Comparative Study of Protein Immobilization Properties on Calixarene Monolayers
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
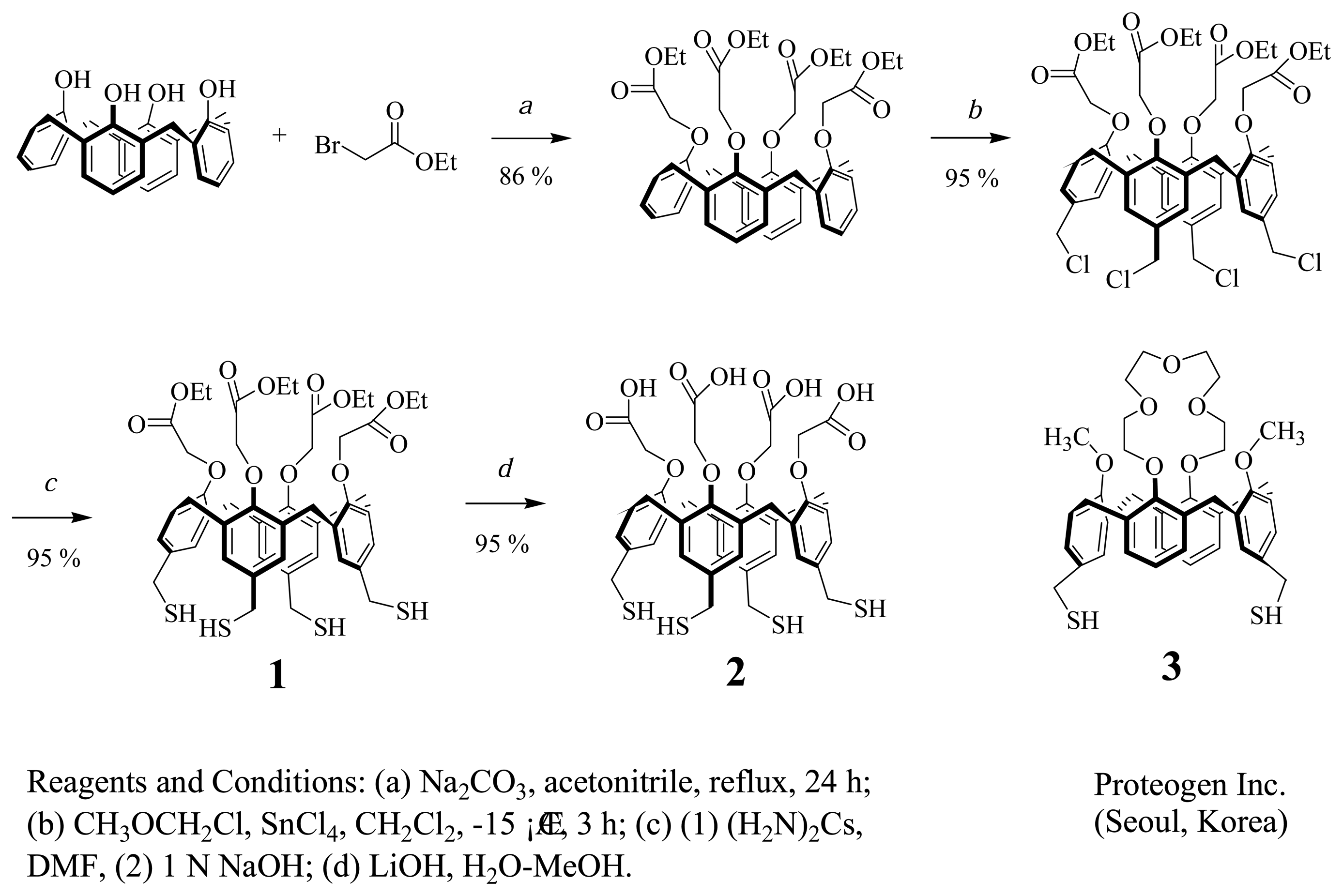
2.2. Synthesis and Characterization of Cal-4 Derivative 1 and 2
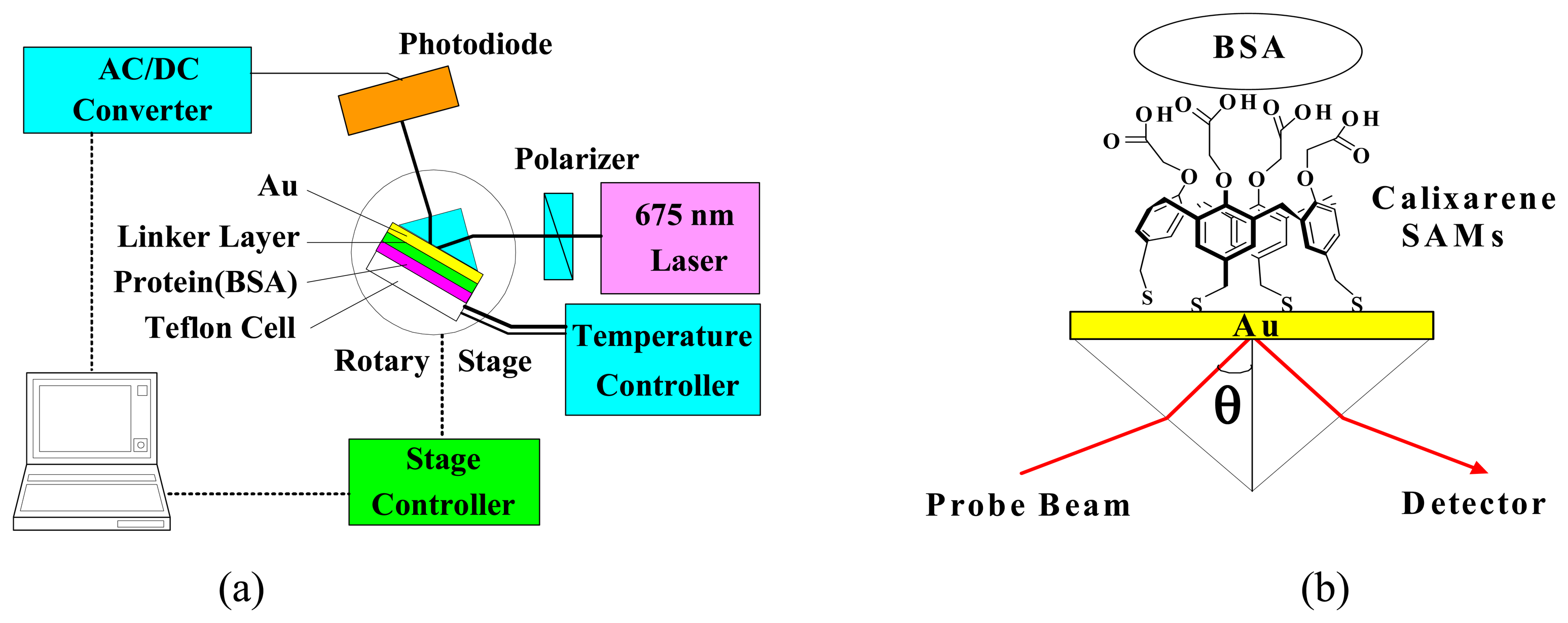
2.3. Formation and Characterization of Cal-4 Derivative SAMs
2.4. SPR Spectroscopic Measurement
2.5. BSA Immobilization and Theoretical Calculation
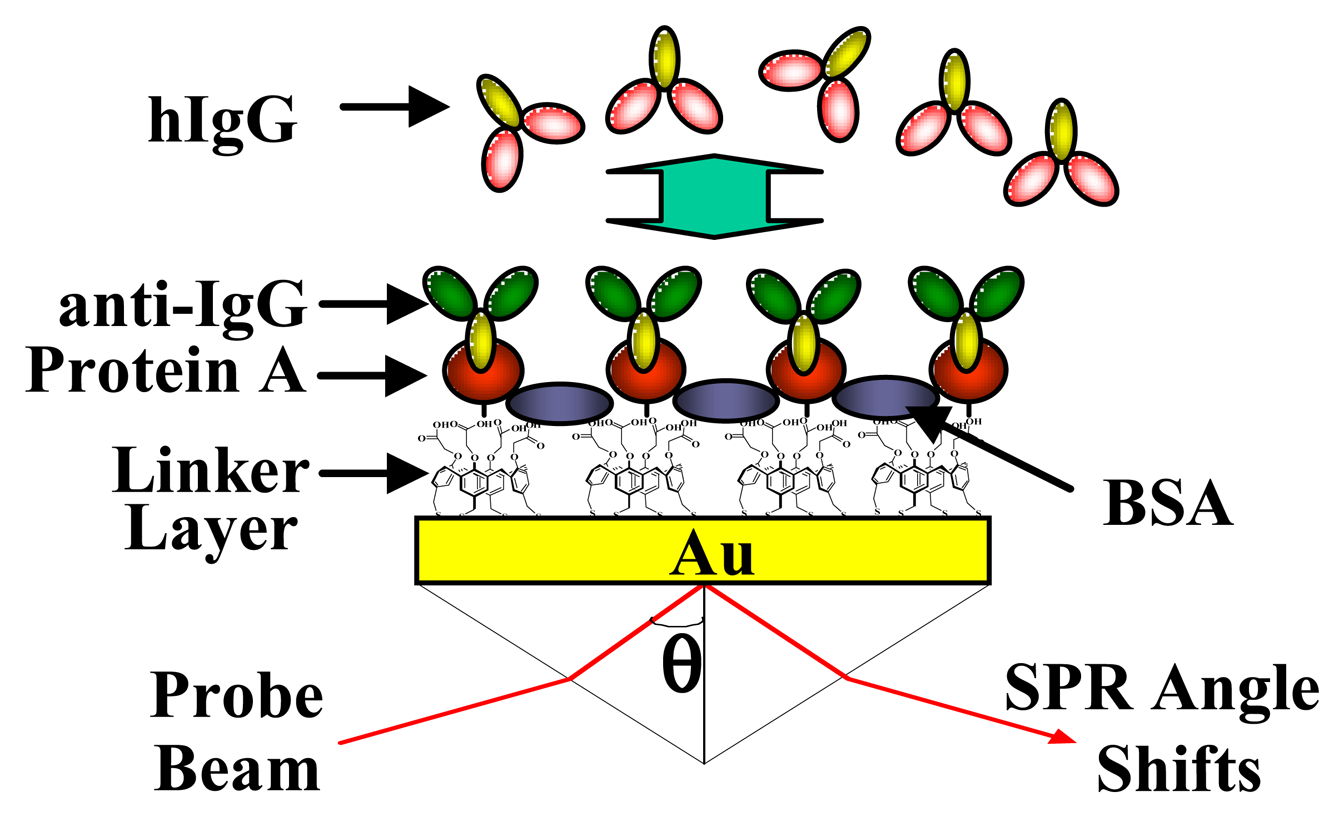
2.6. Application to Biochip
3. Results and Discussion
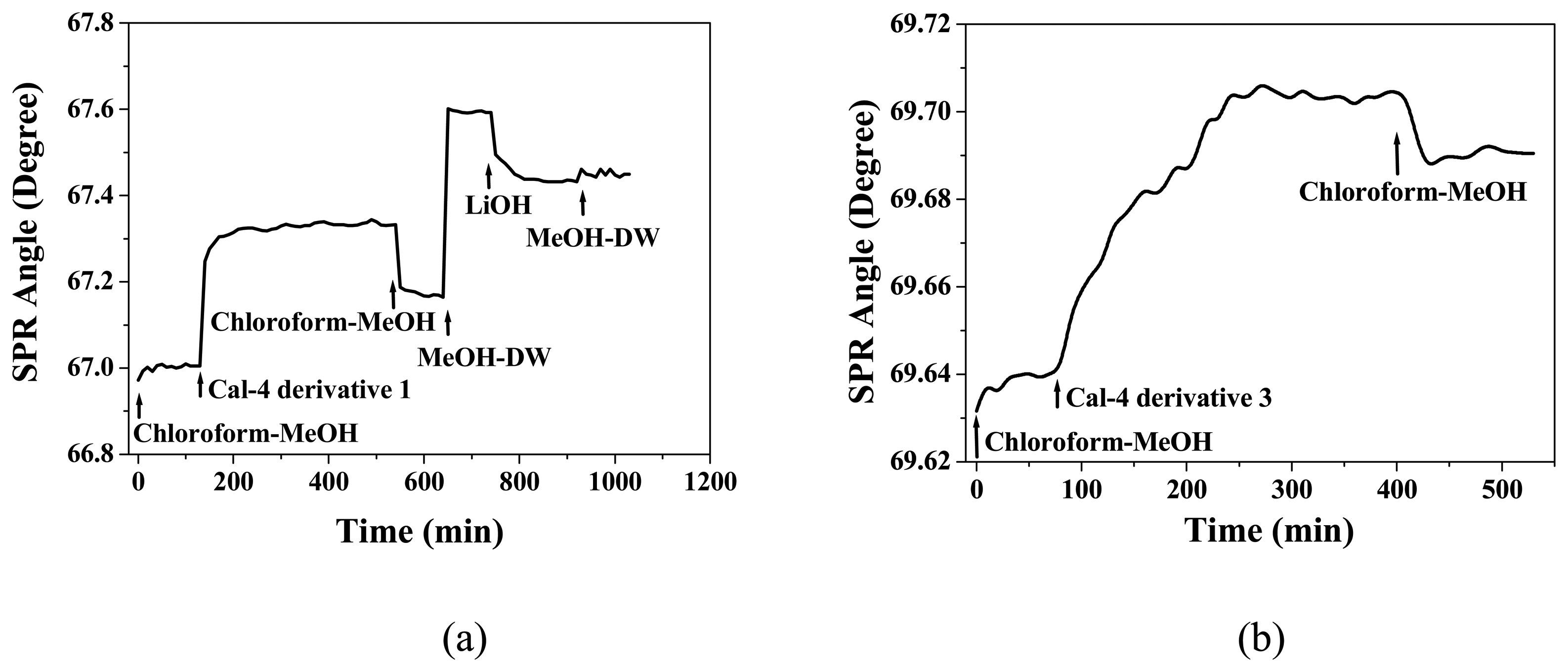
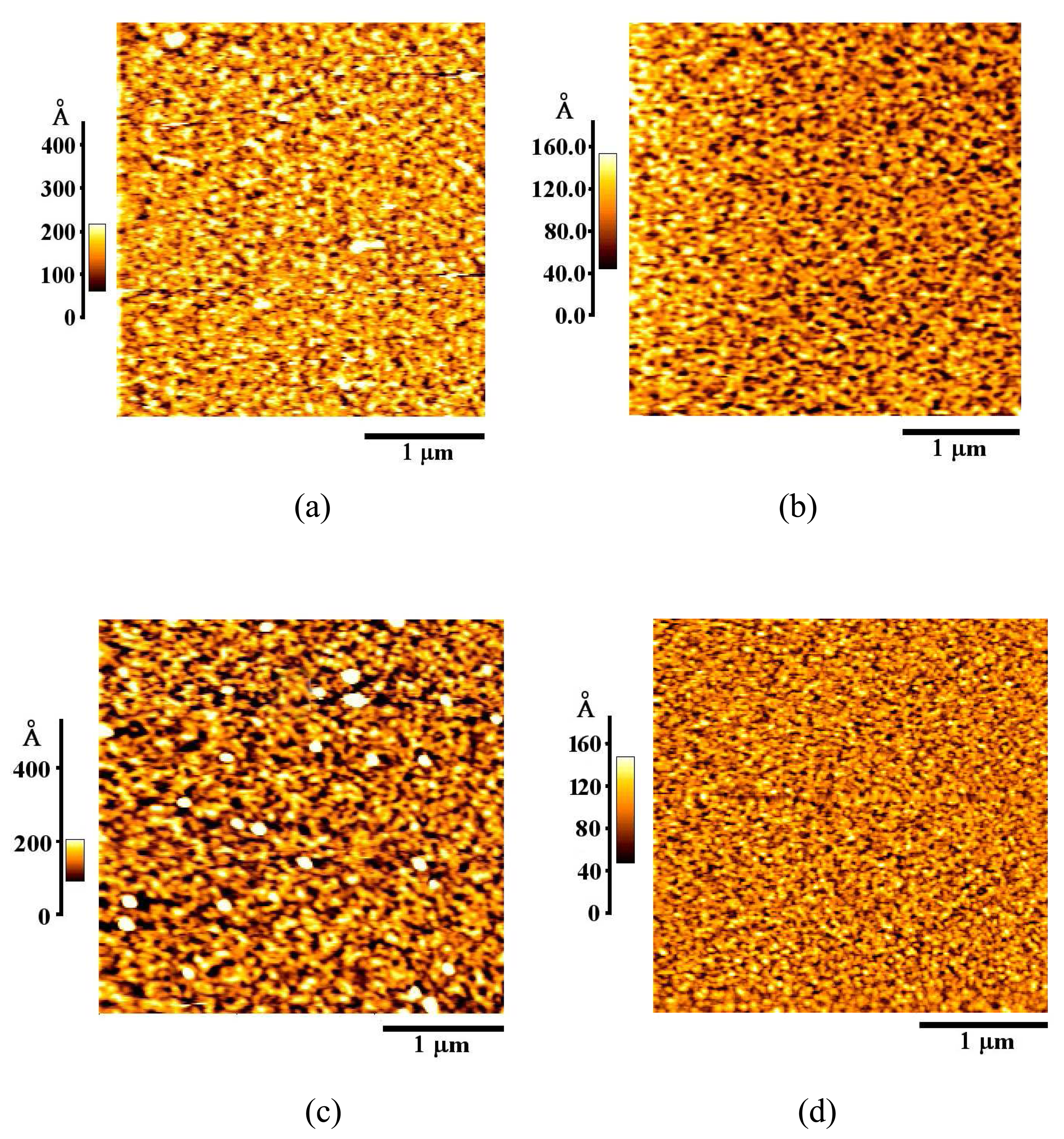
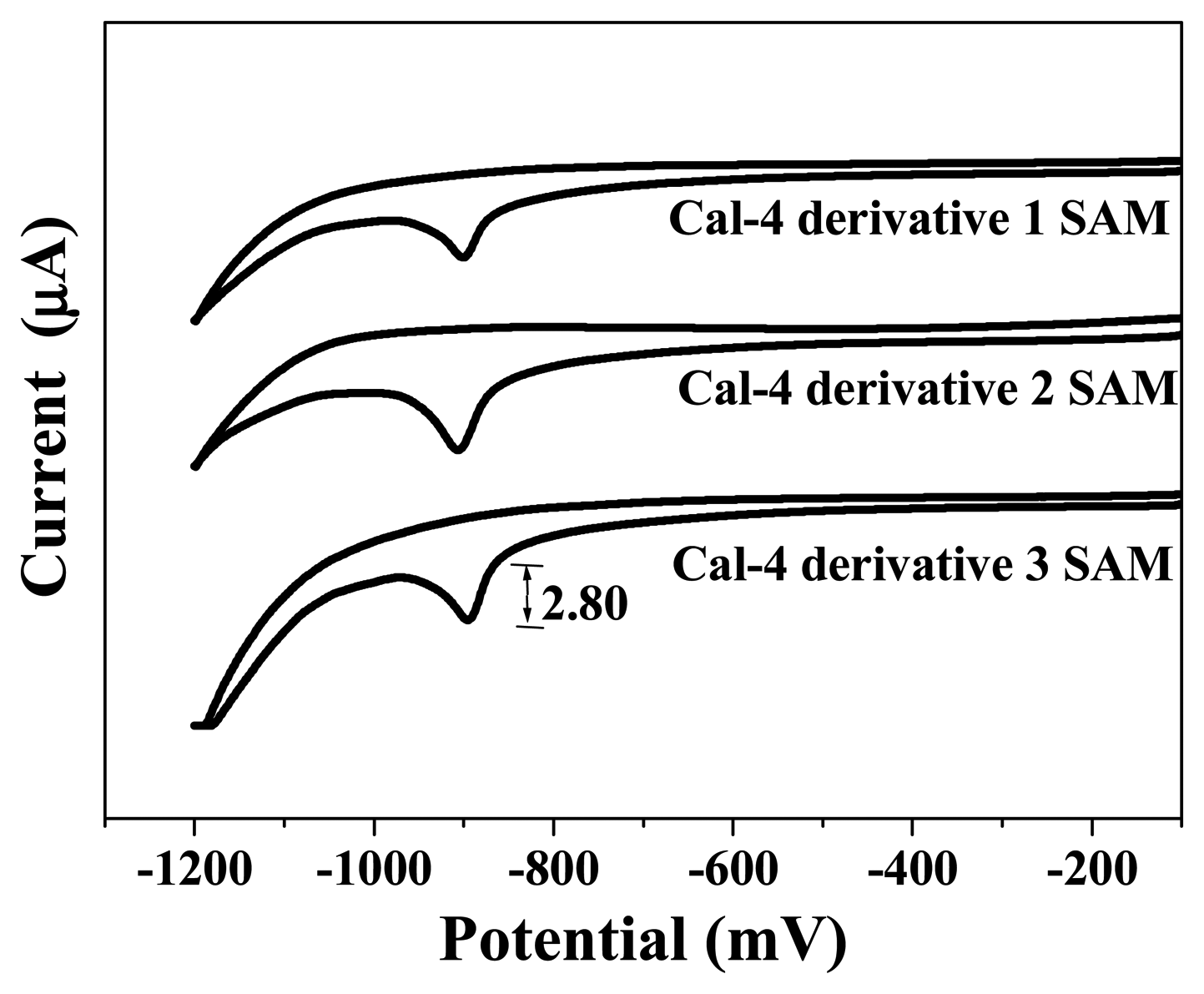
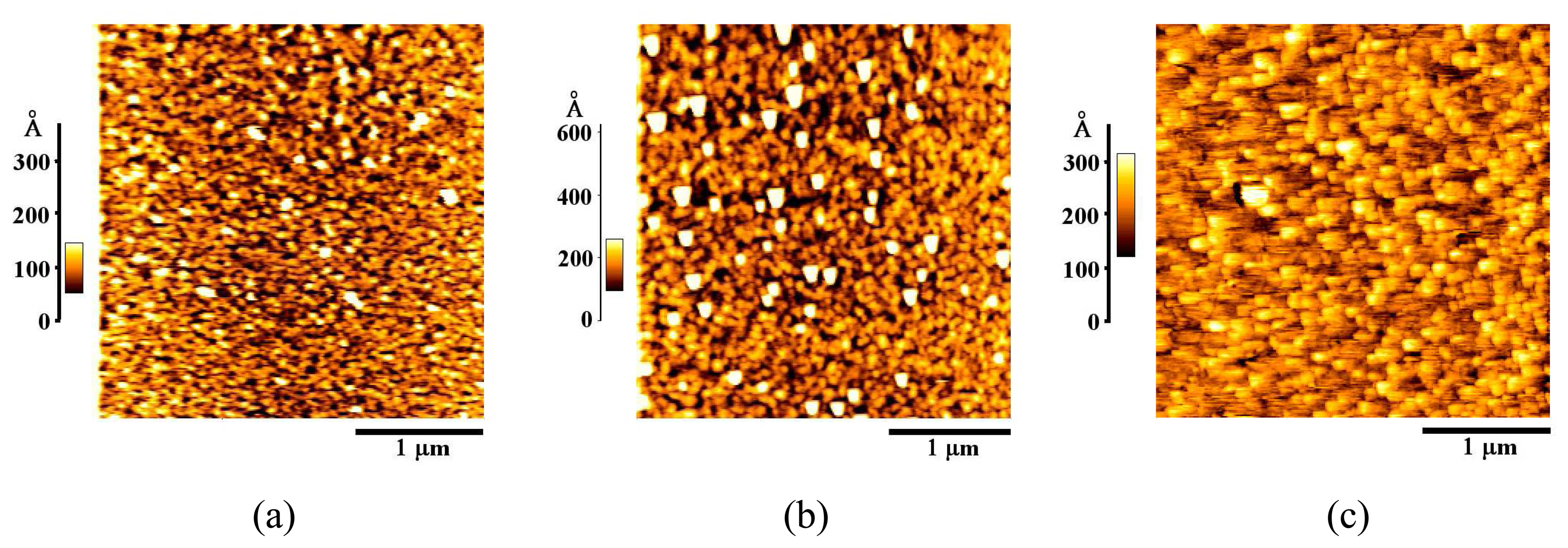
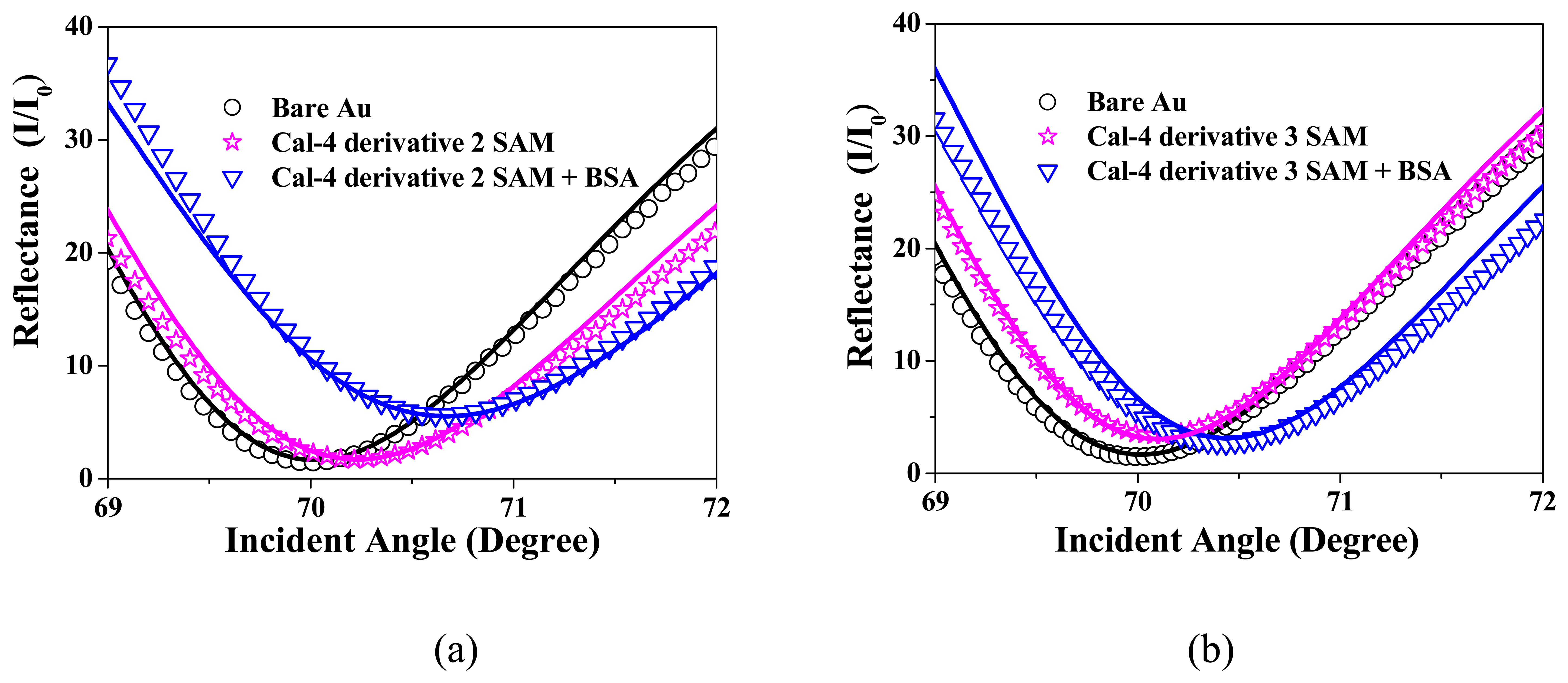
3.1. Formation and Characterization of Cal-4 Derivative SAMs
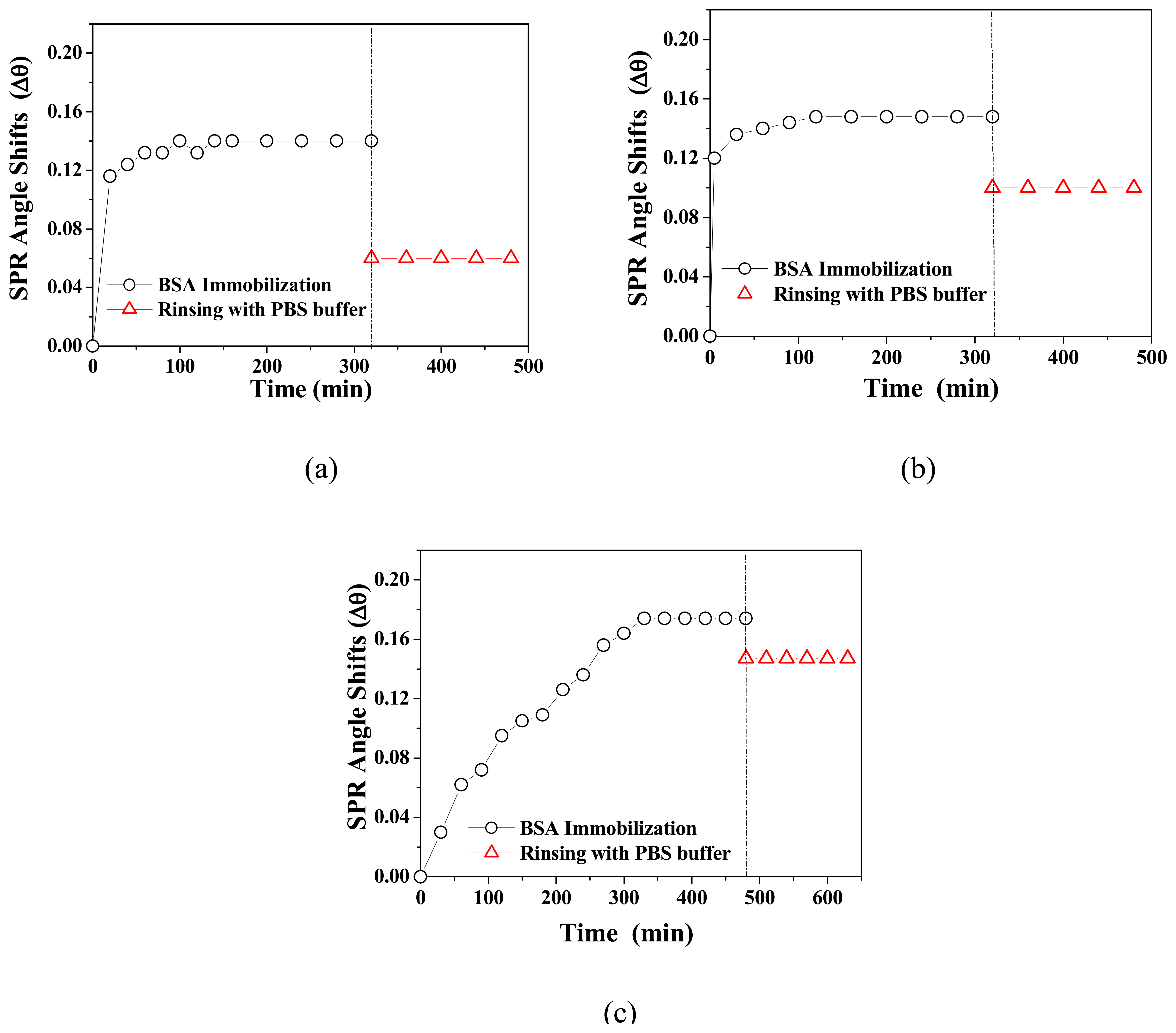
3.2. BSA Immobilization and Theoretical Calculation
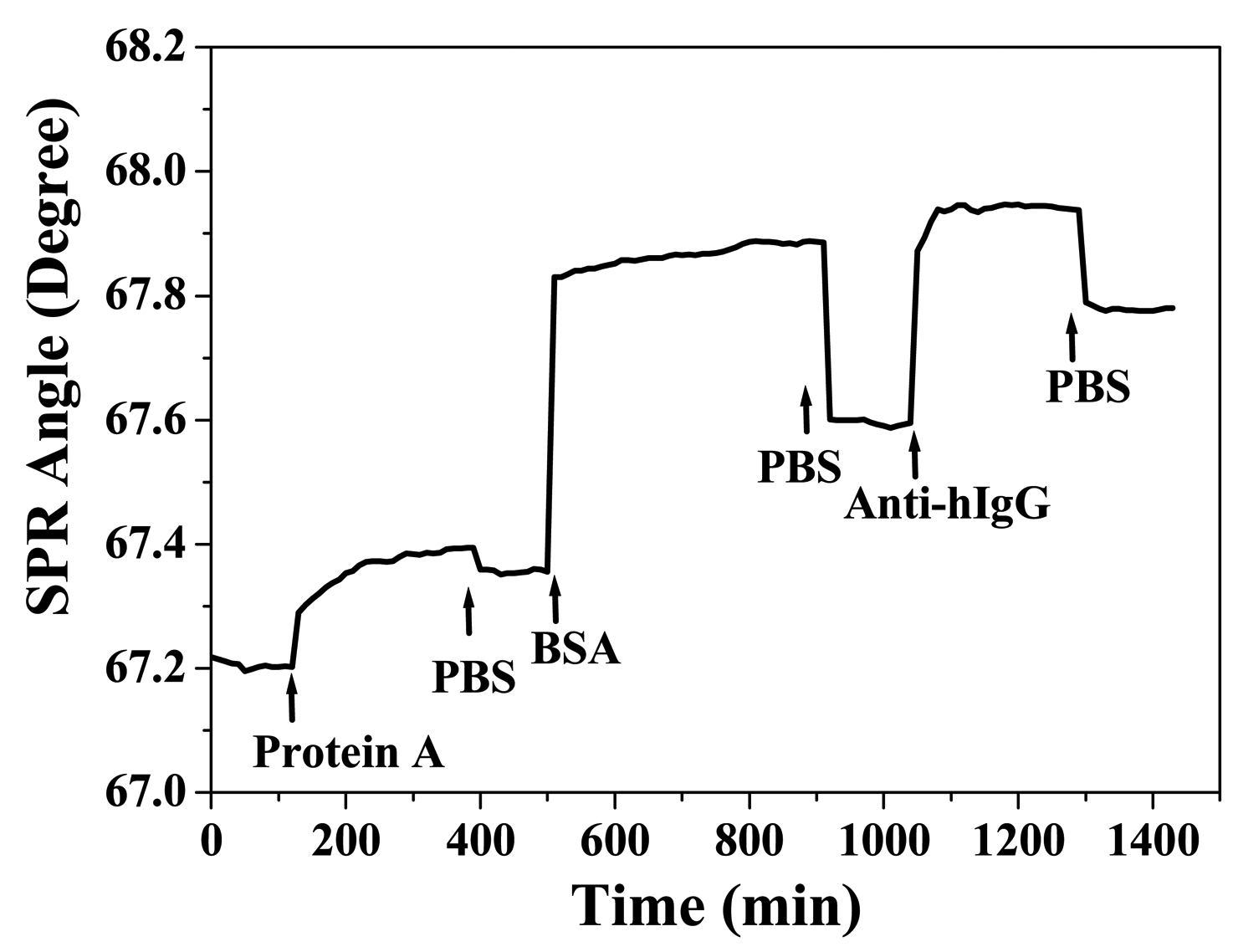
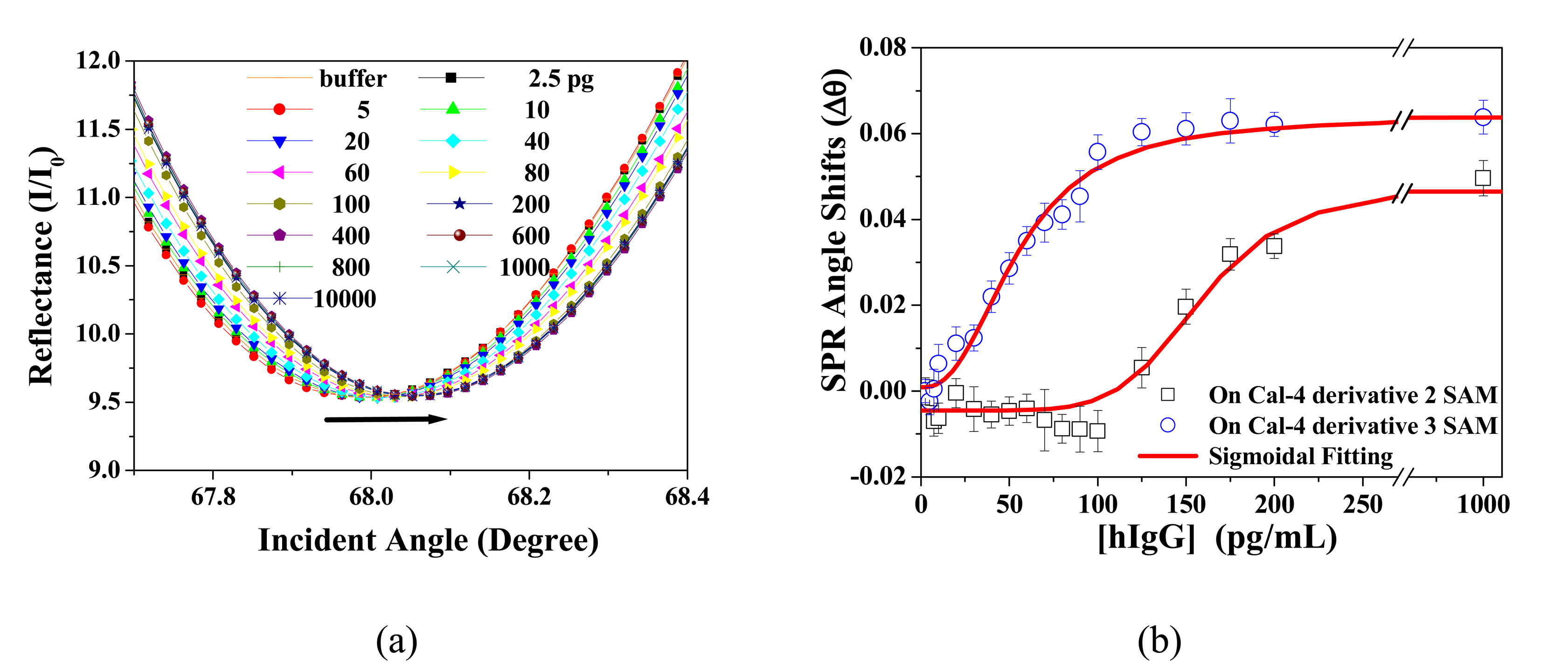
3.3 Application to Biochip
4. Conclusion
Acknowledgments
References and Notes
- Vitalii, S.; Howard, W.; David, J. V. SPR Studies of the Nonspecific Adsorption Kinetics of Human IgG and BSA on Gold Surfaces Modified by Self-Assembled Monolayers (SAMs). J. Colloid Interface Sci. 1997, 185, 94–103. [Google Scholar]
- Ji, H. F.; Brown, G. M.; Dabestani, R. Calix[4]arene-based Cs+ selective optical sensor. Chem. Commun. 1999, 7, 609–610. [Google Scholar]
- Bohmer, V. Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew Chemie 1995, 107, 785–818. [Google Scholar]
- Koh, K.; Araki, K.; Shinkai, S.; Asfari, Z.; Vicens, J. Cation Binding Properties of a Novel 1,3-Alternate Calix[4]biscrown. Formation of 1:1 and 1:2 Complexes and Unique Cation Tunneling across a Calix[4]arene Cavity. Tetrahedron Letters 1995, 36, 6095–6098. [Google Scholar]
- Lo, H. S.; Yip, S. K.; Wong, K. M. C.; Zhu, N.; Yam, V. W. W. Selective Luminescence Chemosensing of Potassium Ions Based on a Novel Platinum (II) Alkynylcalix[4]crown-5 Complex. Organometallics 2006, 25, 3537–3540. [Google Scholar]
- Doutea-guevel, N.; Coleman, A. W.; Morel, J. P.; Morel-Desrosiers, N. Complexation of the basic amino acids lysine and arginine by three sulfonatocalix[n]arenes (n = 4, 6 and 8) in water: microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation. J. Chem. Soc., Perkin Trans. II 1999, 3, 629–634. [Google Scholar]
- Grote Gansey, M. H. B.; De Haan, A. S.; Bos, E. S.; Verboom, W.; Reinhoudt, D. N. Conjugation, Immunoreactivity, and Immunogenicity of Calix[4]arenes; Model Study to Potential Calix[4]arene-Based Ac3+ Chelators. Bioconjug. Chem. 1999, 10, 613–623. [Google Scholar]
- Yilmaz, A.; Memon, S.; Yilmaz, M. Synthesis and study of allosteric effects on extraction behavior of novel calixarene-based dichromate anion receptors. Tetrahedron 2002, 58, 7735–7740. [Google Scholar]
- Memon, S.; Yilmaz, A.; Roundhill, D. M.; Yilmaz, M. Synthesis of Polymeric Calix[4]arene Dinitrile and Diamino-Derivatives: Exploration of Their Extraction Properties Towards Dichromate Anion. J. Macromolecular Sci., Part A. Pure and Applied Chemistry 2004, 41, 433–447. [Google Scholar]
- Tabakci, M.; Tabakci, B.; Yilmaz, M. Design and Synthesis of New Chiral Calix[4]arenes as Liquid Phase Extraction Agents for alfa-Amino Acid Methylesters and Chiral alfa-Amines. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 51–56. [Google Scholar]
- Esplandiú, M. J.; Hagenström, H.; Kolb, D. M. Functionalized Self-Assembled Alkanethiol Monolayers on Au(111) Electrodes: 1. Surface Structure and Electrochemistry. Langmuir 2001, 17, 828–838. [Google Scholar]
- O'Shannessy, D. J.; Brigham-Burke, M.; Peck, P. Immobilization chemistries suitable for use in the BIAcore surface plasmon resonance detector. Anal. Biochem. 1992, 205, 132–136. [Google Scholar]
- Nelson, R. W.; Krone, J. R. Advances in surface plasmon resonance biomolecular interaction analysis mass spectrometry (BIA/MS). J. Mol. Recognit. 1999, 12, 77–93. [Google Scholar]
- Gomes, P.; Giralt, E.; Andreu, D. Direct single-step surface plasmon resonance analysis of interactions between small peptide analytes and immobilised monoclonal antibodies. J. Immunol. Methods 2000, 325, 101–111. [Google Scholar]
- Corsel, J. W.; Willems, G. M.; Kop, J. M. M.; Cuypers, P. A.; Hermens, W. T. The role of intrinsic binding rate and transport rate in the adsorption of prothrombin, albumin and fibrinogen to phospholipid bilayers. J. Colloid Interface Sci. 1986, 111, 544–554. [Google Scholar]
- Iki, N.; Narumi, F.; Fujimoto, T.; Morohashi, N.; Miyano, S. Selective synthesis of three conformational isomers of tetrakis[(ethoxycarbonyl)methoxy]thiacalix[4]arene and their complexation properties towards alkali metal ions. J. Chem. Soc. Perkin Trans. 1998, 2, 2745–2750. [Google Scholar]
- 1H NMR (400 MHz, DMF-d6) δ6.72 (s, 8 H), 4.75 (overlapped with NH2), 4.67 (s, 8 H), 4.16 (s, 8 H), 4.12 (q, J = 6.6 Hz, 8 H), 3.20 (d, J = 11.5 Hz, 4 H), 1.20 (t, J = 6.6 Hz, 12 H); 13C NMR (100 MHz, CDCl3) δ185.9, 172.7, 171.7, 157.7, 136.9, 131.1, 129.8, 73.0, 62.3, 36.8, 32.7, 15.0.
- Kretschmann, E. Determination of optical constants of metals by excitation of surface plasmons. Phys. 1971, 241, 313–324. [Google Scholar]
- Philips, A. V.; Robbins, D. J.; Coleman, M. S.; Barkley, M. D. Immunoaffinity purification and fluorescence studies of human adenosine deaminase. Biochemistry 1987, 26, 2893–2903. [Google Scholar]
- Suri, C. R.; Jain, P. K.; Mishra, G. C. Development of piezoelectric crystal based microgravimetric immunoassay for determination of insulin concentration. J. Biotechnil. 1995, 39, 27–34. [Google Scholar]
- Kanno, S.; Yanagida, Y.; Haruyama, T.; Kobatake, E.; Aizawa, M. Assembling of engineered IgG-binding protein on gold surface for highly oriented antibody immobilization. J. Biotechnol. 2000, 76, 207–214. [Google Scholar]
- Whitesides, G. M.; Laibinis, P. E. Wet chemical approaches to the characterization of organic surfaces: self-assembled monolayers, wetting, and the physical-organic chemistry of the solid-liquid interface. Langmuir 1990, 6, 87–96. [Google Scholar]
- Bryant, M. A.; Pemberton, J. E. Surface Raman scattering of self-assembled monolayers formed from 1-alkanethiols: behavior of films at gold and comparison to films at silver. J. Am. Chem. Soc. 1991, 113, 8284–8293. [Google Scholar]
- Bard, A. J.; Faulkner, L. R. Electrochemical Methods; John Wiley & Sons: New York, 2001; pp. 239–243. [Google Scholar]
- Walczak, M. M.; Popenoe, D. D.; Deinhammer, R. S.; Lamp, B. D.; Chung, C.; Porter, M. D. Reductive desorption of alkanethiolate monolayers at gold: a measure of surface coverage. Langmuir 1991, 7, 2687–2693. [Google Scholar]
- Nonogaki, T.; Xu, S.; Kugimiya, S.; Sato, S.; Miyata, I.; Yonese, M. Two Dimensional Auto-Organized Nanostructure Formation of Hyaluronate on Bovine Serum Albumin Monolayer and Its Surface Tension. Langmuir 2000, 16, 4272–4278. [Google Scholar]
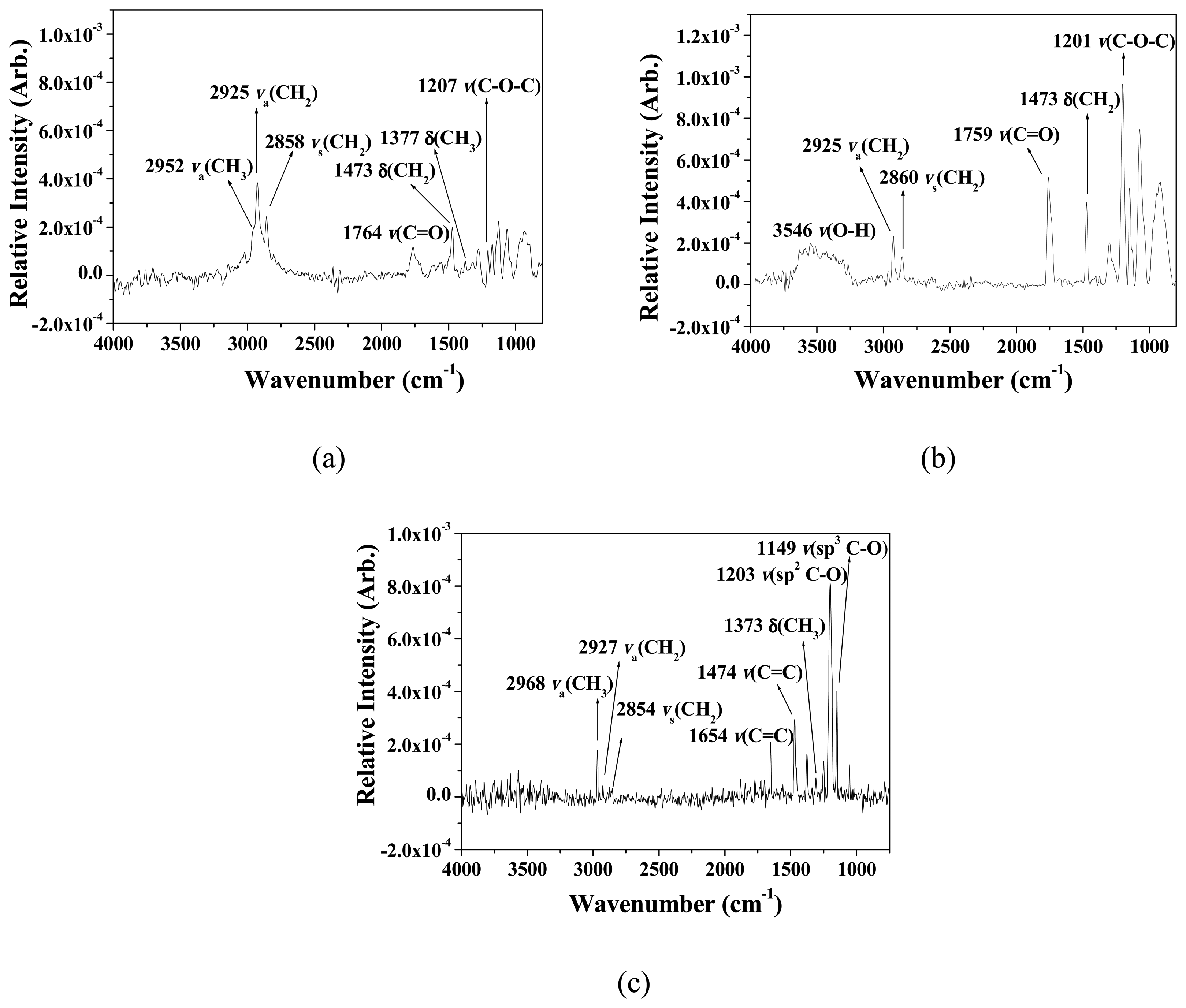
| Stretching Mode | Wavenumber (cm-1) | ||
|---|---|---|---|
| Cal-4 derivative 1 SAM | Cal-4 derivative 2 SAM | Cal-4 derivative 3 SAM | |
| ν(O-H) | 3546 | ||
| νa(CH3) | 2952 | 2968 | |
| νa(CH2) | 2925 | 2925 | 2927 |
| νs(CH2) | 2858 | 2860 | 2854 |
| ν(C=O) | 1764 | 1759 | |
| ν(C=C) | 1473 | 1473 | 1474 |
| δ(CH3) | 1377 | 1373 | |
| ν(sp2C-O) | 1207 | 1201 | 1203 |
| ν(sp3C-O) | 1149 | ||
| Monolayer | RMS Roughness (Å) |
|---|---|
| Bare Au | 20.1 |
| Cal-4 derivative 1 SAM | 35.1 |
| Cal-4 derivative 2 SAM | 39.1 |
| Cal-4 derivative 3 SAM | 18.1 |
| Monolayer | Surface Coverage (Γ) (×10-11 mole/cm2) |
|---|---|
| Cal-4 derivative 1 SAM | 7.076 |
| Cal-4 derivative 2 SAM | 6.647 |
| Cal-4 derivative 3 SAM | 22.092 |
| Monolayer | Bare Au | Cal-4 Linker Layer | BSA layer | Surface concentration (ng/cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | k | d | n | k | d | n | k | d | ||
| Cal-4 derivative 1 SAM | 0.233 | 3.8 | 48 | 1.60 | 0.24 | 1.4 | 1.45 | 0 | 1.3 | 98 |
| Cal-4 derivative 2 SAM | 0.227 | 3.8 | 48 | 1.59 | 0.15 | 1.1 | 1.45 | 0.05 | 2.4 | 163 |
| Cal-4 derivative 3 SAM | 0.221 | 3.8 | 49 | 1.60 | 0.08 | 1.4 | 1.45 | 0 | 2.7 | 184 |
| Proteins | SPR Angle Shift (Δθ) | |
|---|---|---|
| Cal-4 derivative 2 SAM | Cal-4 derivative 3 SAM | |
| protein A (500 μg/mL) | 0.113 | 0.153 |
| BSA (30 mg/mL) | 0.475 | 0.239 |
| anti-hIgG (150 μg/mL) | 0.229 | 0.185 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Chen, H.; Lee, M.; Choi, S.; Kim, J.-H.; Choi, H.-J.; Kim, S.-H.; Lee, J.; Koh, K. Comparative Study of Protein Immobilization Properties on Calixarene Monolayers. Sensors 2007, 7, 1091-1107. https://doi.org/10.3390/s7071091
Chen H, Lee M, Choi S, Kim J-H, Choi H-J, Kim S-H, Lee J, Koh K. Comparative Study of Protein Immobilization Properties on Calixarene Monolayers. Sensors. 2007; 7(7):1091-1107. https://doi.org/10.3390/s7071091
Chicago/Turabian StyleChen, Hongxia, Minsu Lee, Sungwook Choi, Jae-Ho Kim, Heung-Jin Choi, Sung-Hoon Kim, Jeabeom Lee, and Kwangnak Koh. 2007. "Comparative Study of Protein Immobilization Properties on Calixarene Monolayers" Sensors 7, no. 7: 1091-1107. https://doi.org/10.3390/s7071091




