Synthesis, Characterization and Metal Ion Detection of Novel Fluoroionophores Based on Heterocyclic Substituted Alanines
Abstract
:1. Introduction
2. Results and Discussion
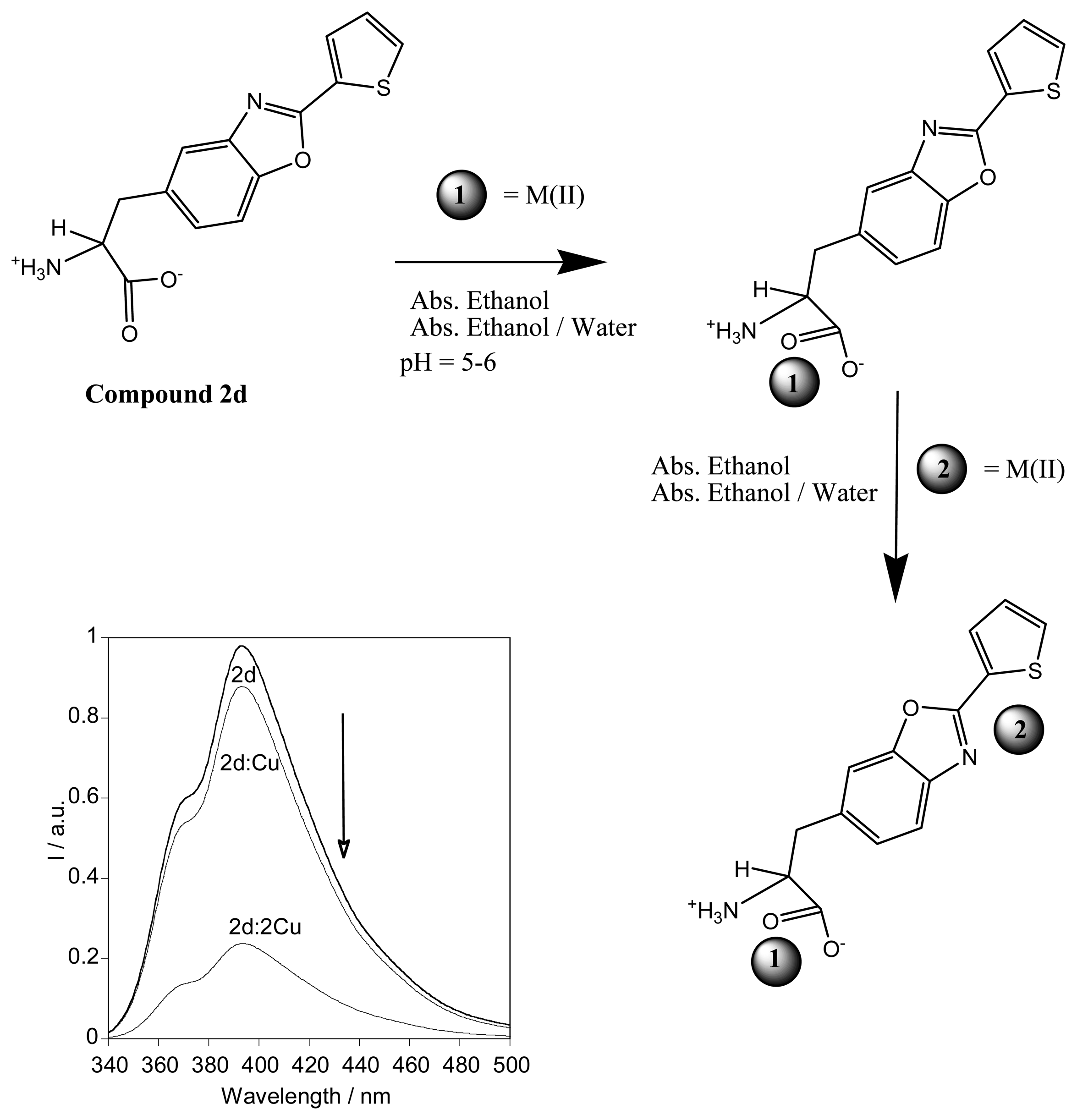
2.1. Synthesis
2.2. Photophysical study
2.3. Spectrofluorimetric titrations and metal sensing effect
2.3.1. Protonation effects
2.3.2. Deprotonation effects
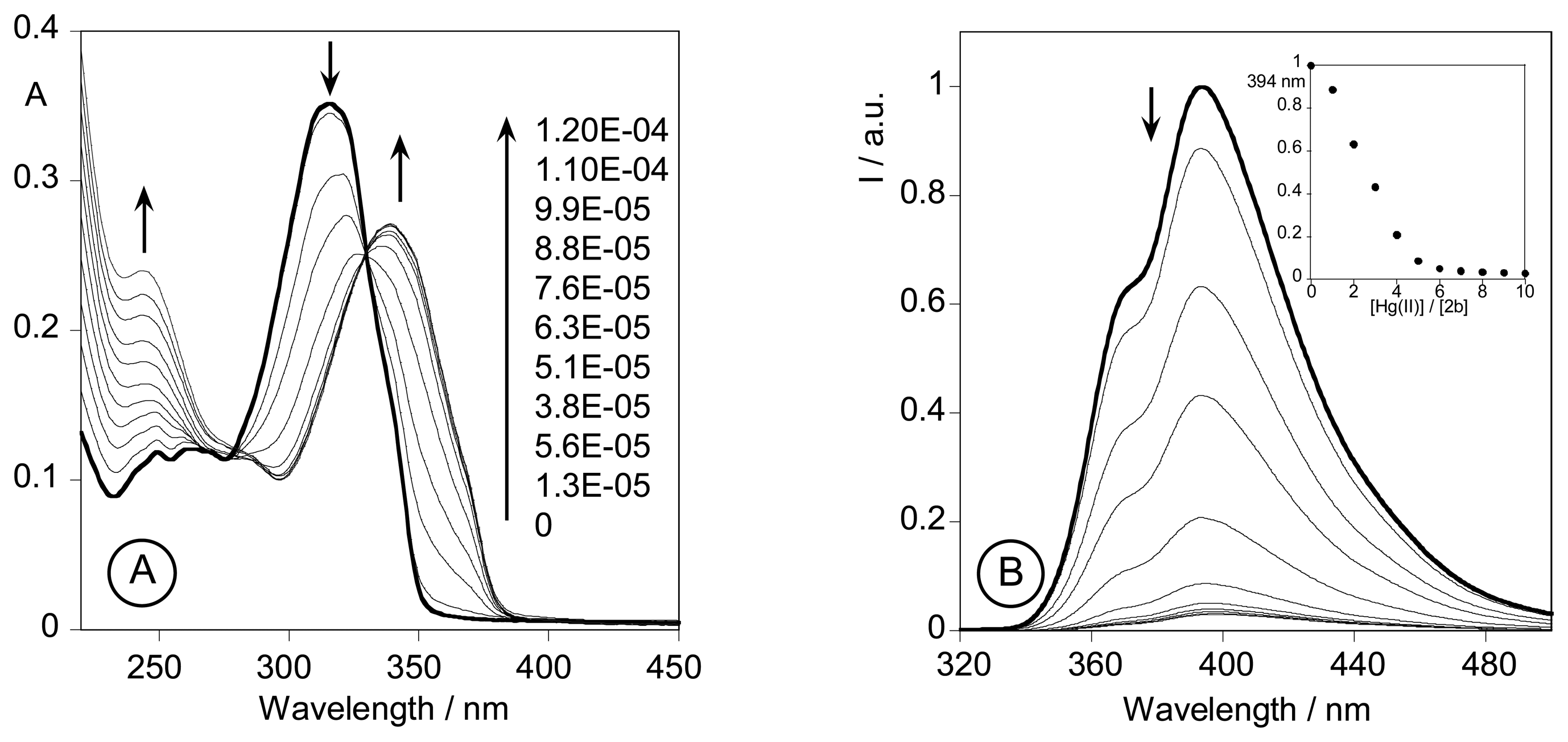
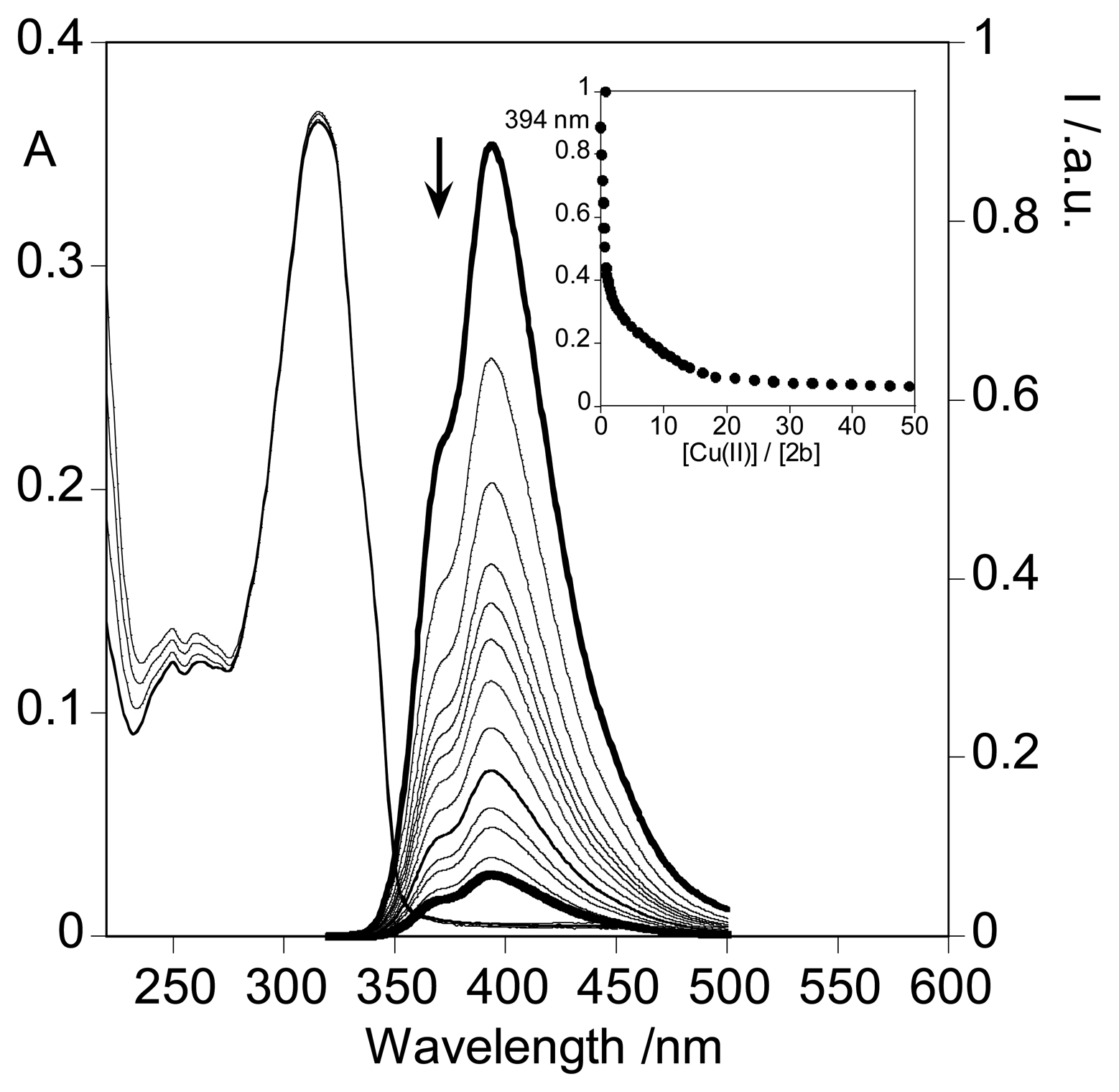
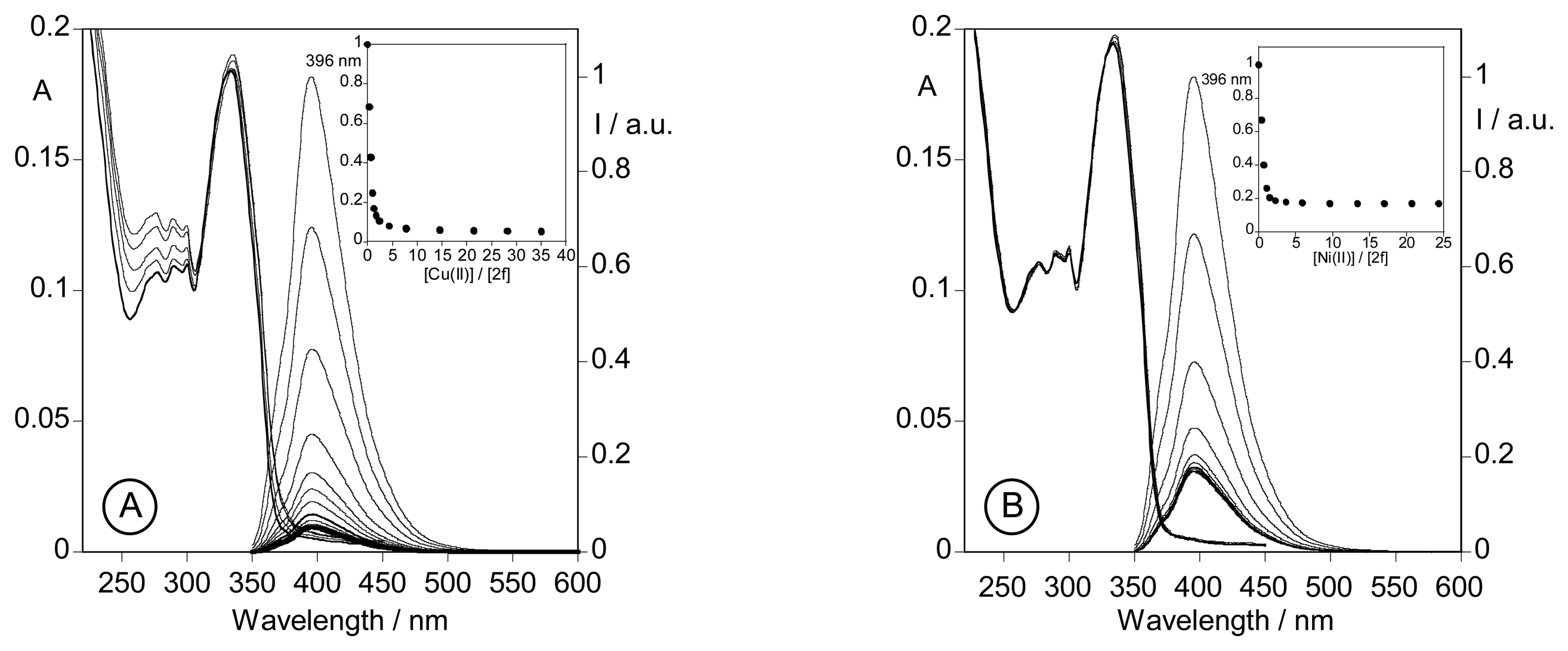
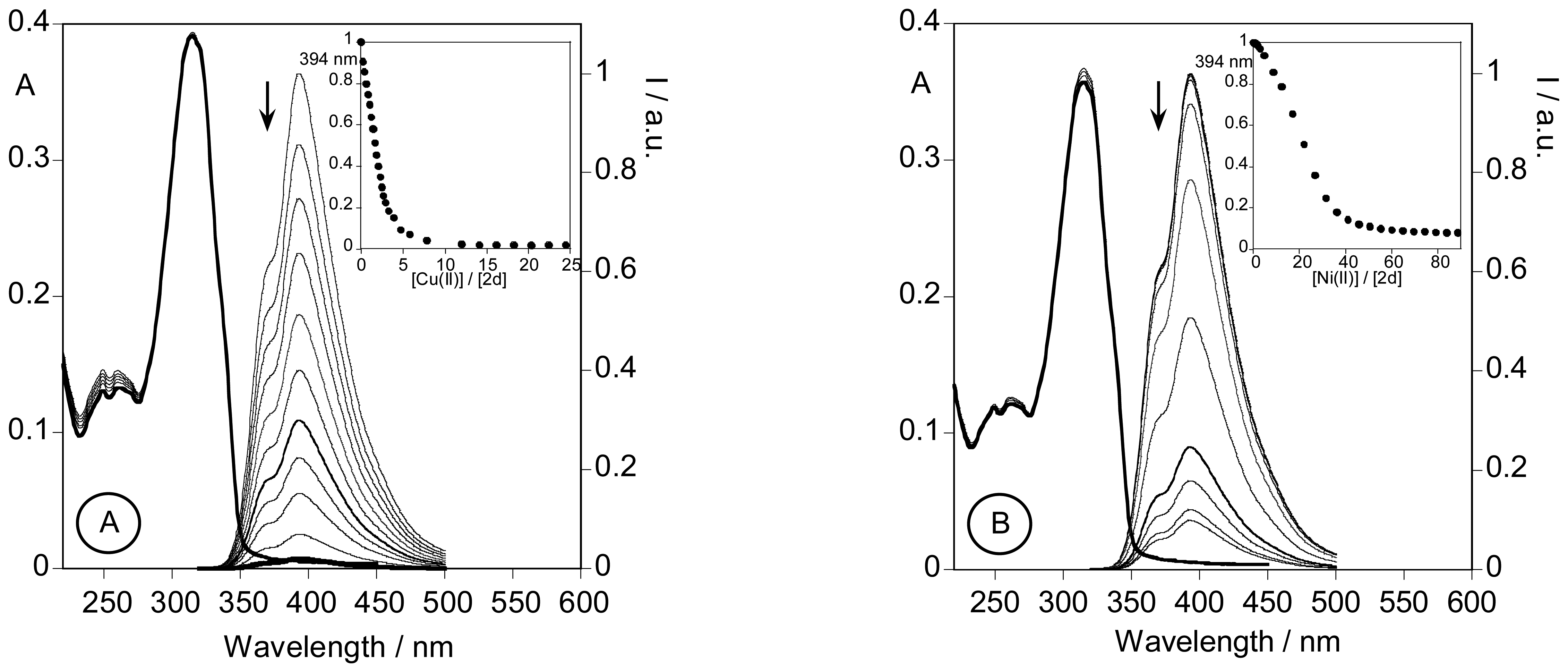
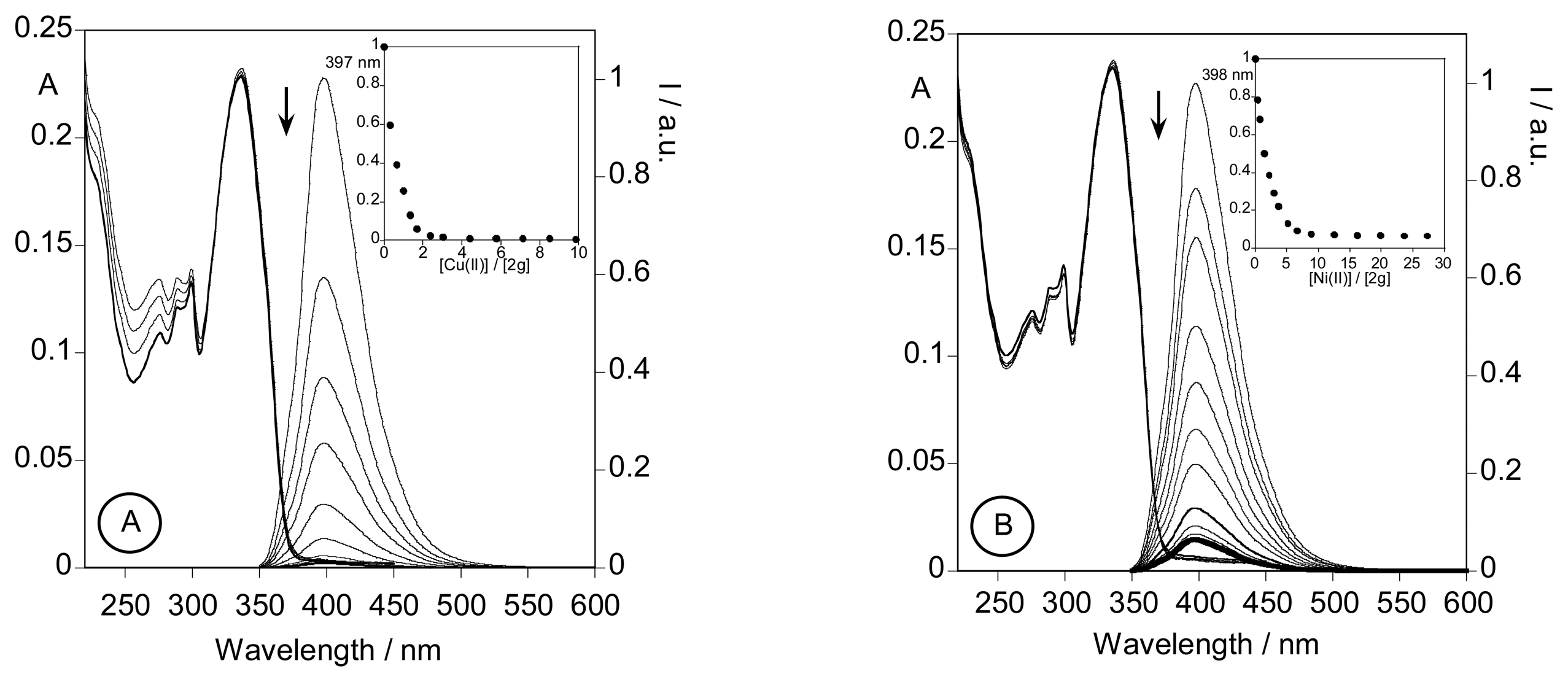
2.3.3. Metal sensing effects
2.4. Conclusions
3. Experimental Section
3.1. Synthesis general
3-Nitro-l-tyrosine methyl ester hydrochloride (1a)
N-tert-Butyloxycarbonyl-3-nitro-l-tyrosine methyl ester (1b)
N-tert-Butyloxycarbonyl-3-amino-l-tyrosine methyl ester (1c)
N-tert-Butyloxycarbonyl-3-[(thien-2′-ylmethylene)amino]-l-tyrosine methyl ester (1d)
N-tert-Butyloxycarbonyl-3-[(2′,4′,5′-trimethoxyphenylmethylene)amino]-l-tyrosine methyl ester (1e)
N-tert-Butyloxycarbonyl [2-(thien-2′-yl)benzoxazol-5-yl]-l-alanine methyl ester (2a)
N-tert-Butyloxycarbonyl [2-(thien-2′-yl)benzoxazol-5-yl]-l-alanine (2b)
[2-(Thien-2′-yl)benzoxazol-5-yl]-l-alanine methyl ester (2c)
[2-(Thien-2′-yl)benzoxazol-5-yl]-l-alanine (2d)
N-tert-Butyloxycarbonyl-3-[2-(2′,4′,5′-trimethoxyphenyl)benzoxazol-5-yl]-l-alanine methyl ester (2e)
N-tert-Butyloxycarbonyl-3-[2-(2′,4′,5′-trimethoxyphenyl)benzoxazol-5-yl]-l-alanine (2f)
3-[2-(2′,4′,5′-Trimethoxyphenyl)benzoxazol-5-yl]-l-alanine (2g)
3.2. Spectrofluorimetric titrations
Acknowledgments
References and Notes
- Rida, S.M.; Ashour, F.A.; El-Hawash, S.A.M.; ElSemary, M.M.; Badr, M.H.; Shalaby, M.A. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 949 a, nd references cited therein.. [Google Scholar]
- Okamura, N.; Suemoto, T.; Shimazu, H.; Suzuki, M.; Shiomitsu, T.; Akatsu, H.; Yamamoto, T.; Staufenbiel, M.; Yanai, K.; Arai, H.; Sasaki, H.; Kudo, Y.; Sawada, T. Styrylbenzoxazole Derivatives for In Vivo Imaging of Amyloid Plaques in the Brain. J. Neurosci. 2004, 24, 2535. [Google Scholar]
- Allagile, D.; Baldwin, R.M.; Tamagnan, G.D. One-step synthesis of 2-arylbenzothiazole (‘BTA’) and -benzoxazole precursors for in vivo imaging of β-amyloid plaques. Tetrahedron Lett. 2005, 46, 1349. [Google Scholar]
- Leopoldo, M.; Lacivita, E.; De Giorgio, P.; Colabufo, N.A.; Niso, M.; Berardi, F.; Perrone, R. Design, Synthesis, and Binding Affinities of Potential Positron Emission Tomography (PET) Ligands for Visualization of Brain Dopamine D3 Receptors. J. Med. Chem. 2006, 49, 358. [Google Scholar]
- Aquilina, J.A.; Carver, J.A.; Truscott, R.J.W. Polypeptide Modification and Cross-Linking by Oxidized 3-Hydroxykynurenine. Biochemistry 2000, 39, 16176. [Google Scholar]
- McGrath, M.E.; Sprengeler, P.A.; Hill, C.M.; Martichonok, V.; Cheung, H.; Somoza, J.R.; Palmer, J.T.; Janc, J.W. Peptide Ketobenzoxazole Inhibitors Bound to Cathepsin K. Biochemistry 2003, 42, 15018. [Google Scholar]
- Lima, P.G.; Caruso, R.R.B.; Alves, S.O.; Pessôa, R.F.; Mendonça-Silva, D.L.; Nunes, R.J.; Nöel, F.; Castro, N.G.; Costa, P.R.R. Stereoselective synthesis and preliminary evaluation of new D-3-heteroarylcarbonylalanines as ligands of the NMDA receptor. Bioorg. Med. Chem. Lett. 2004, 14, 4399. [Google Scholar]
- Cagnoli, R.; Lanzi, M.; Mucci, A.; Parenti, F.; Schenetti, L. Polymerization of cysteine functionalized thiophenes. Polymer 2005, 46, 3588 a, nd references cited therein.. [Google Scholar]
- Wang, Y.; Wang, K.; Liu, W.; Shen, G.; Yu, R. Optical Chemical Sensors for Pharmaceutical Analysis Using 1,4-Bis(1,3-benzoxazol-2-yl)benzene as Sensing Material. Analyst 1997, 122, 69 a, nd references cited therein.. [Google Scholar]
- Taki, M.; Wolford, J.L.; O'Halloran, T.V. Emission Ratiometric Imaging of Intracellular Zinc: Design of a Benzoxazole Fluorescent Sensor and Its Application in Two-Photon Microscopy. J. Am. Chem. Soc. 2003, 126, 712. [Google Scholar]
- Shamsipur, M.; Poursaberi, T.; Karami, A.R.; Hosseini, M.; Momeni, A.; Alizadeh, N.; Yousefi, M.; Ganjali, M.R. Development of a new fluorimetric bulk optode membrane based on 2,5-thiophenylbis(5-tert-butyl-1,3-benzexazole) for nickel(II) ions. Anal. Chim. Acta. 2004, 501, 55. [Google Scholar]
- Ohshima, A.; Momotake, A.; Arai, T. A new fluorescent metal sensor with two binding moieties. Tetrahedron Lett. 2004, 45, 9377. [Google Scholar]
- Lee, J.K.; Na, J.; Kim, T.H.; Kim, Y.-S.; Park, W.H.; Lee, T.S. Synthesis of polyhydroxybenzoxazole-based colorimetric chemosensor for anionic species. Mat. Sci. Eng. C- Bio S. 2004, 24, 261. [Google Scholar]
- Guzow, K.; Milewska, M.; Wróblewski, D.; Gieldón, A.; Wiczk, W. 3-[2-(8-Quinolinyl)benzoxazol-5-yl]alanine derivative - a specific fluorophore for transition and rare-earth metal ion detection. Tetrahedron 2004, 60, 11889. [Google Scholar]
- Milewska, M.; Skwierawska, A.; Guzow, K.; Szmigiel, D.; Wiczk, W. 3-[2-(2-Quinoxalinyl)benzoxazol-5-yl]alanine derivative – A specific fluoroionophore for Ni(II). Inorg. Chem. Commun. 2005, 8, 947. [Google Scholar]
- Ohshima, A.; Momotake, A.; Arai, T. Photochemical and complexation studies for new fluorescent and colored chelator. Sci. Tech. Adv. Mat. 2005, 6, 633. [Google Scholar]
- Zang, X.-B.; Peng, J.; He, C.-L.; Shen, G.-L.; Yu, R.-Q. A highly selective fluorescent sensor for Cu2+ based on 2-(2′-hydroxyphenyl)benzoxazole in a poly(vinyl chloride) matrix. Anal. Chim. Acta. 2006, 567, 189. [Google Scholar]
- Batista, R.M. F.; Costa, S.P.G.; Raposo, M.M.M. Synthesis of new fluorescent 2-(2′,2′-bithienyl)-1,3-benzothiazoles. Tetrahedron Lett. 2004, 45(13), 2825. [Google Scholar]
- Costa, S.P.G.; Batista, R.M.F.; Cardoso, P.; Belsley, M.; Raposo, M.M.M. 2-Arylthienyl-substituted 1,3-benzothiazoles as new nonlinear optical chromophores. Eur. J. Org. Chem. 2006, 17, 3938. [Google Scholar]
- Costa, S. P. G.; Batista, R. M. F.; Sousa, A. M. R. C.; Raposo, M. M. M. New fluorescent heterocyclic materials: synthesis, solvatochromic and fluorescence properties. Mater. Sci. Forum 2006, 514-516, 147. [Google Scholar]
- Batista, R.M. F.; Costa, S.P.G.; Malheiro, E.L.; Belsley, M.; Raposo, M.M.M. Synthesis and characterization of new thienylpyrrolyl-benzothiazoles as efficient and thermally stable nonlinear optical chromophores. Tetrahedron 2007, 63(20), 4258–4265. [Google Scholar]
- Pina, J.; Seixas de Melo, J.; Burrows, H.D.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. Spectral and photophysical characterization of donor-π-acceptor arylthienyl- and bithienyl-benzothiazole derivatives in solution and solid state. J. Phys. Chem. A. 2007, 111, 8574–8578. [Google Scholar]
- Batista, R.M. F.; Costa, S.P.G.; Belsley, M.; Raposo, M.M.M. Synthesis and second-order nonlinear optical properties of new chromophores containing benzimidazole, thiophene and pyrrole heterocycles. Tetrahedron 2007, 63(39), 9842–9849. [Google Scholar]
- Batista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and ion sensing properties of new colorimetric and fluorimetric chemosensors based on bithienyl-imidazo-anthraquinone chromophores. Org. Lett. 2007, 9(17), 3201–3204. [Google Scholar]
- Guzow, K.; Szabelski, M.; Malicka, J.; Wiczk, W. Synthesis of a new, highly fluorescent amino acid derivative: N-[(tert-butoxy)carbonyl]-3-[2-(1H-indol-3-yl)benzoxazol-5-yl]-l-alanine methyl ester. Helv. Chim. Acta. 2001, 84, 1086 a, nd references cited therein.. [Google Scholar]
- Stephens, F. F.; Bower, J. D. The preparation of benziminazoles and benzoxazoles from Schiff's bases. Part I. J. Chem. Soc. 1949, 2971.
- Guzow, K.; Zielińska, J.; Mazurkiewicz, K.; Karolczak, J.; Wiczk, W. Influence of substituents in the phenyl ring on photophysical properties of 3-[2-(phenyl)benzoxazol-5-yl]alanine derivatives. J. Photochem. Photobiol. A. 2005, 175, 57. [Google Scholar]
- Czarnik, A. W. Fluorescent Chemosensors for Ion and Molecule Recognition; American Chemical Society: Washington, DC, 1993. [Google Scholar]
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515. [Google Scholar]
- Valeur, B. Molecular Fluorescence. Principles and Applications; Wiley-VCH: Weinheim, 2002. [Google Scholar]
- Rurack, K. Flipping the light switch ‘ON’ – the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions. Spect. Acta A. 2001, 57, 2161. [Google Scholar]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533. [Google Scholar]
- SPECFIT/32 Global Analysis System; 3.0 Spectrum Software Associates: Malborough, MA, USA.
- Berlman, B. Handbook of Fluorescence Spectra of Aromatic Molecules, 2nd ed.; Academic Press: New York, 1971. [Google Scholar]
- Lakowicz, J. R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Publisher: New York, 1999. [Google Scholar]



| Compd | Yield (%) | IR (cm−1) | 1H NMR (δ, ppm) |
|---|---|---|---|
| 1d | 98 | 3375, 1754 | 8.76 (N=CH) 6.90-6.95 (H-2), 7.05 (H-5) |
| 1e | 97 | 3430, 1741 | 9.01 (N=CH) 6.88-6.94 (H-2), 6.99 (H-5) |
| 2a | 56 | 3358, 1734 | 7.48 (H-4), 7.45 (H-7) |
| 2b | 84 | 3357, 1752 | 7.63 (H-4), 7.48 (H-7) |
| 2c | 74 | 3310, 1741 | 7.56-7.59 (H-4), 7.49 (H-7) |
| 2d | 54 | 3413, 1620 | 7.93-7.96 (H-4), 7.62-7.67 (H-7) |
| 2e | 65 | --- | 7.50 (H-4), 7.45 (H-7) |
| 2f | 82 | 3340, 1761 | 7.66 (H-4), 7.50 (H-7) |
| 2g | 44 | 3400, 1618 | 7.95(H-4), 7.60 (H-7) |
| Compound | UV-vis λmax (nm) | λem (nm) | Fluorescence ΦF | Stokes' shift (nm) |
|---|---|---|---|---|
| 2a | 315 | 394 | 0.80 | 79 |
| 2b | 316 | 393 | 0.66 | 77 |
| 2c | 315 | 394 | 0.77 | 79 |
| 2d | 315 | 393 | 0.76 | 78 |
| 2e | 334 | 396 | 0.47 | 62 |
| 2f | 334 | 395 | 0.44 | 61 |
| 2g | 336 | 398 | 0.26 | 62 |
| Compd. | Metal complex | Log K | M.L.Ha |
|---|---|---|---|
| 2b | 2bHg | 5.01 ± 4.0E-02 9.37 ± 7.0E-02 | 1.1.0 2.1.0 |
| 2d | 2dHg | 7.78 ± 2.0E-02 | 2.1.0 |
| 2d | 2dCu | 9.77 ± 2.0E-02 | 2.1.0 |
| 2f | 2fHg | 7.09 ± 8.0E-02 | 2.1.0 |
| 2f | 2fCu | 6.22 ± 2.0E-02 | 1.1.0 |
| 2f | 2fNi | 6.67 ± 6.0E-02 | 1.1.0 |
| 2g | 2gCu | 6.10 ± 6.0E-02 | 1.1.0 |
| 2g | 2gNi | 10.26 ± 8.0E-02 | 2.1.0 |
© 2007 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Costa, S.P.G.; Oliveira, E.; Lodeiro, C.; Raposo, M.M.M. Synthesis, Characterization and Metal Ion Detection of Novel Fluoroionophores Based on Heterocyclic Substituted Alanines. Sensors 2007, 7, 2096-2114. https://doi.org/10.3390/s7102096
Costa SPG, Oliveira E, Lodeiro C, Raposo MMM. Synthesis, Characterization and Metal Ion Detection of Novel Fluoroionophores Based on Heterocyclic Substituted Alanines. Sensors. 2007; 7(10):2096-2114. https://doi.org/10.3390/s7102096
Chicago/Turabian StyleCosta, Susana P. G., Elisabete Oliveira, Carlos Lodeiro, and M. Manuela M Raposo. 2007. "Synthesis, Characterization and Metal Ion Detection of Novel Fluoroionophores Based on Heterocyclic Substituted Alanines" Sensors 7, no. 10: 2096-2114. https://doi.org/10.3390/s7102096





