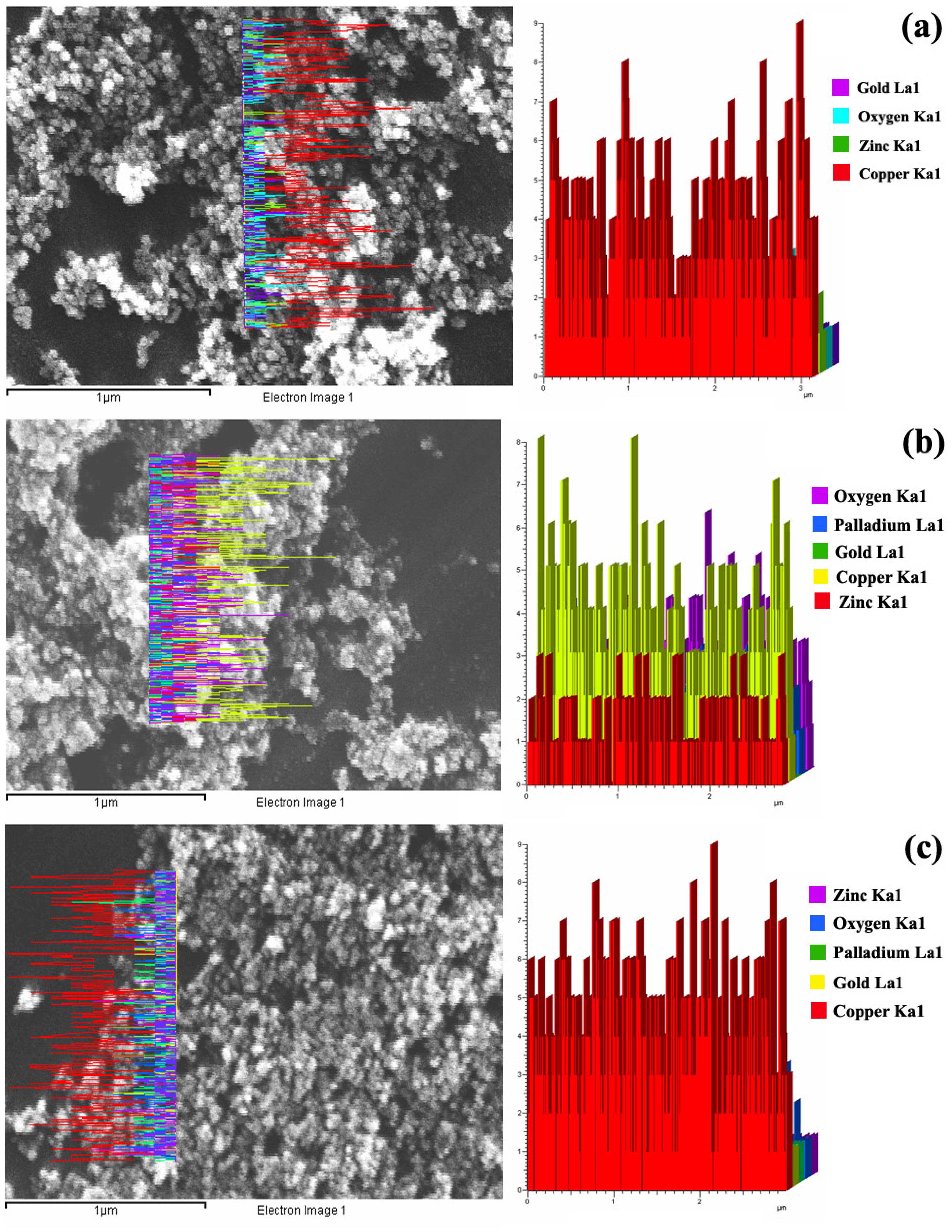
3.1.3. Scanning Electron Microscopy (SEM) Analysis
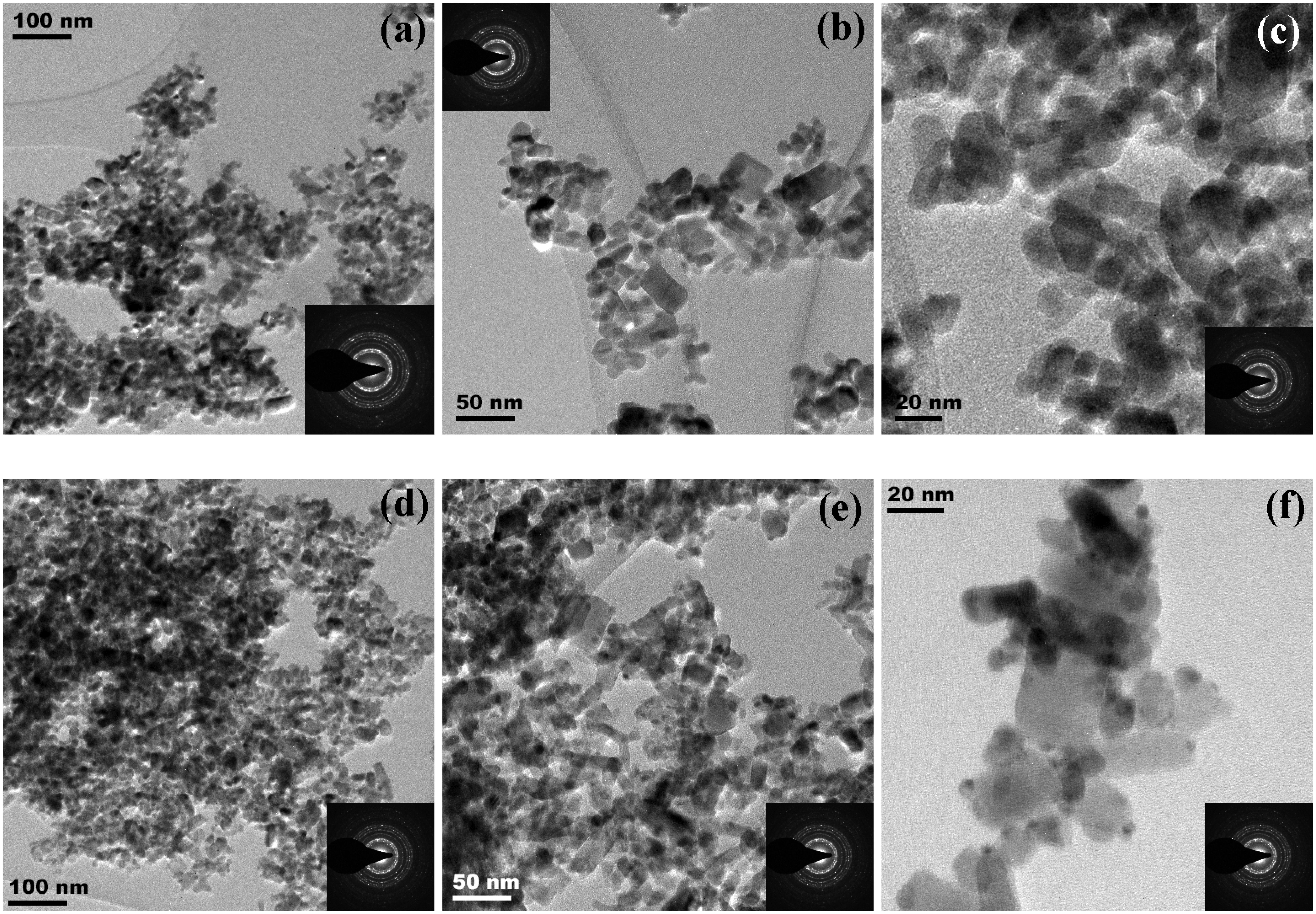
3.1.4. High Resolution-Transmission Electron Microscopy (HR-TEM) Analysis
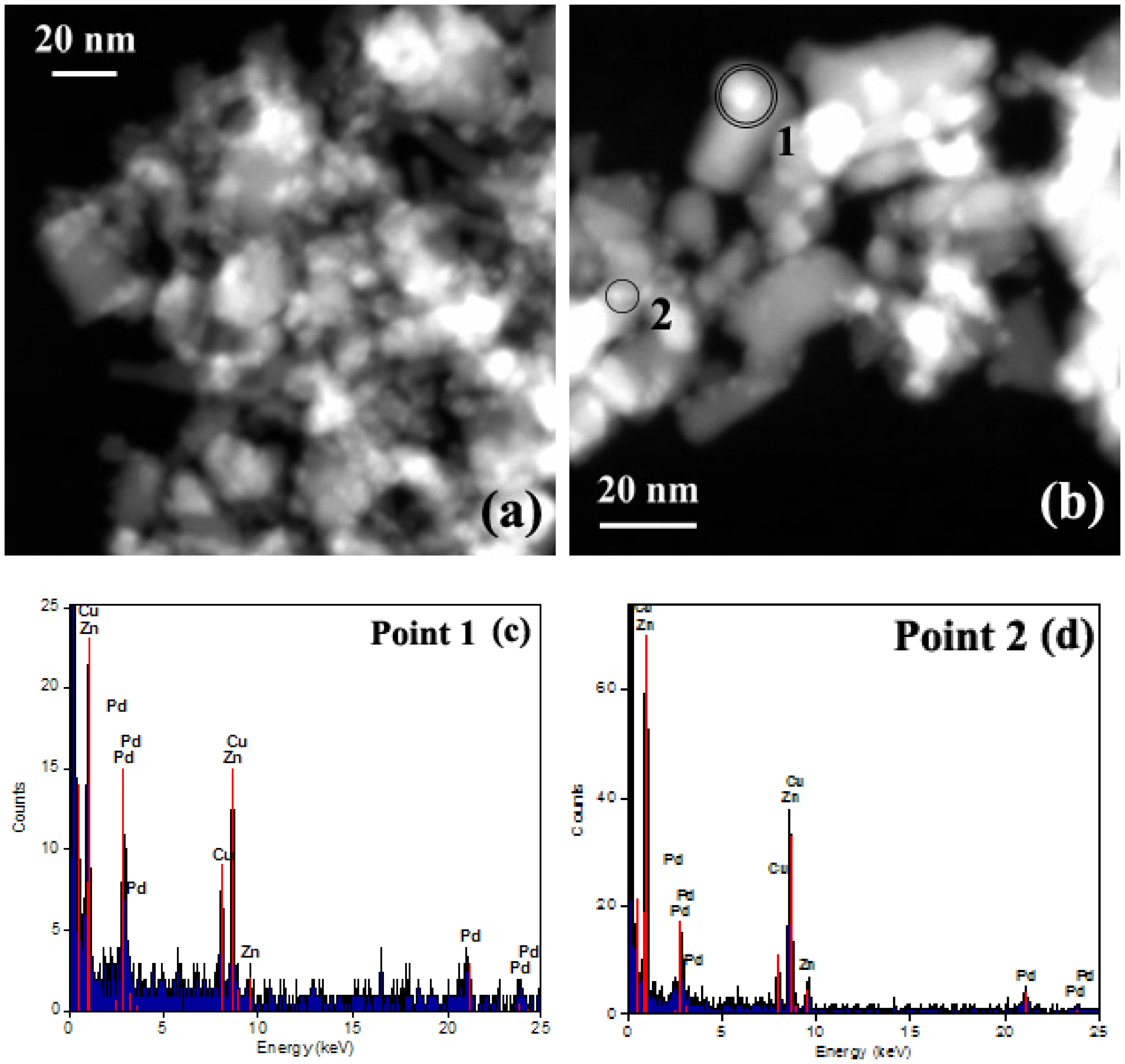
3.1.5. Scanning-Transmission Electron Microscopy (STEM) Analysis
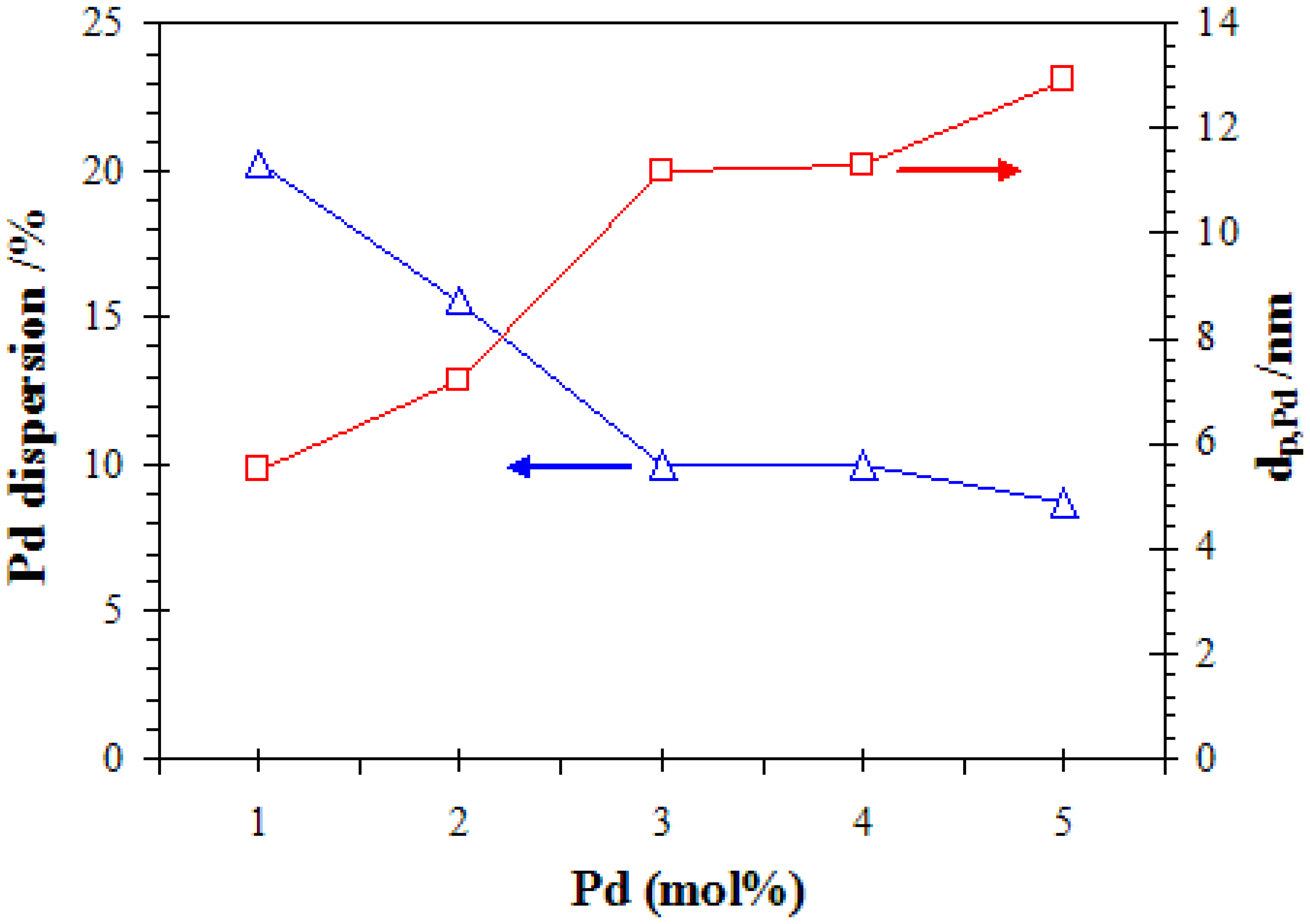
3.1.6. CO-pulse Chemisorption Measurement
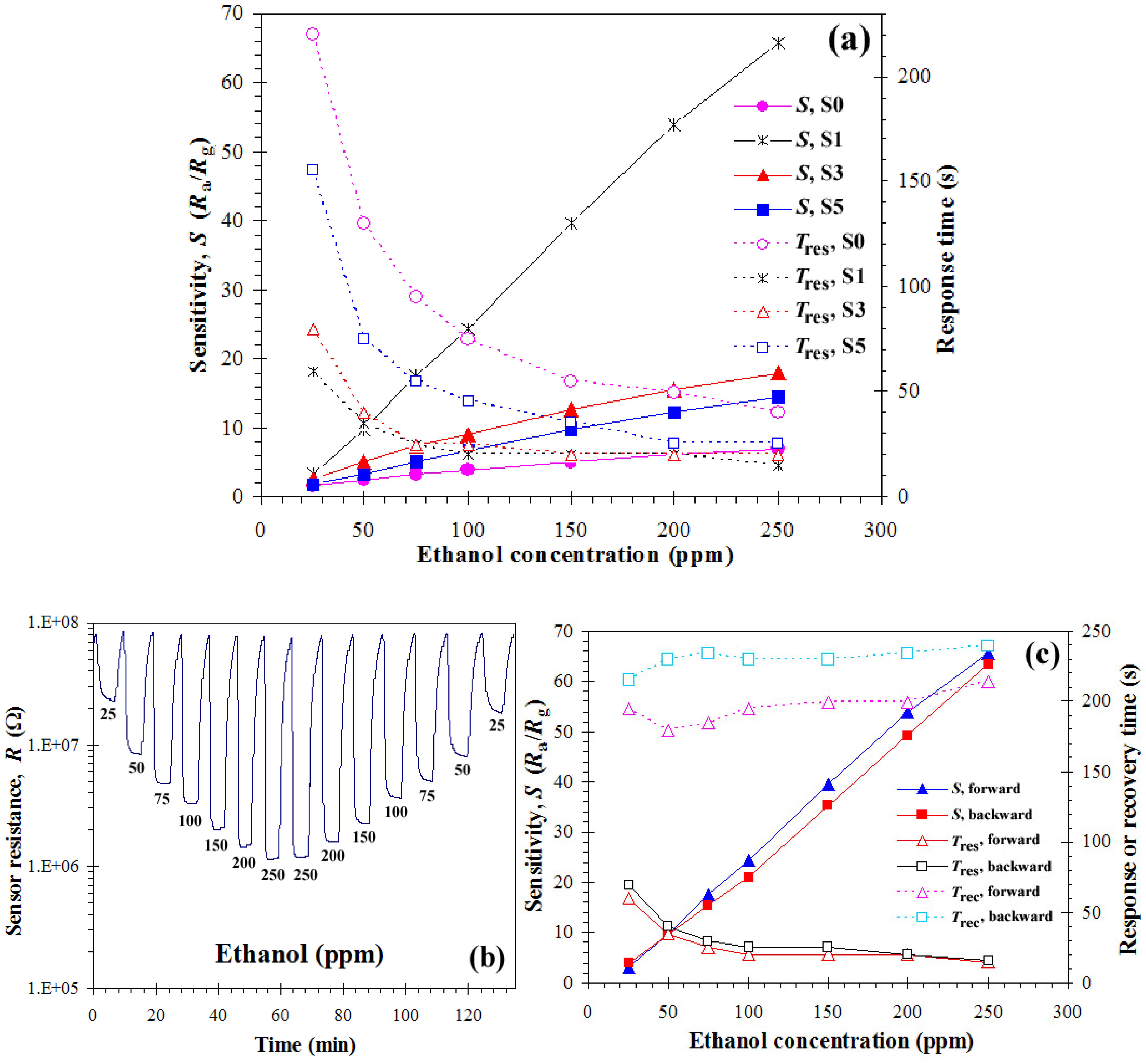
3.2. Gas Sensing Properties
- -
- Nanostructuring of the based-semiconducting oxide also contributes to the improvement in the gas response by larger surface area because here the complete particle contributes to the gas sensing phenomenon (D ~ 2L, where D is the particle diameter and L is the Debye length). The larger surface area of the materials synthesized also facilitates the gas detection at much lower temperatures [43]. In addition, palladium is known to have a catalytic effect due to its excellent oxidation capability to convert hydrocarbons at lower temperatures making the sensor selective to hydrocarbons [43-45].
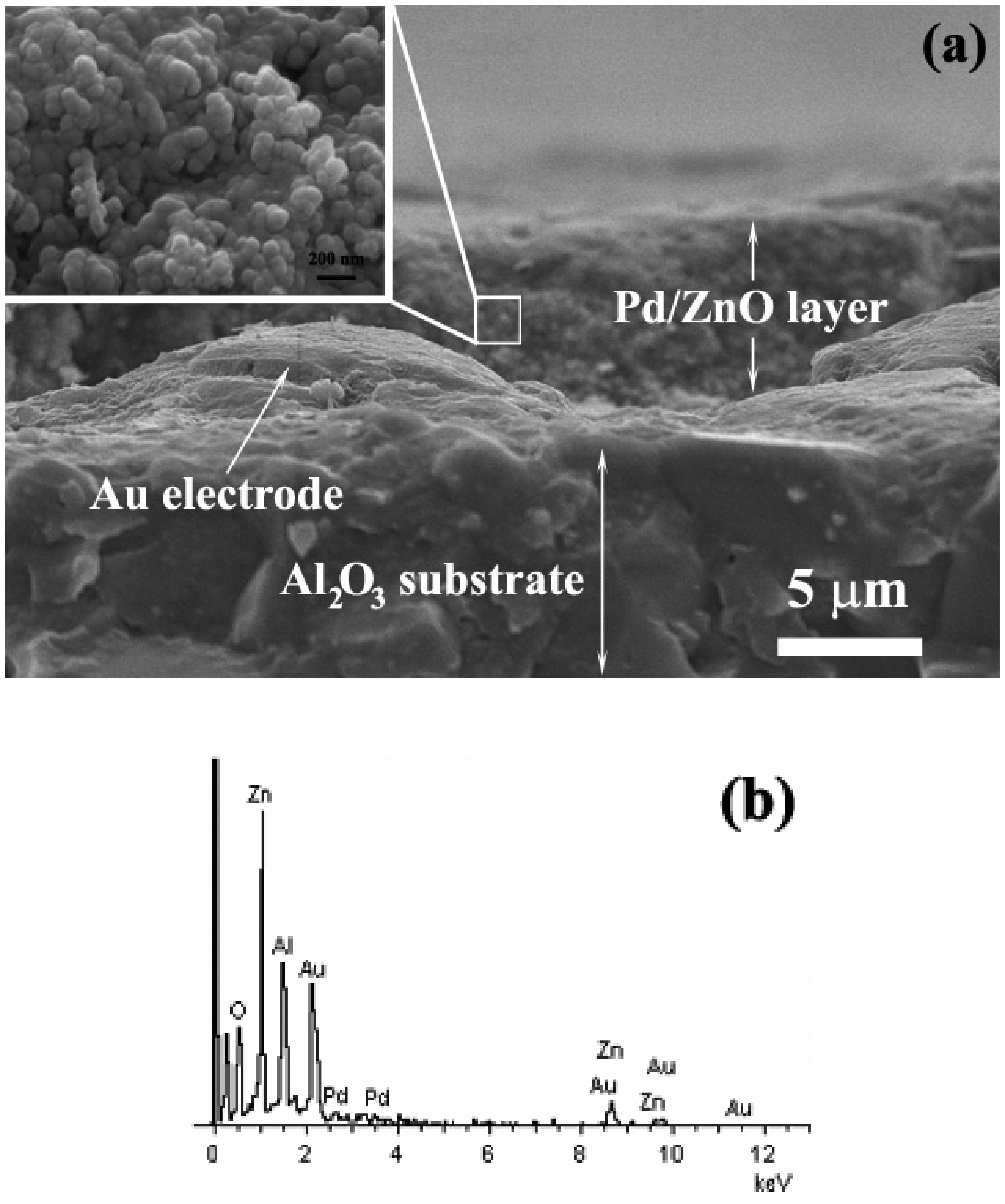
3.3. SEM-Film thickness sensing layer
4. Conclusions
Acknowledgments
References
- Sahm, T.; Mädler, L.; Gurlo, A.; Barsan, N.; Pratsinis, S.E.; Weimar, U. Flame spray synthesis of tin dioxide nanoparticles for gas sensing. Sens. Actuators B 2004, 98, 148–153. [Google Scholar]
- Neri, G.; Bonavita, A.; Micali, G.; Donato, N.; Deorsola, F.A.; Mossino, P.; Amato, I.; Benedetti, B.D. Ethanol sensors based on Pt-doped tin oxide nanopowders synthesised by gel-combustion. Sens. Actuators B 2006, 117, 196–204. [Google Scholar]
- Li, F.; Xu, J.; Yu, X.; Chen, L.; Zhu, J.; Yang, Z.; Xin, X. One-step solid-state reaction synthesis and gas sensing property of tin oxide nanoparticles. Sens. Actuators B 2002, 81, 165–169. [Google Scholar]
- Rella, R.; Serra, A.; Siciliano, P.; Vasanelli, L.; De, G.; Licciulli, A.; Quirini, A. Tin oxide-based gas sensors prepared by the sol-gel process. Sens. Actuators B 1997, 44, 462–467. [Google Scholar]
- Maekawa, T.; Tamaki, J.; Miura, N.; Yamazoe, N. Development of SnO2-based ethanol gas sensor. Sens. Actuators B 1992, 9, 63–69. [Google Scholar]
- Gautheron, B.; Labeau, M.; Delabouglise, G.; Schmatz, U. Undoped and Pd-doped SnO2thin films for gas sensors. Sens. Actuators B 1993, 15-16, 357–362. [Google Scholar]
- Ivanov, P.; Hubalek, J.; Prášek, K.; Vilanova, X.; Llobet, E.; Correig, X. A route toward more selective and less humidity sensitive screen-printed SnO2 and WO3 gas sensitive layers. Sens. Actuators B 2004, 100, 221–227. [Google Scholar]
- Tan, O.K.; Cao, W.; Zhu, W.; Chai, J.W.; Pan, J.S. Ethanol sensors based on nano-sized α-Fe2O3 with SnO2, ZrO2, TiO2 solid solutions. Sens. Actuators B 2003, 93, 396–401. [Google Scholar]
- Mädler, L.; Roessler, A.; Pratsinis, S.E.; Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Direct formation of highly porous gas-sensing films by in situ thermophoretic deposition of flame-made Pt/SnO2 nanoparticles. Sens. Actuators B 2006, 114(1), 283–295. [Google Scholar]
- Ge, J.P.; Wang, J.; Zhang, H.X.; Wang, X.; Peng, Q.; Li, Y.D. High ethanol sensitive SnO2 microspheres. Sens. Actuators B 2006, 113, 937–943. [Google Scholar]
- Fang, Y.K.; Lee, J.J. A tin oxide thin film sensor with high ethanol sensitivity. Thin Solid Films 1989, 169, 51–56. [Google Scholar]
- Heilig, A.; Barsan, N.; Weimar, U.; Göpel, W. Selectivity enhancement of SnO2 gas sensors: simultaneous monitoring of resistances and temperatures. Sens. Actuators B 1999, 58, 302–309. [Google Scholar]
- Makhija, K.K.; Ray, A.; Patel, R.M.; Trivedi, U.B.; Kapse, H.N. Indium oxide thin film based ammonia gas and ethanol vapour sensor. Bull. Mater. Sci. 2005, 28, 9–17. [Google Scholar]
- Xiangfeng, C.; Caihong, W.; Dongli, J.; Chenmou, Z. Ethanol sensor based on indium oxide nanowires prepared by carbothermal reduction reaction. Chem. Phys. Lett. 2004, 399, 461–464. [Google Scholar]
- Teleki, A.; Pratsinis, S.E.; Kalyanasundaram, K.; Gouma, P.I. Sensing of organic vapors by flame-made TiO2 nanoparticles. Sens. Actuators B 2006, 119, 683–690. [Google Scholar]
- Sberveglieri, G.; Comini, E.; Faglia, G.; Atashbar, M.Z.; Wlodarski, W. Titanium dioxide thin films prepared for alcohol microsensor applications. Sens. Actuators B 2000, 66, 139–141. [Google Scholar]
- Mabrook, M.; Hawkins, P. A rapidly-responding sensor for benzene, methanol and ethanol vapours based on films of titanium dioxide dispersed in a polymer operating at room temperature. Sens. Actuators B 2001, 75, 197–202. [Google Scholar]
- Ruiz, A.M.; Cornet, A.; Morante, J.R. Performances of La-TiO2nanoparticles as gas sensing material. Sens. Actuators B 2005, 111-112, 7–12. [Google Scholar]
- Galatsis, K.; Li, Y.X.; Wlodarski, W.; Comini, E.; Sberveglieri, G.; Cantalini, C.; Santucci, S.; Passacantando, M. Comparison of single and binary oxide MoO3, TiO2 and WO3 sol-gel gas sensors. Sens. Actuators B 2002, 83, 276–280. [Google Scholar]
- Ferroni, M.; Guidi, V.; Martinelli, G.; Roncarati, G.; Comini, E.; Sberveglieri, G.; Vomiero, A.; Della Mea, G. Coalescence inhibition in nanosized titania films and related effects on chemoresistive properties towards ethanol. J. Vac. Sci. Technol. B 2002, 20(2), 523–530. [Google Scholar]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar]
- Liu, Y.; Dong, J.; Hesketh, P.J.; Liu, M. Synthesis and gas sensing properties of ZnO single crystal flakes. J. Mater. Chem. 2005, 15, 2316–2320. [Google Scholar]
- Xiangfeng, C.; Dongli, J.; Djurišic, A.B.; Leung, Y.H. Gas-sensing properties of thick film based on ZnO nano-tetrapods. Chem. Phys. Lett. 2005, 401, 426–429. [Google Scholar]
- Sahay, P.P.; Tewari, S.; Jha, S.; Shamsuddin, M. Sprayed ZnO thin films for ethanol sensor. J. Mater. Sci. 2005, 40, 4791–4793. [Google Scholar]
- Xu, J.; Chen, Y.; Li, Y.; Shen, J. Gas sensing properties of ZnO nanorods prepared by hydrothermal method. J. Mater. Sci. 2005, 40, 2919–2921. [Google Scholar]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; He, X.L.; Li, J.P.; Lin, C.L.; Wang, T.H. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar]
- Xu, J.; Pan, Q.; Shun, Y.; Tian, Z. Grain size control and gas sensing properties of ZnO gas sensor. Sens. Actuators B 2000, 66, 277–279. [Google Scholar]
- Xu, H.; Liu, X.; Cui, D.; Li, M.; Jiang, M. A novel method for improving the performance of ZnO gas sensors. Sens. Actuators B 2006, 114, 301–307. [Google Scholar]
- Arshak, K.; Gaidan, I. Development of a novel gas sensor based on oxide thick films. Mater. Sci. Eng. B 2005, 118, 44–49. [Google Scholar]
- Cheng, X.L.; Zhao, H.; Huo, L.H.; Gao, S.; Zhao, J.G. ZnO nanoparticulate thin film: preparation, characterization and gas-sensing property. Sens. Actuators B 2004, 102, 248–252. [Google Scholar]
- Tang, H.; Li, Y.; Zheng, C.; Ye, J.; Hou, X.; Lv, Y. An ethanol sensor based on cataluminescence on ZnO nanoparticles. Talanta 2007, 72, 1593–1597. [Google Scholar]
- Choopun, S.; Hongsith, N.; Mangkorntong, P.; Mangkornthong, N. Zinc oxide nanobelts by RF sputtering for ethanol sensor. Physica E 2007, in press. [Google Scholar]
- Lv, Y.; Guo, L.; Xu, H.; Chu, X. Gas-sensing properties of well-crystalline ZnO nanorods grown by a simple route. Physica E 2007, 36, 102–105. [Google Scholar]
- Zhou, X.; Jiang, T.; Zhang, J.; Wang, X.; Zhu, Z. Humidity sensor based on quartz tuning fork coated with sol-gel-derived nanocrystalline zinc oxide thin film. Sens. Actuators B 2006, 123, 299–305. [Google Scholar]
- Gao, T.; Wang, T.H. Synthesis and properties of multipod-shaped ZnO nanorods for gas-sensor applications. Appl. Phys. A 2005, 80, 1451–1454. [Google Scholar]
- Feng, X.; Li, Z.J.; Wang, P.; Zhou, Y. Preparation and gas-sensitivity of ultra-fine zinc-oxide powders from roasted zinc-blended. J. Mater. Sci. 2005, 40, 6597–6600. [Google Scholar]
- Feng, P.; Wan, Q.; Wang, T.H. Contact-controlled sensing properties of flowerlike ZnO nanostructure. Appl. Phys. Lett. 2005, 87, 213111–213113. [Google Scholar]
- Delaunay, J.J.; Kakoiyama, N.; Yamada, I. Fabrication of three-dimensional network of ZnO tetrapods and its response to ethanol. Mater. Chem. Phys. 2007, in press. [Google Scholar]
- Stambolova, I.; Konstantinov, K.; Vassilev, S.; Peshev, P.; Tsacheva, Ts. Lanthanum doped SnO2 and ZnO thin films sensitive to ethanol and humidity. Mater. Chem. Phys. 2000, 63, 104–108. [Google Scholar]
- Rout, C.S.; Krishna, S.H.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. Hydrogen and ethanol sensors based on ZnO nanorods, nanowires and nanotubes. Chem. Phys. Lett. 2005, 418, 582–586. [Google Scholar]
- Paraguay, F.D.; Yoshida, M.M.; Morales, J.; Solis, J.; Estrada, W.L. Influence of Al, In, Cu, Fe and Sn dopants on the response of thin films ZnO gas sensor to ethanol vapour. Thin Solid Films 2000, 373, 137–140. [Google Scholar]
- Raju, A.R.; Rao, C.N.R. Gas-sensing characteristics of ZnO and copper-impregnated ZnO. Sens. Actuators B 1991, 3, 305–310. [Google Scholar]
- Baruwati, B.; Kumar, D.K.; Manorama, S.V. Hydrothermal synthesis of highly crystalline ZnO nanoparticles: A competitive sensor for LPG and EtOH. Sens. Actuators B 2006, 119, 676–682. [Google Scholar]
- Rao, B.B. Zinc oxide ceramic semi-conductor gas sensor for ethanol vapour. Mater. Chem. Phys. 2000, 64, 62–65. [Google Scholar]
- Xu, J.; Chen, Y.; Chen, D.; Shen, J. Hydrothermal synthesis and gas sensing characters of ZnO nanorods. Sens. Actuators B 2006, 113, 526–531. [Google Scholar]
- Zhu, B.L.; Zeng, D.W.; Wu, J.; Song, W.L.; Xie, C.S. Synthesis and gas sensitivity of In-doped ZnO nanoparticles. J. Mater. Sci. - Mater. Electron. 2003, 14, 521–526. [Google Scholar]
- Chou, S.M.; Teoh, L.G.; Lai, W.H.; Su, Y.H.; Hon, M.H. ZnO:Al thin film gas sensor for detection of ethanol vapor. Sensor 2006, 6, 1420–1427. [Google Scholar]
- Ge, C.; Xie, C.; Cai, S. Preparation and gas-sensing properties of Ce-doped ZnO thin film sensors by dip-coating. Mater. Sci. Eng. B 2007, 137, 53–58. [Google Scholar]
- Hsueh, T.J.; Hsu, C.L.; Chang, S.J.; Chen, I.C. Laterally grown ZnO nanowire ethanol gas sensors. Sens. Actuators B 2007, in press. [Google Scholar]
- Wegner, K.; Stark, W.J.; Pratsinis, S.E. Flame-nozzle synthesis of nanoparticles with closely controlled size, morphology and crystallinity. Mater. Lett. 2002, 55, 318–321. [Google Scholar]
- Liewhiran, C.; Seraphin, S.; Phanichphant, S. Synthesis of nano-sized ZnO powders by thermal decomposition of zinc acetate using Broussonetia papyrifera (L.) Vent pulp as a dispersant. Curr. Appl. Phys. 2006, 6, 499–502. [Google Scholar]
- Liewhiran, C.; Phanichphant, S. Influence of thickness on ethanol sensing characteristics of doctor-bladed thick film from flame-made ZnO nanoparticles. Sensors 2007, 7, 185–201. [Google Scholar]
- Liewhiran, C.; Phanichphant, S. Improvement of flame-made nanoparticulate thick film morphology for ethanol sensing. Sensors 2007, 7, 650–675. [Google Scholar]
- Purica, M.; Budianu, E.; Rusu, E.; Danila, M.; Gavrila, R. Optical and structural investigation of ZnO thin films prepared by chemical vapor deposition (CVD). Thin Solid Films 2002, 403-404, 485–488. [Google Scholar]
- Dayan, N.J.; Karekar, R.N.; Aiyer, R.C.; Sainkar, S.R. Effect of film thickness and curing temperature on the sensitivity of ZnO:Sb thick-film hydrogen sensor. J. Mater. Sci. - Mater. Electron. 1997, 8, 277–279. [Google Scholar]
- Zheng, L.; Xu, T.; Li, G.; Yin, Q. Influence of thickness on oxygen-sensing properties of TiO2 thin films on Al2O3. Jpn. J. Appl. Phys., Part 1 2002, 41, 4655–4658. [Google Scholar]
- Lee, Y.L.; Sheu, C.Y.; Hsiao, R.H. Gas sensing characteristics of copper phthalocyanine films; effects of film thickness and sensing temperature. Sens. Actuators B 2004, 99, 281–287. [Google Scholar]
- Hossein-Babari, F.; Orvatinia, M. Analysis of thickness dependence of the sensitivity in thin film resistive gas sensors. Sens. Actuators B 2003, 89, 256–261. [Google Scholar]
- Geistlinger, H. Electron theory of thin-film gas sensors. Sens. Actuators B 1993, 17, 47–60. [Google Scholar]
- López, M.A.R.; Peiteado, M.; Fernández, J.F.; Caballero, A.C.; Holc, J.; Drnovsek, S.; Kuscer, D.; Macek, S.; Kosec, M. Thick film ZnO based varistors prepared by screen printing. J. Eur. Ceram. Soc. 2006, 26, 2985–2989. [Google Scholar]
- Chang, J.F.; Kuo, H.H.; Leu, I.C.; Hon, M.H. The effects of thickness and operation temperature on ZnO:Al thin film CO gas sensor. Sens. Actuators B 2002, 84, 258–264. [Google Scholar]
- Christoulakis, S.; Suchea, M.; Koudoumas, E.; Katharakis, M.; Katsarakis, N.; Kiriakidis, G. Thickness influence on surface morphology and ozone sensing properties of nanostructured ZnO transparent thin films grown by PLD. Appl. Surf. Sci. 2006, 252, 5351–5354. [Google Scholar]
- Sakai, G.; Baik, N.S.; Miura, N.; Yamazoe, N. Gas sensing properties of tin oxide thin films fabricated from hydrothermally treated nanoparticles dependence of CO and H2 response on film thickness. Sens. Actuators B 2001, 77, 116–121. [Google Scholar]
- Pratsinis, S.E. Flame aerosol synthesis of ceramic powders. Prog. Energy Comust. Sci. 1998, 24, 197–219. [Google Scholar]
- Tani, T.; Mädler, L.; Pratsinis, S.E. Homogeneous ZnO nanoparticles by flame spray pyrolysis. J. Nanopart. Res. 2002, 4, 337–343. [Google Scholar]
- Mädler, L.; Kammler, H.K.; Mueller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aeros. Sci. 2002, 33, 369–389. [Google Scholar]
- Mueller, R.; Mädler, L.; Pratsinis, S.E. Nanoparticle synthesis at high production rates by flame spray pyrolysis. Chem. Eng. Sci. 2003, 58, 1969–1976. [Google Scholar]
- Mädler, L. Liquid-fed aerosol reactors for one-step synthesis of nano-structured particles. KONA 2004, 22, 107–120. [Google Scholar]
- Kammler, H.K.; Mädler, L.; Pratsinis, S.E. Flame synthesis of nanoparticles. Chem. Eng. Tech. 2001, 24, 583–596. [Google Scholar]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Rapid synthesis of stable ZnO quantum dots. J. Appl. Phys. 2002, 92, 6537–6540. [Google Scholar]
- Height, M.J.; Mädler, L.; Krumeich, F.; Pratsinis, S.E. Nanorods of ZnO made by flame spray pyrolysis. Chem. Mater. 2006, 18, 572–578. [Google Scholar]
- Height, M.J.; Pratsinis, S.E.; Mekasuwandumrong, O.; Praserthdam, P. Ag-ZnO catalysts for UV-photodegradation of methylene blue. Appl. Catal., B 2005, 63, 305–312. [Google Scholar]
- Kaelin, M.; Zogg, H.; Tiwari, A.N.; Wilhelm, O.; Pratsinis, S.E.; Meyer, T.; Meyer, A. Electrosprayed and selenized Cu/In metal particles films. Thin Solid Films 2004, 457, 391–396. [Google Scholar]
- Kaelin, M.; Rudmann, D.; Tiwari, A.N. Low cost processing of CIGS thin film solar cells. Sol. Energy. 2004, 77, 749–756. [Google Scholar]
- Wagh, M.S.; Patil, L.A.; Seth, T.; Amalnerkar, D.P. Surface cupricated SnO2-ZnO thick films as a H2S gas sensor. Mater. Chem. Phys. 2004, 84, 228–233. [Google Scholar]
- Strobel, R.; Grunwaldt, J.D.; Camenzind, A.; Pratsinis, S.E.; Baiker, A. Flame-made alumina supported Pd-Pt nanoparticles: structural properties and catalytic behavior in methane combustion. Catal. Lett. 2005, 104, 9–16. [Google Scholar]
- Cheary, R.W.; Coelho, A.A. Axial divergence in a conventional X-ray powder diffractometer. I. Theoretical foundations. J. Appl. Crystallogr. 1998, 31, 851–861. [Google Scholar]
- Brunauer, S.; Emmett, P.; Teller, P.H. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar]
- Moreno-Marín, J.C.; Abril, I.; Heredia-Avalos, S.; Garcia-Molina, R. Electronic energy loss of swift H+ and He+ ions in solids with material science applications. Nucl. Instrum. Meth. Phys. Res. Sect. B 2006, 249, 29–33. [Google Scholar]
- Canton, P.; Fagherazzi, G.; Battagliarin, M.; Menegazzo, F.; Pinna, F.; Pernicone, N. Pd/CO average chemisorption stoichiometry in highly dispersed supported Pd/γ-Al2O3 catalysts. Langmuir 2002, 18, 6530–6535. [Google Scholar]
- Canton, P.; Menegazzo, F.; Polizzi, S.; Pinna, F.; Pernicone, N.; Riello, P.; Fagherazzi, G. Structure and size of poly-domain Pd nanoparticles supported on silica. Catal. Lett. 2003, 88, 141–146. [Google Scholar]
- Liu, Z.; Chung, D.D.L. Burnout of the organic vehicle in an electrically conductive thick-film paste. J. Elec. Mater. 2004, 33, 1316–1325. [Google Scholar]
- Krishnan, B.; Nampoori, V.P.N. Screen printed nanosized ZnO thick film. Bull. Mater. Sci. 2005, 28, 239–242. [Google Scholar]
- Yun, K.H.; Yun, K.Y.; Cha, G.Y.; Lee, B.H.; Kim, J.C.; Lee, D.D.; Huh, J.S. Gas sensing characteristics of ZnO-doped SnO2sensors for simulants of the chemical agents. Mater. Sci. Forum 2005, 486-487, 9–12. [Google Scholar]
- Kim, K.B.; Song, Y.H.; Hwang, C.S.; Chung, C.H.; Lee, J.H.; Choi, I.S.; Park, J.H. Efficient electron emissions from printed carbon nanotubes by surface treatments. J. Vac. Sci. Technol. B 2004, 22, 1331–1334. [Google Scholar]
- Kim, C.J.; Kim, K.B.; Kuk, I.H.; Hong, G.W.; Park, S.D.; Yang, S.W.; Shin, H.S. Fabrication and properties of YBa2Cu3O7-δ-Ag composite superconducting wires by plastic extrusion technique. J. Mater. Sci. 1997, 32, 5233–5242. [Google Scholar]
- Arshaka, K.; Twomey, K.; Egan, D. A ceramic thick film humidity sensor based on MnZn ferrite. Sensors 2002, 2, 50–61. [Google Scholar]
- Kisi, E.H.; Elcombe, M.M. u Parameters for the wurtzite structure of ZnS and ZnO using powder neutron diffraction. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1989, 45, 1867–1870. [Google Scholar]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of the structure of hexagonal ZnO. Acta Crystallogr., Sect. B: Struct. Sci. 1969, 25, 1233–1236. [Google Scholar]
- Liu, R.S.; Shi, W.C.; Cheng, Y.C.; Huang, C.Y. Crystal structures and peculiar magnetic properties of alpha- and gamma-(Al2O3) powders. Mod. Phys. Lett. B 1997, 11, 1169–1174. [Google Scholar]
- Swanson, H.E.; Tatge, E. Standard X-ray diffraction powder patterns. National Bureau of Standards 1953, 359, 1–1. [Google Scholar]Z. Angew. Phys.; 1956; Volume 8, pp. 202–205.
- Cullity, B.D. Structure of polycrystalline aggregates; Elements of X-ray diffraction; Addison-Wesley: Massachusetts, 1978; Volume 2, Chapter 9; p. 284. [Google Scholar]

© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Liewhiran, C.; Phanichphant, S. Effects of Palladium Loading on the Response of Thick Film Flame-made ZnO Gas Sensor for Detection of Ethanol Vapor. Sensors 2007, 7, 1159-1184. https://doi.org/10.3390/s7071159
Liewhiran C, Phanichphant S. Effects of Palladium Loading on the Response of Thick Film Flame-made ZnO Gas Sensor for Detection of Ethanol Vapor. Sensors. 2007; 7(7):1159-1184. https://doi.org/10.3390/s7071159
Chicago/Turabian StyleLiewhiran, Chaikarn, and Sukon Phanichphant. 2007. "Effects of Palladium Loading on the Response of Thick Film Flame-made ZnO Gas Sensor for Detection of Ethanol Vapor" Sensors 7, no. 7: 1159-1184. https://doi.org/10.3390/s7071159



