Tripodal Receptors for Cation and Anion Sensors
Abstract
:1. Introduction
2. Cation Recognition
2.1. Alkali and Alkaline Earth Metal Ions
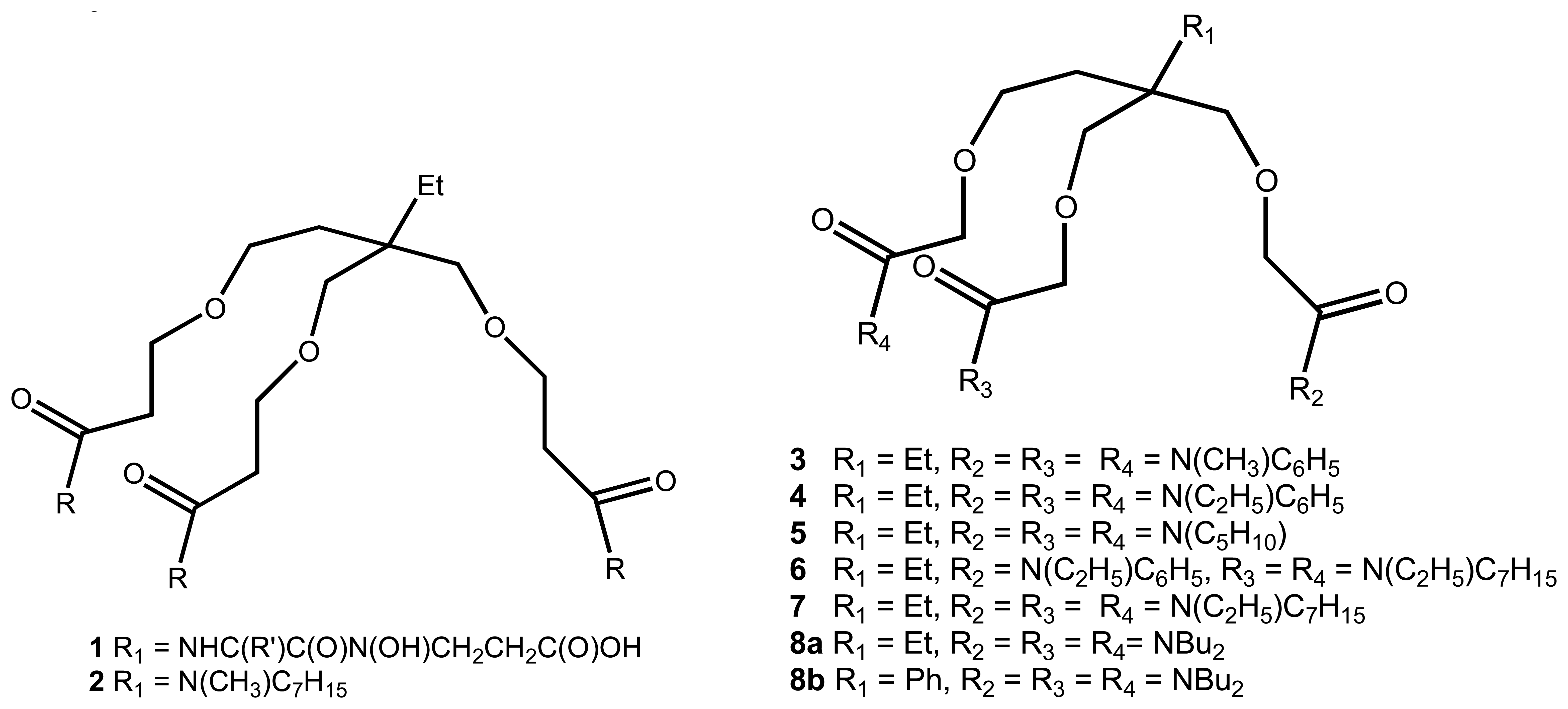
2.2. Heavy Metals


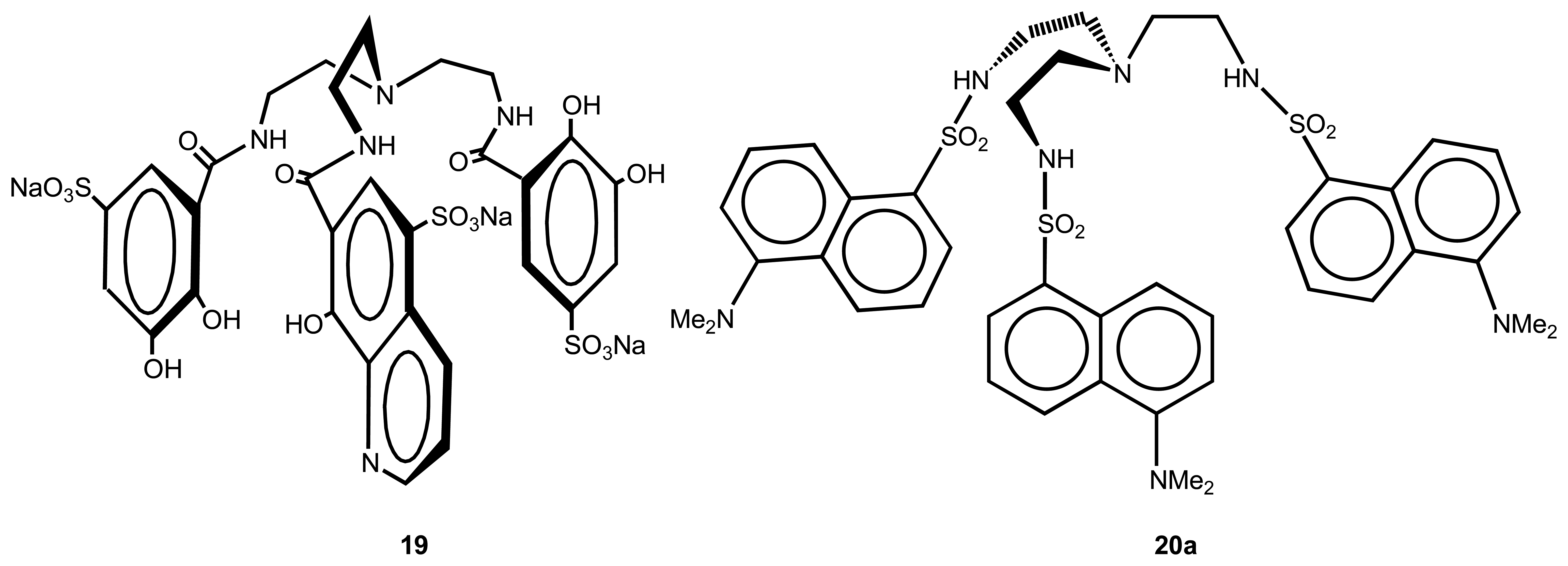

2.3. Actinides and Lanthanides
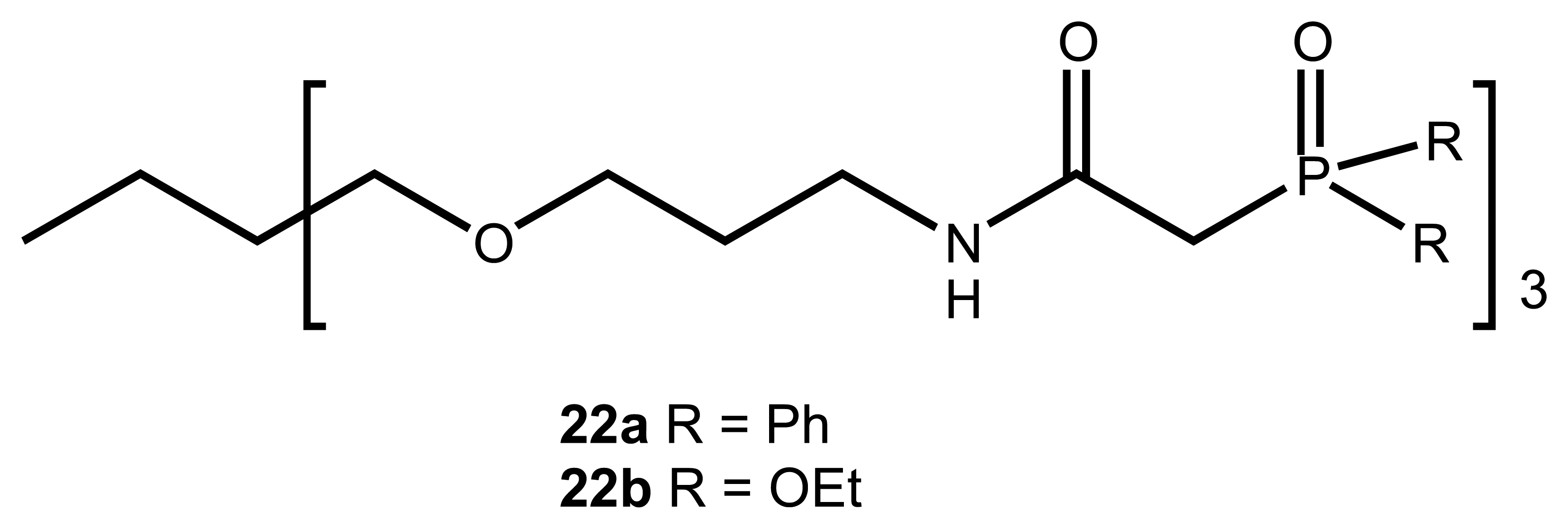


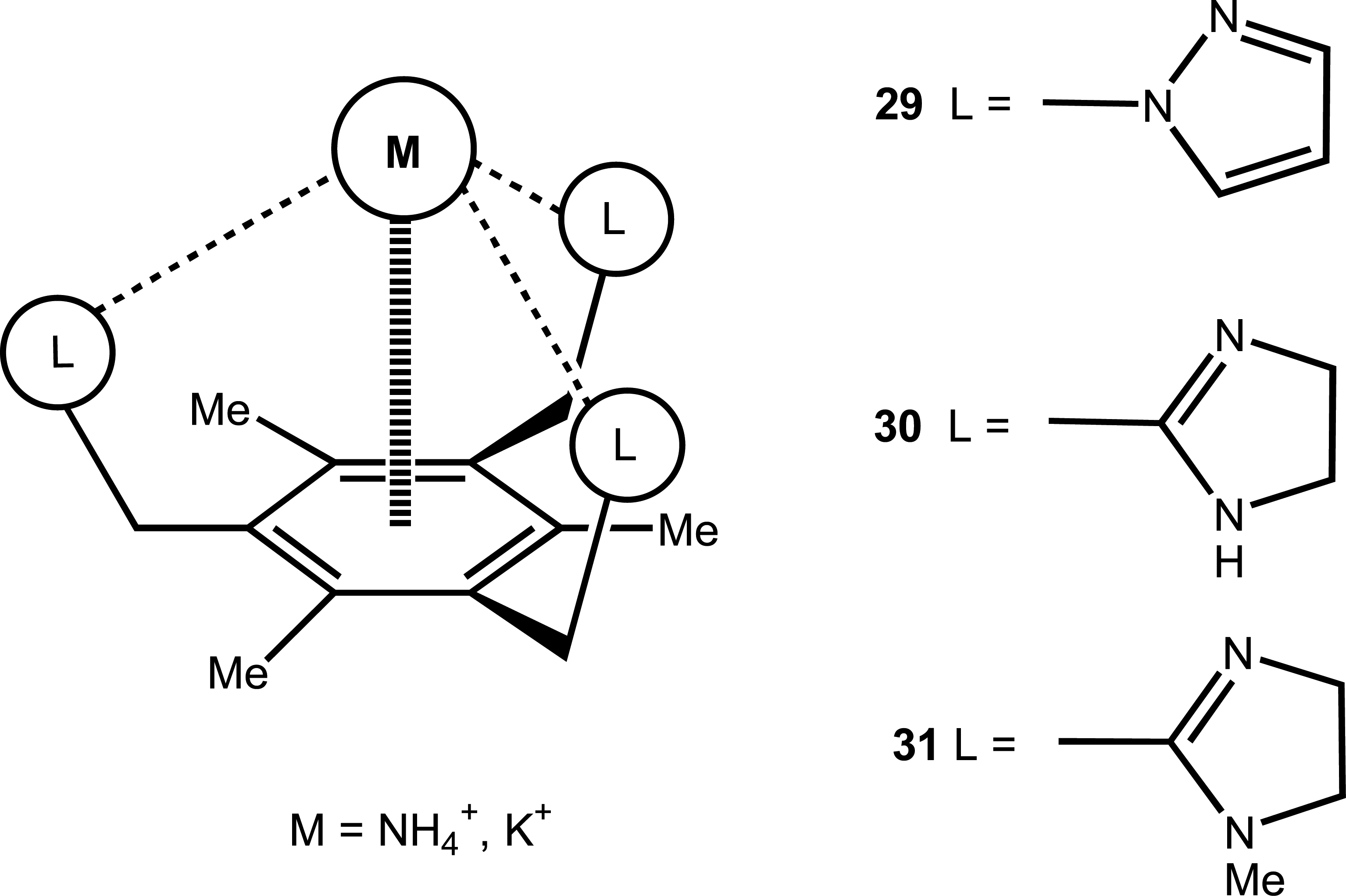
2.4. Ammonium and other Cations
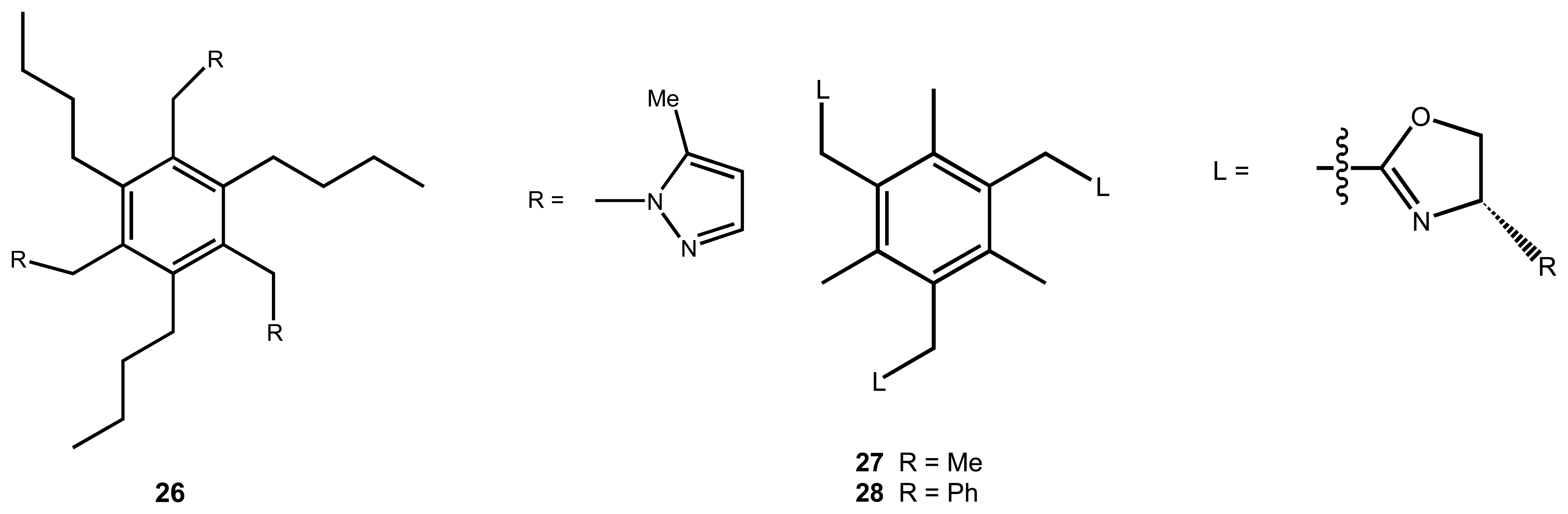

3. Anion Recognition
3.1. Halide Anions


3.2. Phosphates

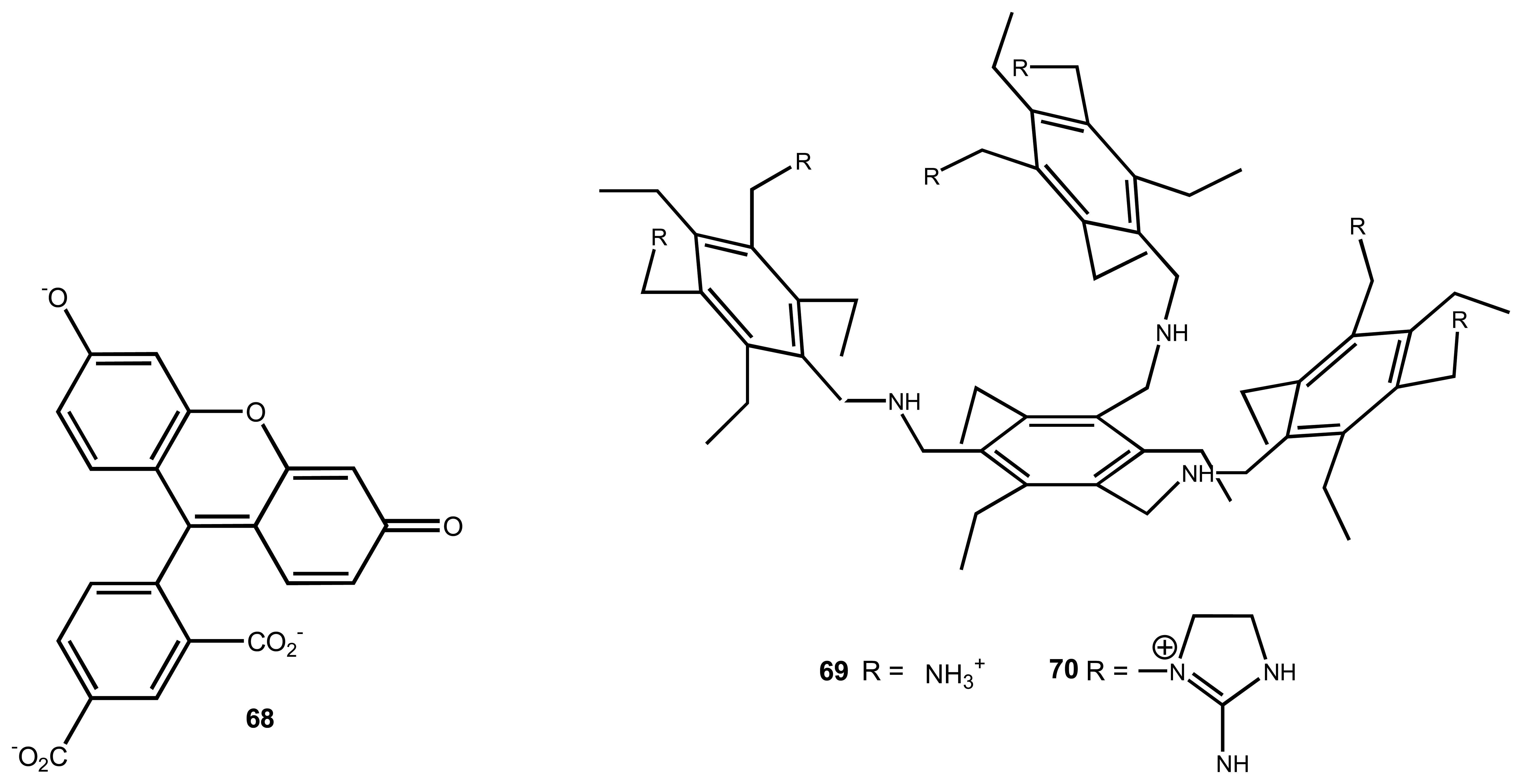

3.3. Sulphates


3.4. Citrate, Tartrate, and Malate
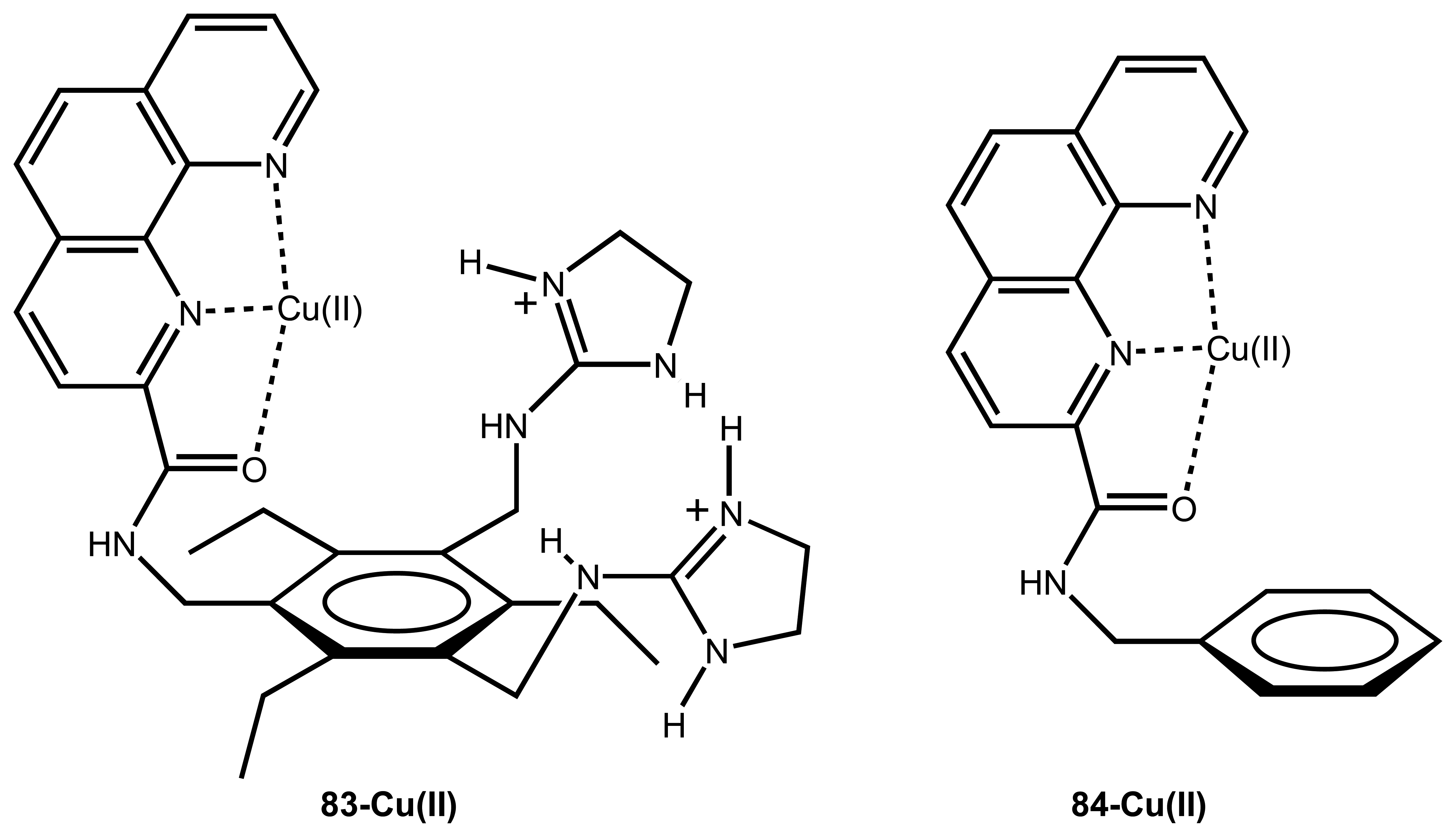
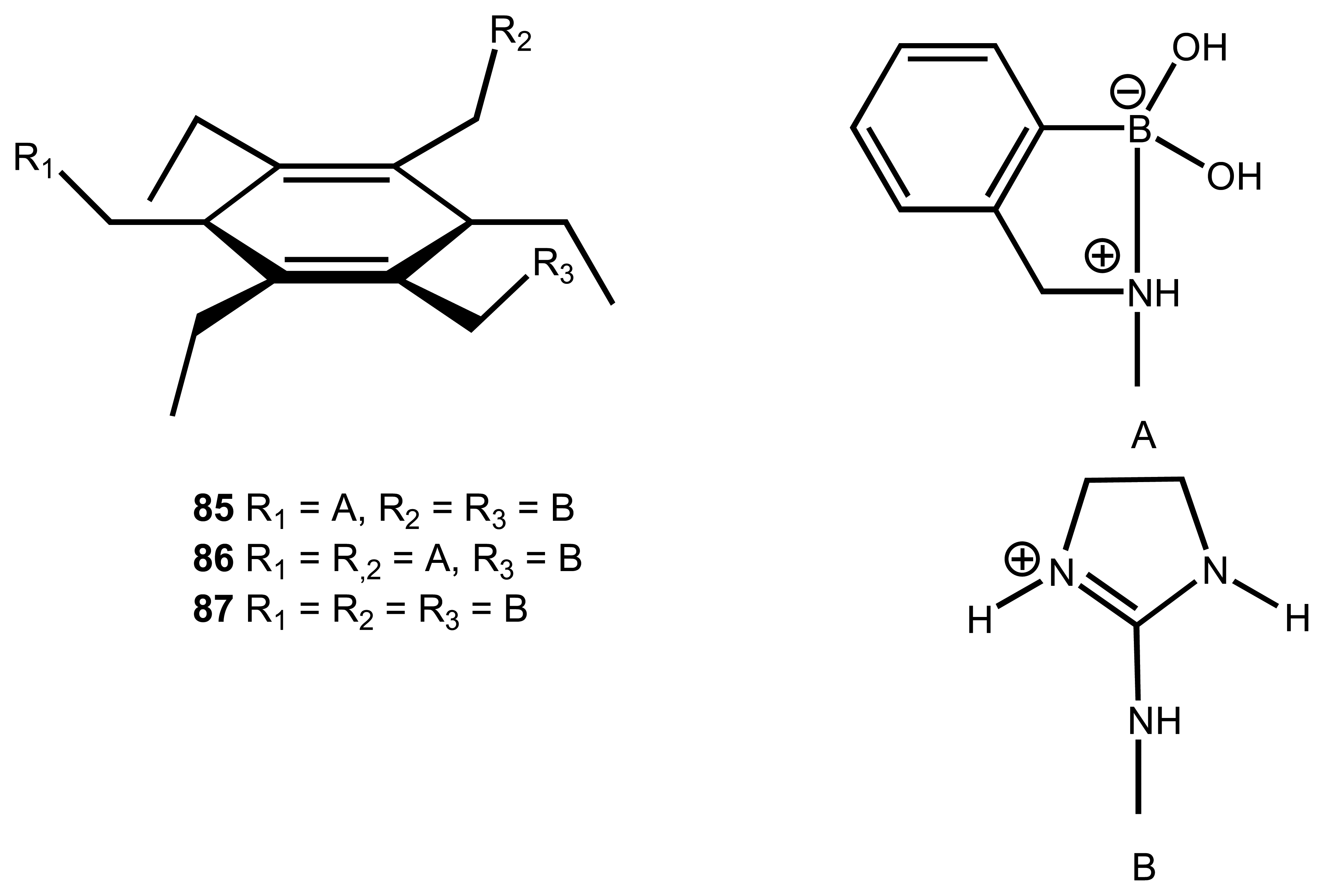
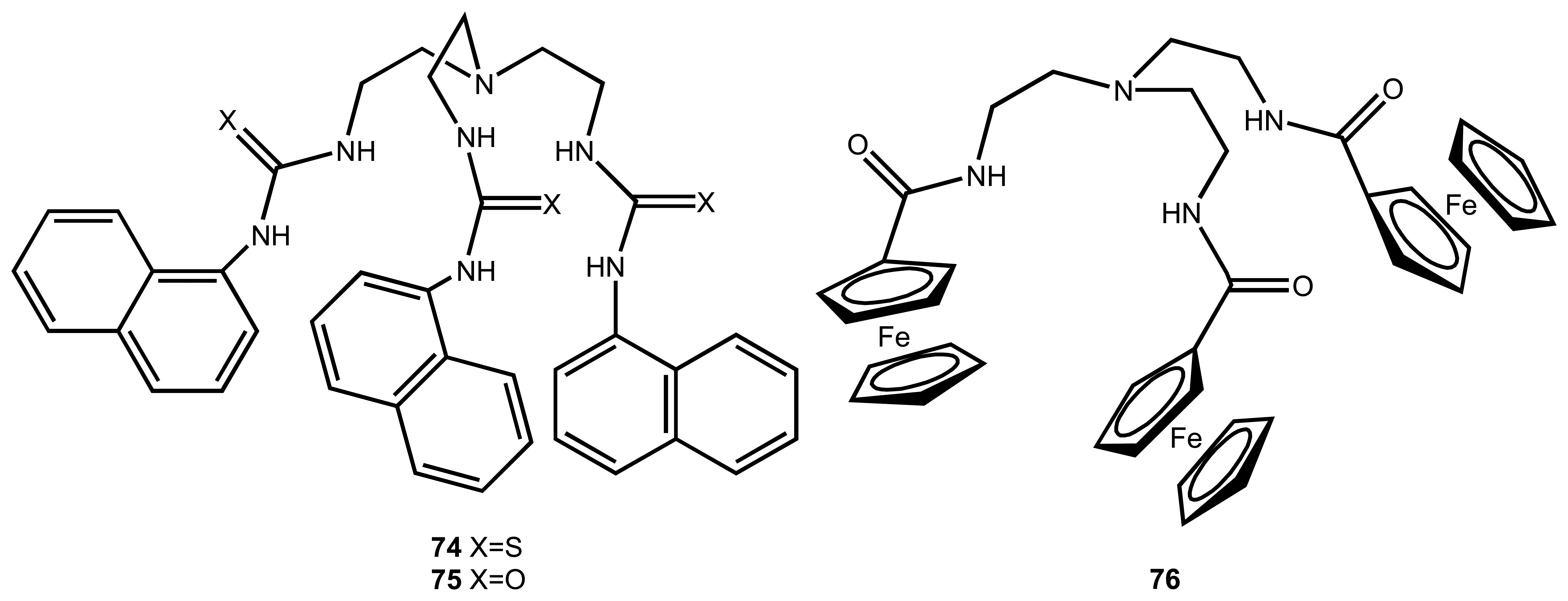
3.5. Carbonates and other Anions


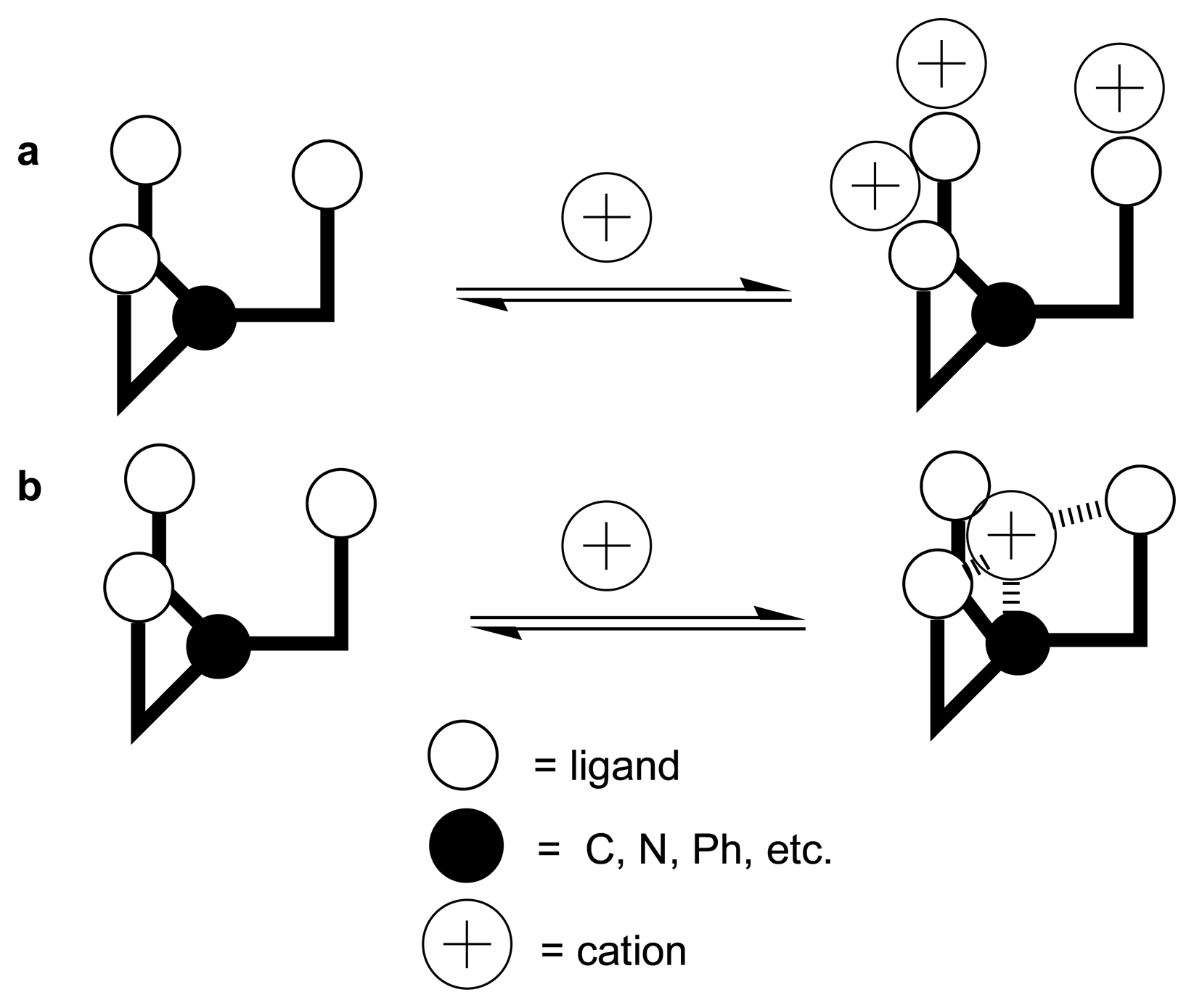
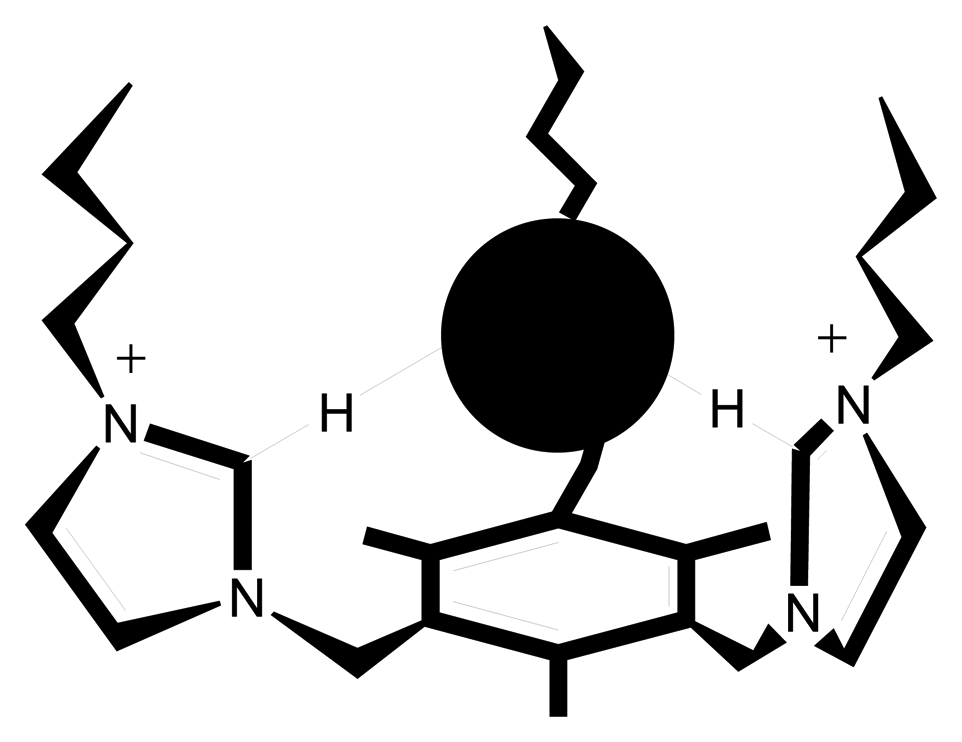
4. Conclusion and Outlook
Acknowledgments
References
- Diamond, D.; Nolan, K. Calixarenes: Designer ligands for chemical sensors. Anal. Chem. 2001, 73, 22A–29A. [Google Scholar]
- Ludwig, R.; Dzung, N. T. K. Calixarene based molecules for cation recognition. Sensors 2002, 2, 397–416. [Google Scholar]
- Beer, P. D.; Gale, P. A. Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed. Engl. 2001, 40, 486–516. [Google Scholar]
- Antonisse, M. M. G.; Reinhoudt, D. N. Potentiometric anion selective sensors. Electroanalysis 1999, 11, 1035–1048. [Google Scholar]
- Antonisse, M. M. G.; Reinhoudt, D. N. Neutral anion receptors: design and application. Chem. Commun. 1998, 443–448. [Google Scholar]
- Berocal, M. J.; Cruz, A.; Badr, I. H. A.; Bachas, L. G. Tripodal ionophore with sulphate recognition properties for anion-selective electrode. Anal. Chem. 2000, 72, 5295–5299. [Google Scholar]
- Reinoso-Garcia, M. M.; Dijkman, A.; Verboom, W.; Reinhoudt, D. N.; Malinoswka, E.; Wojciechowska, D.; Pietrzak, M.; Selucky, P. Metal complexation by tripodal N- acyl(thio)urea and picolin(thio)amide compounds: synthesis/extraction and potentiometric studies. Eur. J. Org. Chem. 2005, 2131–2138. [Google Scholar]
- Kim, S.-G.; Kim, K.-H.; Jung, J.; Shin, S. K.; Ahn, K. H. Unprecedented chiral molecular recognition in a C-3-symmetric environment. J. Am. Chem. Soc. 2002, 124, 591–597. [Google Scholar]
- Kim, Y.-K.; Ha, J.; Cha, G. S.; Ahn, K. H. Synthesis of tripodal trifluoroacetophenone derivatives and their evaluation as ion-selective electrode membranes. Bull. Korean Chem. Soc. 2002, 23, 1420–1424. [Google Scholar]
- Sasaki, S.; Ozawa, S.; Citterio, D.; Iwasawa, N.; Suzuki, K. Phosphate anion sensing based on preorganised tripodal ionophores. Anal. Sci. 2001, 17, i1659–1661. [Google Scholar]
- Reinoso-Garcia, M. M.; Janczewski, D.; Reinhoudt, D. N.; Verboom, W.; Malinoswka, E.; Pietrzak, M.; Hill, D.; Baca, J.; Gruner, B.; Selucky, P; Gruttner, C. CMP(O) tripodands: Synthesis, potentiometric studies and extractions. New J. Chem. 2006, 30. in press. [Google Scholar]
- Schmuck, C.; Schwegmann, M. A naked-eye sensing ensemble for selective detection of citrate-but not-tartrate or malate-in water based on a tris-cationic receptor. Org. Biomol. Chem. 2006, 4, 836–838. [Google Scholar]
- Wei, L. H.; He, Y. B.; Wu, J. L.; Qin, H. J.; Xu, K. X.; Meng, L. Z. Color response of a tripodal colorimetric sensor toward anions. Chin. J. Chem. 2005, 23, 608–612. [Google Scholar]
- Niikura, K.; Bisson, A. P.; Anslyn, E. V. Optical sensing of inorganic anions employing a synthethic receptor and ionic colorimetric dyes. J. Chem. Soc., Perkin Trans. 2 1999, 1111–1114. [Google Scholar]
- Wiskur, S. L.; Ait-Haddou, H.; Lavigne, J. J.; Anslyn, E. V. Teaching old indicators new tricks. Acc. Chem. Res. 2001, 34, 963–972. [Google Scholar]
- Sato, K.; Arail, S.; Yamagishi, T. A new tripodal anion receptor with C-H—X hydrogen bonding. Tetrahedron Lett. 1999, 40, 5219–5222. [Google Scholar]
- Ballester, P.; Costa, A.; Deyii, P. M.; Vega, M.; Morey, J. Influence of remote intramolecular hydrogen bonds on the thermodynamics of molecular recognition of cis-1,3,5-cyclohexanetricarboxylic acid. Tetrahedron Lett. 1999, 40, 171–174. [Google Scholar]
- Fan, A. L.; Hong, H. K.; Valiyaveettil, S.; Vittal, J. J. A urea-incorporated receptor for aromatic carboxylate anion recognition. J. Supramol. Chem. 2002, 2, 247–254. [Google Scholar]
- Borovik, A. S. Bioinspired hydrogen bond motifs in ligand design: the role of noncovalent interactions in metal ion mediated activation of dioxygen. Acc. Chem. Res. 2005, 38, 54–61. [Google Scholar]
- Martinez-Manez, R.; Sancenon, F. Fluorogenic and chromogenic chemosensors and reagents for anions. Chem. Rev. 2003, 103, 4419–4476. [Google Scholar]
- Lu, H.-J.; Fan, Y.-T.; Wu, Y.-J; Yin, M.-C. Tripodal lipophilic ionophores: synthesis, cation binding and transport through liquid membranes. Polyhedron 2001, 20, 3281–3286. [Google Scholar]
- Goodall, M.; Kelly, P. M.; Peter, D. Selective cation binding with cis, cis-1,3,5-trioxycyclohexyl based ligands. J. Chem. Soc., Perkin Trans. 2 1997, 59–63. [Google Scholar]
- Teulade-Fichou, M. P.; Vigeron, J. P.; Lehn, J.-M. Detection of organic anions in water through complexation enhanced fluorescence of a macrobicyclic tris-acridine cryptand. J. Chem. Soc., Perkin Trans. 2 1996, 2169–21672. [Google Scholar]
- Amendola, V.; Bastianello, E.; Fabrizzi, L. Halide-ion encapsulation by a flexible dicopper(II) bis-tren cryptate. Angew. Chem., Int. Ed. Engl. 2000, 39, 2917–2920. [Google Scholar]
- Ge, Q.–C.; Guo, Y.-H.; Lin, H.; Lin, H.–K.; Zhu, S.–R. Stoichiometrical coordination behaviour of hexaza tripodal ligands towards zinc(II), copper(II), nickel(II) and cobalt(II). Trans. Met. Chem. 2003, 28, 572–578. [Google Scholar]
- Meijler, M. M.; Arad-Yellin, R.; Cabantchik, Z. I.; Shanzer, A. Synthesis and evaluation of iron chelators with masked hydrophilic moieties. J. Am. Chem. Soc. 2002, 124, 12666–12668. [Google Scholar]
- Steiner, R. A.; Oehme, M.; Ammann, D.; Simon, W. Neutral carrier sodium ion-selective microelectrode for intracellular studies. Anal. Chem. 1979, 51, 351–357. [Google Scholar]
- Yan, Z.; Fan, Y.; Gao, Q.; Lu, H.; Hou, H. Tripodal compound 1,1,1-tris(N-ethyl-N-phenylamino-carboxylmethoxymethyl)propane as an ionophore for alkali and alkaline earth metal cations-selective electrode. Talanta 2002, 57, 81–88. [Google Scholar]
- Kataky, R.; Parker, D.; Teasdale, A. Comparative study of tripodal oxa-amides and oxa-esters as ionophores in potentiometric ion-selective electrodes for alkali and alkaline earth cations. Anal. Chim. Acta 1993, 276, 353–360. [Google Scholar]
- Tuntulani, T.; Thavornyutikarn, P.; Poompradub, S.; Jaiboon, N.; Ruangpornvisuti, V.; Chaichit, N.; Asfari, Z.; Vicens, J. Synthesis of tripodal azacrown ether calix[4]arenes and their supramolecular chemistry with transition, alkali metal ions and anions. Tetrahedron 2002, 58, 10277–10285. [Google Scholar]
- Hayashi, M.; Ishii, M.; Hiratani, K.; Saigo, K. Synthesis and binding properties of new tripodal hexadentate ligands having three quinolinol moieties for trivalent metal cations. Tetrahedron Lett. 1998, 39, 6215–6218. [Google Scholar]
- Hayashi, M.; Hiratani, K.; Kina, S.-I.; Ishii, M.; Saigo, K. Synthesis and binding property of a novel tripodal hexadentate ligand having catechol moieties. Tetrahedron Lett. 1998, 39, 6211–6214. [Google Scholar]
- Mulon, J.-B.; Destandau, É.; Alain, V.; Bardez, É. How can aluminium(III) generate fluorescence? J. Inorg. Biochem. 2005, 99, 1749–1755. [Google Scholar]
- Prodi, L.; Bolletta, F.; Montalti, M.; Zaccheroni, N. Searching for new luminescent sensors: synthesis and photophysical properties of a tripodal ligand incorporating the dansyl chromophore and its metal complexes. Eur. J. Inorg. Chem. 1999, 455–459. [Google Scholar]
- Malval, J. P.; Lapouyade, R.; Leger, J.-M.; Jarry, C. Tripodal ligand incorporating a dual fluorescent ionophores: a coordinative control of photoinduced electron transfer. Photochem. Photobiol. Sci. 2003, 2, 259–266. [Google Scholar]
- Arnaud-Neu, F.; Böhmer, V.; Dozol, J. F.; Gruttner, C.; Jakobi, R. A.; Kraft, D.; Mauprivez, O.; Rouquette, H.; Schwing-Weill, M. J.; Simon, N.; Vogt, W. Calixarenes with diphenylphosphoryl acetamide functions at the upper rim. A new class of highly efficient extractants for lanthanides and actinides. J. Chem. Soc., Perkin Trans. 2 1996, 1175–1179. [Google Scholar]
- Matthews, S. E.; Saadioui, M.; Böhmer, V.; Barboso, S.; Arnaud-Neu, F.; Schwing-Weill, M.-J.; Garcia Carrera, A.; Dozol, J.-F. Conformationally mobile wide rim carbamoylmethylphosphine oxide (CMPO)-calixarenes. J. Prakt Chem. 1999, 341, 264–273. [Google Scholar]
- Arduini, A.; Böhmer, V.; Delmau, L.; Desreux, J.-F.; Dozol, J.-F.; Carrera, M. A. G.; Lambert, B.; Musigmann, C.; Pochini, A.; Shivanyuk, A.; Ugozzoli, F. Rigidified calixarenes bearing four carbamoylmethylphosphineoxide or carbamoylmethylphosphoryl functions at the wide rim. Chem. Eur. J. 2000, 6, 2135–2144. [Google Scholar]
- Schmidt, C.; Saadioui, M.; Böhmer, V.; Host, V.; Spirlet, M.-R.; Desreux, J.-F.; Brisach, F.; Arnaud-Neu, F.; Dozol, J.-F. Modification of calix[4]arenes with CMPO-functions at the wide rim. Synthesis, solution behavior, and separation of actinides from lanthanides. Org. Biomol. Chem. 2003, 1, 4089–4095. [Google Scholar]
- Wang, P.; Saadioui, M.; Schmidt, C.; Böhmer, V.; Host, V.; Desreux, J.-F.; Dozol, J.-F. Dendritic octa-CMPO derivatives of calix[4]arenes. Tetrahedron 2004, 60, 2509–2515. [Google Scholar]
- Boerrigter, H.; Verboom, W.; Reinhoudt, D. N. Novel resorcinarene cavitand-based CMP(O) cation ligands: Synthesis and extraction properties. J. Org. Chem. 1997, 62, 7148–7155. [Google Scholar]
- Reinoso Garcia, M. M.; Verboom, W.; Reinhoudt, D. N.; Brisach, F.; Arnaud-Neu, F.; Liger, K. Solvent extraction of actinides and lanthanides by CMP(O)- and N-acyl(thio)urea-tetrafunctionalized cavitands: strong synergistic effect of cobalt bis(dicarbollide) ions. Solv. Extr. Ion Exch. 2005, 23, 425–437. [Google Scholar]
- Peters, M. W.; Werner, E. J.; Scott, M. J. Enhanced selectivity for actinides over lanthanides with CMPO ligands secured to a C-3-symmetric triphenoxymethane platform. Inorg. Chem. 2002, 41, 1707–1711. [Google Scholar]
- Rudzevich, V.; Schollmeyer, D.; Braekers, D.; Desreux, J.-F.; Diss, R.; Wipff, G.; Böhmer, V. Carbamoylmethylphosphinoxide derivatives based on the triphenylmethane skeleton. synthesis and extraction Properties. J. Org. Chem. 2005, 70, 6027–6033. [Google Scholar]
- Ghosh, P.; Shukla, A. D.; Das, A. Cerium ion-induced fluorescence enhancement of a tripodal fluoroionophore. Tetrahedron Lett. 2002, 43, 7419–7422. [Google Scholar]
- Wietzke, R.; Mazzanti, M.; Latour, J.-M.; Pecaut, J. Strong intramoleculer π-π interactions favor the formation of 2:1 (L:M) lanthanide complexes of tris(2-benzimidazolylmethyl)amine. Chem. Commun. 1999, 209–210. [Google Scholar]
- Sahoo, S. K.; Baral, M.; Kanungo, B. K. Potentiometric, spectrophotometric, theoretical studies and binding properties of a novel tripodal polycatechol-amine ligand with lanthanide(III) ions. Polyhedron 2006, 25, 722–736. [Google Scholar]
- Sasaki, S.; Amano, T.; Monma, G.; Otsuka, T.; Iwasawa, N.; Citterio, D.; Hisamoto, H.; Suzuki, K. Comparison of two molecular design strategies for the development of an ammonium ionophore more highly selective than nonactin. Anal. Chem. 2002, 74, 4845–4848. [Google Scholar]
- Ahn, K. H.; Kim, S.-G.; Kim, K.-H.; Jung, J.; Kim, J.; Chin, J.; Kim, K. Selective recognition of NH4+ over K+with tripodal oxazoline receptors. Chem. Lett. 2000, 170–171. [Google Scholar]
- Kim, S.-G.; Ahn, K. H. Novel artificial receptors for alkylammonium ions with remarkable selectivity and affinity. Chem. Eur. J. 2000, 6, 3399–3403. [Google Scholar]
- Kim, H.-S.; Kim, D.-H.; Kim, K. S.; Choi, H.-J.; Shim, J. H.; Jeong, I. S.; Cha, G. S.; Nam, H. Synthesis and properties of 1,3,5-tris(phenoxymethyl)-2,4,6-triethylbenzenes as NH4+ ionophores. J. Incl. Phenom. Macrocycl. Chem. 2003, 46, 201–205. [Google Scholar]
- Hennrich, G.; Lynch, V. M.; Anslyn, E. V. Novel C3-symmetric molecular scaffolds with potential facial differentiation. Chem. Eur. J. 2002, 8, 2274–2278. [Google Scholar]
- Niikura, K.; Metzger, A.; Anslyn, E. V. Chemosensor ensemble with selectivity for inositol-triphosphate. J. Am. Chem. Soc. 1998, 120, 8533–8534. [Google Scholar]
- Chin, J.; Walsdorff, C.; Stranix, B.; Oh, J.; Chung, H. J.; Park, S.-M.; Kim, K. A rational approach to selective recognition of NH4+ over K+. Angew. Chem., Int. Ed. Engl. 1999, 38, 2756–2759. [Google Scholar]
- Lavigne, J. L.; Anslyn, E. V. Teaching old indicators new tricks: a colorimetric chemosensing ensemble for tartrate/malate in beverages. Angew. Chem., Int. Ed. Engl. 1999, 38, 3666–3669. [Google Scholar]
- Kim, H.-J.; Kim, Y.-H.; Hong, J.-I. Sugar recognition by C-3-symmetric oxazoline hosts. Tetrahedron Lett. 2001, 42, 5049–5052. [Google Scholar]
- Ahn, K. H.; Ku, H.-Y.; Kim, Y.; Kim, S.-G.; Kim, Y. K.; Son, H. S.; Ku, J. K. Fluorescence sensing of ammonium and organoammonium ions with tripodal oxazoline receptors. Org. Lett. 2003, 5, 1419–1422. [Google Scholar]
- Hartshorn, C. M.; Steel, P. J. Poly(pyrazol-1-ylmethyl)benzenes: New multidentate ligands. Aust. J. Chem. 1995, 48, 1587–1589. [Google Scholar]
- Hartshorn, C. M.; Steel, P. J. Coelenterands: A new class of metal-encapsulating ligands. Angew. Chem., Int. Ed. Engl. 1996, 35, 2655–2657. [Google Scholar]
- Oh, K. S.; Lee, C.–W.; Choi, H. S.; Lee, S. J.; Kim, K. S. Origin of the high affinity and selectivity of novel receptors for NH4+ over K+: Charged hydrogen bonds vs cation- π interaction. Org. Lett. 2000, 2, 2679–2681. [Google Scholar]
- Cleland, W. W.; Frey, P. A.; Gerlt, J. A. The low barrier hydrogen bond in enzymatic catalysis. J. Biol. Chem. 1998, 273, 25529–25532. [Google Scholar]
- Ma, J. C.; Dougherty, D. A. The cation-pi interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar]
- Mecozzi, S.; West, A. P.; Dougherty, D. A. Cation-pi interactions in simple aromatics: Electrostatics provide a predictive tool. J. Am. Chem. Soc. 1996, 118, 2307–2310. [Google Scholar]
- Choi, H. S.; Suh, S. B.; Cho, S. J.; Kim, K. S. Ionophores and receptors using cation-π interactions: Collarenes. Proc. Natl. Acad. Sci. 1998, 95, 12094–12099. [Google Scholar]
- Lee, H. M.; Kim, J.; Lee, S.; Mhin, B. J.; Kim, K. S. Aqua-potassium(I) complexes: Ab initio study. J. Chem. Phys. 1999, 111, 3995–3999. [Google Scholar]
- Kim, S. K.; Singh, N. J.; Kim, S. J.; Kim, H. G.; Kim, J. K.; Lee, J. W.; Kim, K. S.; Yoon, J. New fluorescent photoinduced electron transfer chemosensor for the recognition of H2PO4-. Org. Lett. 2003, 5, 2083–2086. [Google Scholar]
- Schmidtchen, F. P.; Berger, M. Artificial organic host molecules for anions. Chem. Rev. 1997, 97, 1609–1646. [Google Scholar]
- Gale, P. A.; Sessler, J. L.; Kral, V.; Lynch, V. Calix[4]pyrroles: old yet new anion-binding agents. J. Am. Chem. Soc. 1996, 118, 5140–5141. [Google Scholar]
- Reetz, M. T.; Niemeyer, C. M.; Harms, K. Crown ethers with a Lewis acidic center: A new class of heterotopic host molecules. Angew. Chem., Int. Ed. Engl. 1991, 30, 1472–1474. [Google Scholar]
- Morzherin, Y.; Rudkevich, D. M.; Verboom, W.; Reinhoudt, D. N. Chlorosulfonated calix[4]arenes: precursors for neutral anion receptors with a selectivity for hydrogen sulphate. J. Org. Chem. 1993, 58, 7602–7605. [Google Scholar]
- Casnati, A.; Fochi, M.; Minari, P.; Pochini, A.; Reggiani, M.; Ungaro, R.; Reinhoudt, D. N. Upper-rim urea-derivatised calix[4]arenes as neutral receptors for monocarboxylate anions. Gazz. Chim. Ital. 1996, 99–106. [Google Scholar]
- Cameron, B. R.; Loeb, S. J. Bis(amido)calix[4]arene in the pinched cone conformation as tuneable hydrogen-bonding anion receptors. J. Chem. Soc., Chem. Commun. 1997, 573–574. [Google Scholar]
- Beer, P. D.; Hazlewood, C.; Hesek, D.; Hodacova, J.; Stokes, S. E. Anion recognition by acyclic redox-responsive amide-linked cobaltocenium receptor. J. Chem. Soc., Dalton. Trans. 1993, 1327–1332. [Google Scholar]
- Raposo, C.; Perez, N.; Almaraz, M.; Luisa Mussons, M.; Cruz Cabarello, M.; Moran, J. R. A cyclohexane spacer for phosphate receptors. Tetrahedron Lett. 1995, 36, 3255–3258. [Google Scholar]
- Beer, P. D.; Keefe, A. D. A new approach to the coordination of anions, novel polycobalticinium macrocyclic receptor molecules. J. Organomet. Chem. 1989, 375, C40–C42. [Google Scholar]
- Beer, P. D.; Hesek, D.; Hodacova, J.; Stokes, S. E. Acyclic redox responsive anion responsive amide-linked cobaltocenium moieties. J. Chem. Soc., Chem. Commun. 1992, 270–272. [Google Scholar]
- Metzger, A.; Lynch, V. M.; Anslyn, E. V. A synthetic receptor selective for citrate. Angew. Chem., Int. Ed. Engl. 1997, 36, 862–865. [Google Scholar]
- Cabell, L. A.; Monahan, M.-K.; Anslyn, E. V. A competition assay for determining glucose-6-phosphate concentration with a tris-boronic acid receptor. Tetrahedron Lett. 1999, 40, 7753–7756. [Google Scholar]
- Beer, P. D.; Gale, P. A.; Chen, G. Z. Electrochemical molecular recognition: pathways between complexation and sample. Coord. Chem. Rev. 1999, 3, 185–186. [Google Scholar]
- Scheerder, J.; Engbersen, J. F. J.; Reinhoudt, D. N. Synthetic receptors for anion complexation. Recl. Trav. Chim. Pays-Bas 1996, 115, 307–320. [Google Scholar]
- Kral, V.; Rusin, O.; Shishkanova, T.; Volf, R.; Matejka, P.; Volka, K. Anion binding: from supramolecules to sensors. Chem. Listy 1999, 93, 546–553. [Google Scholar]
- Beer, P. D.; Gale, P. A. Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed. Engl. 2001, 40, 486–516. [Google Scholar]
- Beer, P. D. Transition-metal receptor system for the selective recognition and sensing of anionic guest species. Acc. Chem. Res. 1998, 31, 71–80. [Google Scholar]
- Hoffmann, R. W.; Hettche, F.; Harms, K. Energies and selectivities for anion binding as a function of host conformational preorganisation. Chem. Commun. 2002, 782–783. [Google Scholar]
- Hettche, F.; Hoffmann, R. W. A tri-armed sulfonamide host for selective binding of chloride. New J. Chem. 2003, 27, 172–177. [Google Scholar]
- Bai, Y.; Zhang, B.-G.; Xu, J.; Duan, C.-Y.; Dang, D.-B.; Liu, D.-J.; Meng, Q.-J. Conformational switching fluorescent chemosensor for chloride anion. New J. Chem. 2005, 29, 777–779. [Google Scholar]
- Yoon, J.; Kim, S. K.; Singh, N. J.; Lee, J. W.; Yang, Y. J.; Chellappan, K.; Kim, K. S. Highly effective fluorescent sensor for H2PO4. J. Org. Chem. 2004, 69, 581–584. [Google Scholar]
- Yin, Z.; Zhang, Y.; Hea, J.; Cheng, J.-P. A new tripodal anion receptor with selective binding for H2PO4− and F− ions. Tetrahedron 2006, 62, 765–770. [Google Scholar]
- Sasaki, S.; Mizuno, M.; Naemura, K.; Tobe, Y. Synthesis and anion-selective complexation of cyclophane-based cyclic thioureas. J. Org. Chem. 2000, 65, 275–283. [Google Scholar]
- Snellink-Ruel, B. H. M.; Antonisse, M. G.; Engbersen, J. F. J.; Timmerman, P.; Reinhoudt, D. N. Neutral anion receptors with multiple urea-binding sites. Eur. J. Org. Chem. 2000, 165–170. [Google Scholar]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1685. [Google Scholar]
- Sasaki, S.; Citterio, D.; Ozawa, S.; Suzuki, K. Design and synthesis of preorganized tripodal fluororeceptors based on hydrogen bonding of thiourea groups for optical phosphate ion sensing. J. Chem. Soc., Perkin Trans. 2 2001, 2309–2313. [Google Scholar]
- Raposo, C.; Almaraz, M.; Martin, M.; Weinrich, V.; Musson, M.; Alcazar, V.; Cruz Cabarello, M.; Moran, J. R. Tris(2-aminoethyl)amine, a suitable spacer for phosphate and sulphate receptors. Chem. Lett. 1995, 36, 3255–3258. [Google Scholar]
- Wu, F.-Y.; Li, Z.; Wen, Z.-C.; Zhou, N.; Zhao, Y.-F.; Jiang, Y.-B. A novel thiourea-based dual fluorescent anion receptor with a rigid hydrazine spacer. Org. Lett. 2002, 4, 2325–3205. [Google Scholar]
- Bianchi, A.; Bowman-James, K.; Garcia-Espana, E. (Eds.) Supramolecular Chemistry of Anions; 1997; Willey-CH: New York.
- Valiyaveettil, S.; Engbersen, J. F. J.; Verboom, W.; Reinhoudt, D. N. Synthesis and complexation studies of neutral anion receptors. Angew. Chem. Int. Ed. Engl. 1993, 32, 900–901. [Google Scholar]
- Stibor, I.; Hafeed, D. S. M.; Lhoták, P.; Hodacova, J.; Koca, J.; Cajan, M. From the amide bond activation to simultaneous recognition of anion-cation couple. Gazz. Chim. Ital. 1997, 127, 673–685. [Google Scholar]
- Xie, H.; Yi, S.; Wu, S. Study on host-guest complexation of anions based on tripodal naphthylthiourea derivatives. J. Chem. Soc., Perkin Trans. 2 1999, 2751–2754. [Google Scholar]
- Xie, H.; Yi, S.; Yang, X.; Wu, S. Study on host-guest complexation of anions based on a tripodal naphthylthiourea derivative. New J. Chem. 1999, 23, 1105–1110. [Google Scholar]
- Beer, P. D.; Hopkins, P. K.; McKinney, J. D. Cooperative halide, perrhenate anion-sodium cation binding and pertechnetate extraction and transport by a novel tripodal tris(amido benzo-15-crown-5) ligand. Chem. Commun. 1999, 1253–1254. [Google Scholar]
- Beer, P. D.; Chen, Z.; Goulden, A. J.; Graydon, A. R.; Stokes, S. E.; Wear, T. Calixarene-based anion receptors. J. Chem. Soc., Chem. Commun. 1993, 1834–1839. [Google Scholar]
- Beer, P. D.; Drew, M. G. B.; Hesek, D.; Jagessar, R. Spectral and electrochemical anion sensing by a novel 5,10,15,20-tetrakis(R-substituted) porphyrin receptor. J. Chem. Soc., Chem. Commun. 1995, 1187–1189. [Google Scholar]
- Tobey, S. L.; Jone, B. D.; Anslyn, E. V. C3v symmetric receptors show high selectivity and high affinity for phosphate. J. Am. Chem. Soc. 2003, 125, 4026–4027. [Google Scholar]
- Tobey, S. L.; Anslyn, E. V. Determination of inorganic phosphate in serum and saliva using a synthetic receptor. Org. Lett. 2003, 5, 2029–2031. [Google Scholar]
- Berrocal, M. J.; Cruz, A.; Badr, I. H. A.; Bachas, L. G. Tripodal ionophore with sulfate recognition properties for anion-selective electrodes. Anal. Chem. 2000, 72, 5295–5299. [Google Scholar]
- Seong, H. R.; Kim, D.-S.; Kim, S.-G.; Choi, H.-J.; Ahn, K. H. Benzene-based tripodal isothiouronium compounds as sulfate ion receptors. Tetrahedron Lett. 2004, 45, 723–727. [Google Scholar]
- Gale, P. A. Anion coordination and anion-directed assembly: highlights from 1997 and 1998. Coord. Chem. Rev. 2000, 199, 181–188. [Google Scholar]
- Wiskur, S. L.; Floriano, P. N.; Anslyn, E. V.; McDevitt, J. T. A multicomponent sensing ensemble in solution: differentiation between structurally similar analytes. Angew. Chem., Int. Ed. Engl. 2003, 42, 2070–2072. [Google Scholar]
- Metzger, A.; Anslyn, E. V. A chemosensor for citrate in beverages. Angew. Chem. Int. Ed. Engl. 1998, 37, 649–652. [Google Scholar]
- McCleskey, S. C.; Metzger, A.; Simmons, C. S.; Anslyn, E. V. Competitive indicator methods for the analysis of citrate using colorimetric assays. Tetrahedron 2002, 58, 621–628. [Google Scholar]
- Schmuck, C.; Schwegmann, M. A molecular flytrap for the selective binding of citrate and other tricarboxylates in water. J. Am. Chem. Soc. 2005, 127, 3373–3379. [Google Scholar]
- Massou, S.; Albigot, R.; Prats, M. Carboxyfluorescein fluorescence experiments. Biochem. Educ. 2000, 28, 171–173. [Google Scholar]
- Lee, H. J.; Yoon, I. J.; Yoo, C. L.; Pyun, H. -J.; Cha, G. S.; Nam, H. Potentiometric evaluation of solvent polymeric carbonate-selective membranes based on molecular tweezer type neutral carriers. Anal. Chem. 2000, 72, 4694–4697. [Google Scholar]
- Wiskur, S. L.; Anslyn, E. V. Using a synthetic receptor to create an optical-sensing ensemble for a class of analytes: a colorimetric assay for the aging of scotch. J. Am. Chem. Soc. 2001, 123, 10109–10110. [Google Scholar]
- Zhong, Z.; Anslyn, E. V. A colorimetric sensing ensemble for heparin. J. Am. Chem. Soc. 2002, 124, 9014–9015. [Google Scholar]
- Guo, Y.-H.; Ge, Q.-C.; Lin, H.; Lin, H.-K; Zhu, S.-R. Thermodynamic studies on supramolecular interaction of metal ions with nucleotides/tripods ligands. Polyhedron 2002, 21, 1005–1015. [Google Scholar]
- McCleskey, S. C.; Floriano, P. N.; Wiskur, S. L.; Anslyn, E. V.; McDevitt, J. T. Citrate and calcium determination in flavored vodkas using artificial neural networks. Tetrahedron 2003, 50, 10089–10092. [Google Scholar]
- Cabell, L. A.; Best, M. D.; Lavigne, J. J.; Schneider, S. E.; Perreault, D. M.; Monahan, M.-K.; Anslyn, E. V. Metal triggered fluorescence sensing of citrate using a synthetic receptor. J. Chem. Soc., Perkin Trans. 2 2001, 315–323. [Google Scholar]
- Fabbrizzi, L.; Licchelli, M.; Perotti, A.; Poggi, A.; Rabaioli, G.; Sacchi, D.; Taglietti, A. Fluorescent molecular sensing of amino acids bearing an aromatic residue. J. Chem. Soc., Perkin Trans. 2 2001, 2108–2113. [Google Scholar]
- Fabbrizzi, L.; Licchelli, M.; Parodi, L.; Poggi, A.; Taglietti, A. A versatile fluorescent system for sensing of H+, transition metals, and aromatic carboxylates. Eur. J. Inorg. Chem. 1999, 35–39. [Google Scholar]



| No. | Receptors | Cations | Sensing Mode | Note | Ref. |
|---|---|---|---|---|---|
| 1 | 1 | Ca2+ | Potentiometric/ISE | - | 26 |
| 2 | 2 | Na+ | Potentiometric/ISE | commercially available | 27 |
| 3 | 3-5 | Li+ , Na+, Ca2+ | Potentiometric/ISE | also binds K+, Mg2+ | 21 |
| 4 | 6 | Li+ , Na+, Ca2+ | Potentiometric/ISE | good for Ca2+ electrode, near-Nernstian response to Sr2+ and Ba2+ | 28 |
| 5 | 7 | Na+ | Potentiometric/ISE | known as ETH 227 | 29 |
| 6 | 8a | Na+ | Potentiometric/ISE | also binds Li+ , K+, Ca2+ and Mg2+ | 29 |
| 7 | 8b | Ca2+ | Potentiometric/ISE | also binds Li+ , Na+, K+, and Mg2+ | 29 |
| 8 | 10 | Ag+ | Potentiometric/ISE | - | 7 |
| 9 | 13, 14 | Cu2+, Hg2+ | Potentiometric/ISE | forms a strong complex with Cu2+and Hg2+ so that cannot be used for ISE membrane. Useful for extraction. | 7 |
| 10 | 15a, 15b | Cu2+, Zn2+ | Potentiometric/ISE | also binds to Co2+, Ni2+. The binding ability can be switched by varying pH | 30 |
| 11 | 16 | Fe3+ | Potentiometric/ISE | - | 31 |
| 12 | 17 | Ga3+, Fe3+ | Potentiometric/ISE | more selective to Ga3+ | 31 |
| 13 | 18, 19 | Al3+ | Fluorescent (TRF) | - | 33 |
| 14 | 20a | Cu2+, Co2+ Zn2+, Cd2+ | Luminescent | more selective to Cu2+ | 34 |
| 15 | 21 | Zn2+, Cu2+ | Fluorescent | more selective to Zn2+ | 35 |
| 16 | 22a, 22b | UO22+, Am3+, Eu3+ | Potentiometric/ISE | 22a very high complex formation for Eu3+ | 11 |
| 17 | 23 | Ce3+ | Fluorescent/PET | no fluorescence for La3+, Pr3+, Nd3+, Sm3+, Eu3+, Dy3+ | 45 |
| 18 | 24 | La3+, Pr3+, Nd3+, Sm3+, Eu3+, Dy3+ | Potentiometric/ISE | - | 46 |
| 19 | 25 | La3+, Gd3+, Lu3+ | Potentiometric and Spectrophotometric | pH dependent metal-ligand formation | 47 |
| 20 | 26 | NH4+ | Potentiometric/ISE | Ca2+, K+ as interference | 48 |
| 21 | 27, 28 | NH4+, R-NH3+ | Fluorescence | K+, Na+, Mg2+, as interference | 57 |
| 22 | 29 | NH4+ | Potentiometric/ISE | K+ as interference | 54 |
| 23 | 30, 31 | NH4+ | Potentiometric/ISE | - | 49 |
| 24 | 32-40 | n-BuNH3+ | Potentiometric/ISE | also binds t-BuNH3+ | 50 |
| 25 | 41-47 | NH4+ | Potentiometric/ISE | 44-47 selective over Na+ and K+ | 66 |
| No. | Receptors | Anions | Sensing Mode | Note | Ref. |
|---|---|---|---|---|---|
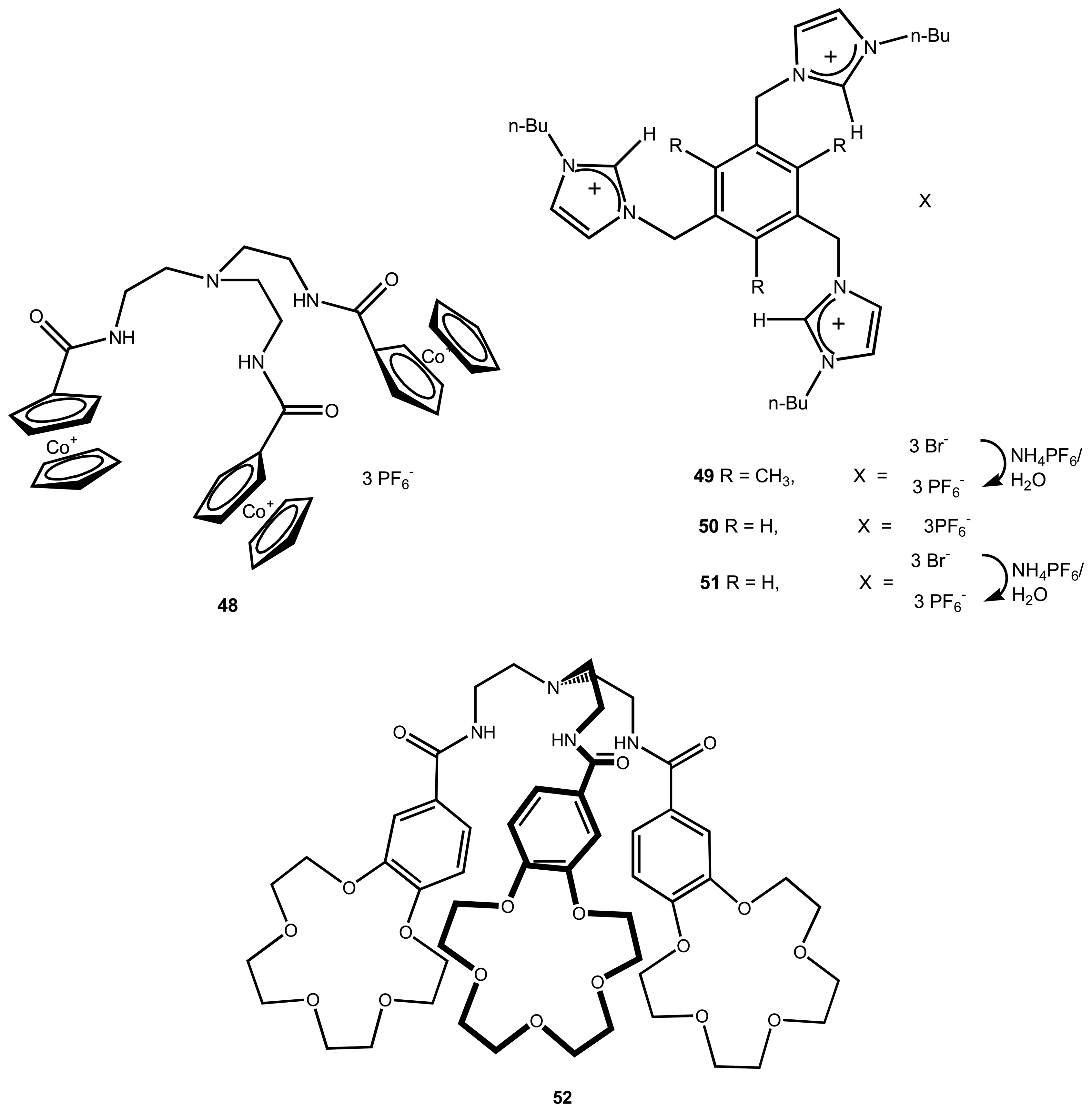
| 1 | 48 | Cl- | Cyclic voltametric | - | 76 |
| 2 | 49 | Cl- , Br-, I- | Potentiometric/ISE | - | 16 |
| 3 | 52 | pertechnetate | - | also binds K+, Mg2+ | 79 |
| 4 | 57 | Cl- | Potentiometric/ISE | also binds Br-, NO3- | 85 |
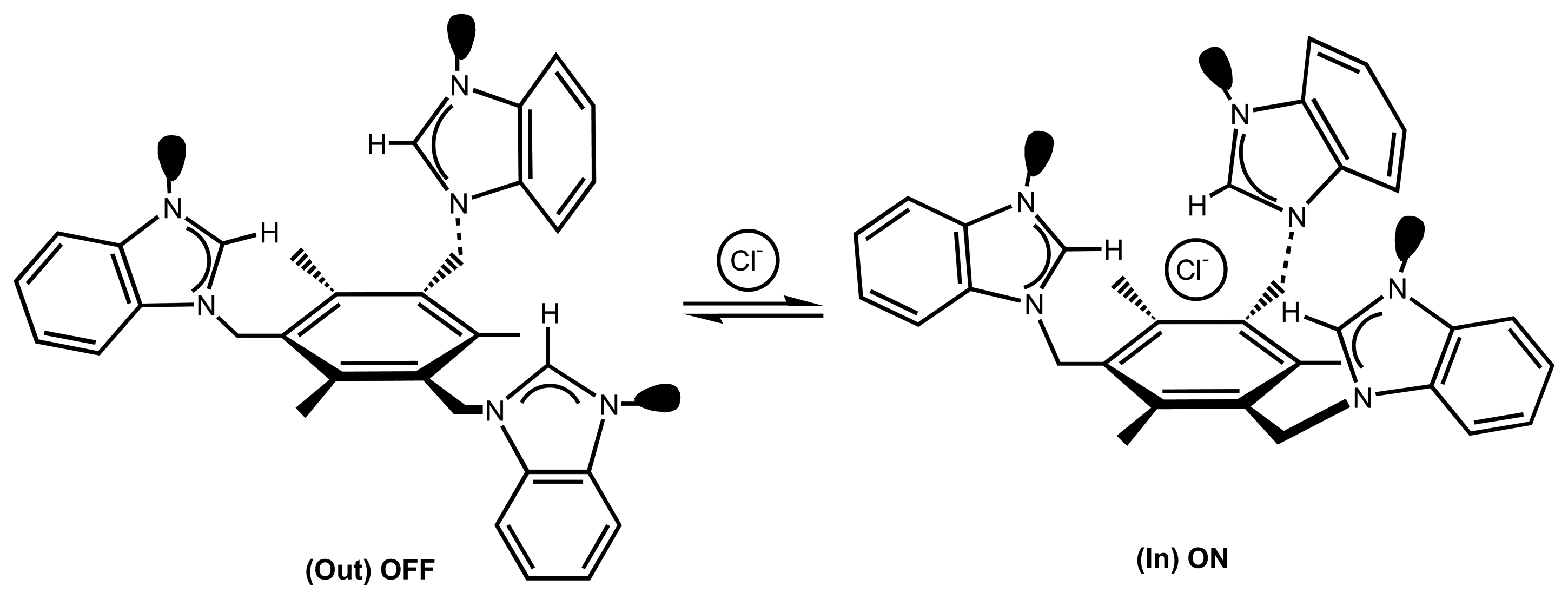
| 5 | 59 | Cl- | Fluorescent (PET) | “off-on” signaling chemical sensor | 86 |
| 6 | 60 | F- | Colourimetric | order of association constants: F-≫ AcO- ≫ Cl-, Br-, I-. Can also be used for acetate | 13 |
| 7 | 61 | F- | Potentiometric/ISE | also binds Cl- , H2PO4-, SO42- | 88 |
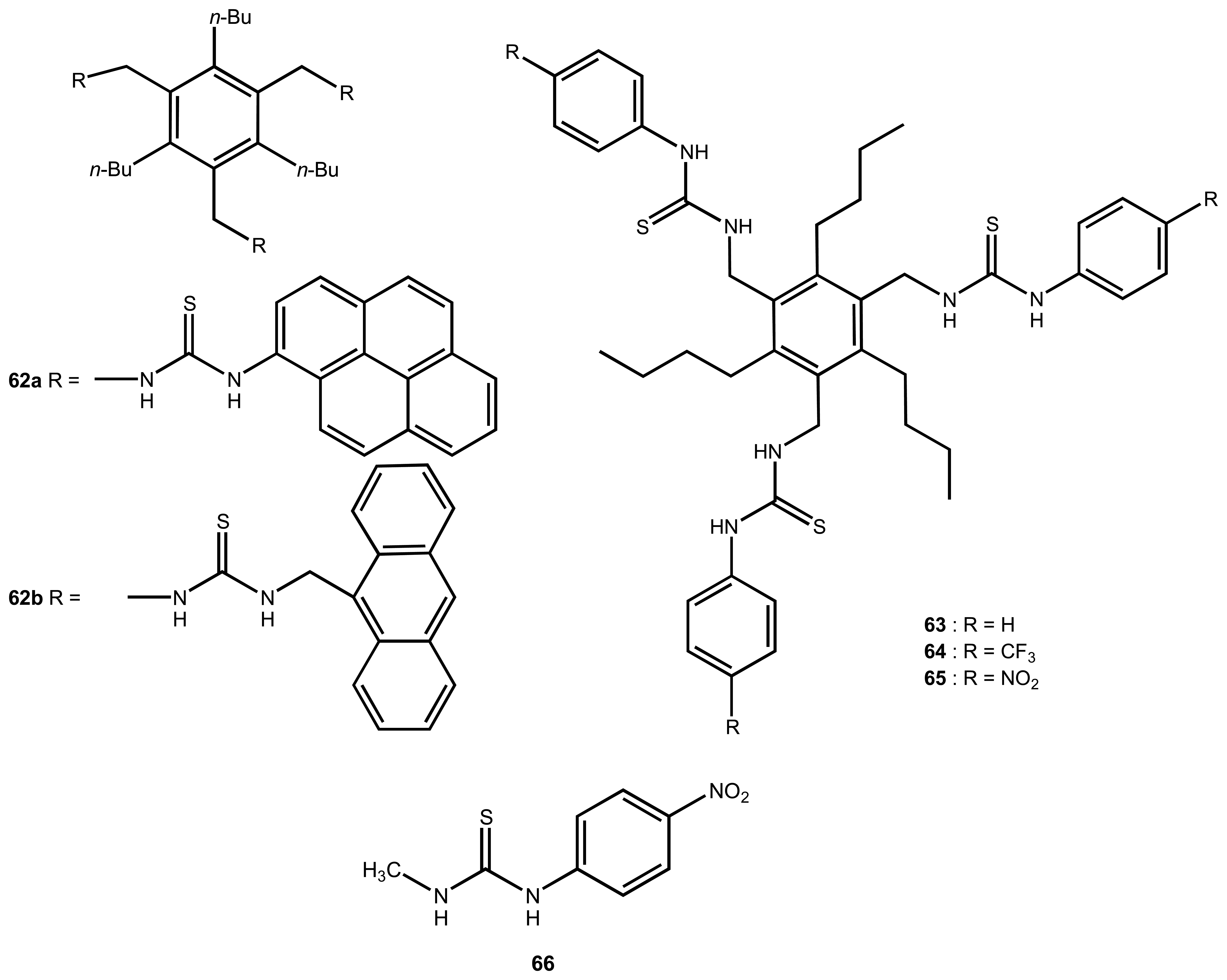
| 8 | 62a | H2PO4- | Fluorescence | interference: AcO-, Cl- | 92 |
| 9 | 62b | H2PO4- | Fluorescence | Order of fluorescence: H2PO4- > AcO- > Cl- | 92 |
| 10 | 63-65 | H2PO4- | Potentiometric/ISE | also binds CH3COO- in CH3CN | 10 |
| 11 | 67a | H2PO4- | Fluorescence | 99 | |
| 12 | 69, 70 | inositol-triphosphate (IP3) | Fluorescence | displacement assay using 68 | 53 |
| 13 | 71 | glucose-6-phosphate | Colourimetric/ Absorbance | displacement assay using 68 | 33 |
| 14 | 72 | H2PO4- | 1H-NMR titration | - | 97 |
| 15 | 73 | HSO4- | 1H-NMR titration | also binds H2PO4- | 97 |
| 16 | 74 | H2PO4- | Colourimetric/ Absorbance | - | 98 |
| 17 | 75 | H2PO4- | Fluorescence | - | 99 |
| 18 | Fe(III)-76 | H2PO4- | Potentiometric/ISE | also binds Cl-, HSO4- | 101 |
| 20 | Cu(II)-77 | H2PO4- | Potentiometric/ISE | - | 103 |
| 21 | Cu(II)-78 | H2PO4- | Colourimetric | displacement assay using 5 (6) carboxy-fluorescein | 104 |
| 22 | 79, 80 | HSO4- | Potentiometric/ISE | 79 shows anti-Hofmeister | 105 |
| 23 | 81 | HSO4- | Calorimetric | also binds H2PO4- | 106 |
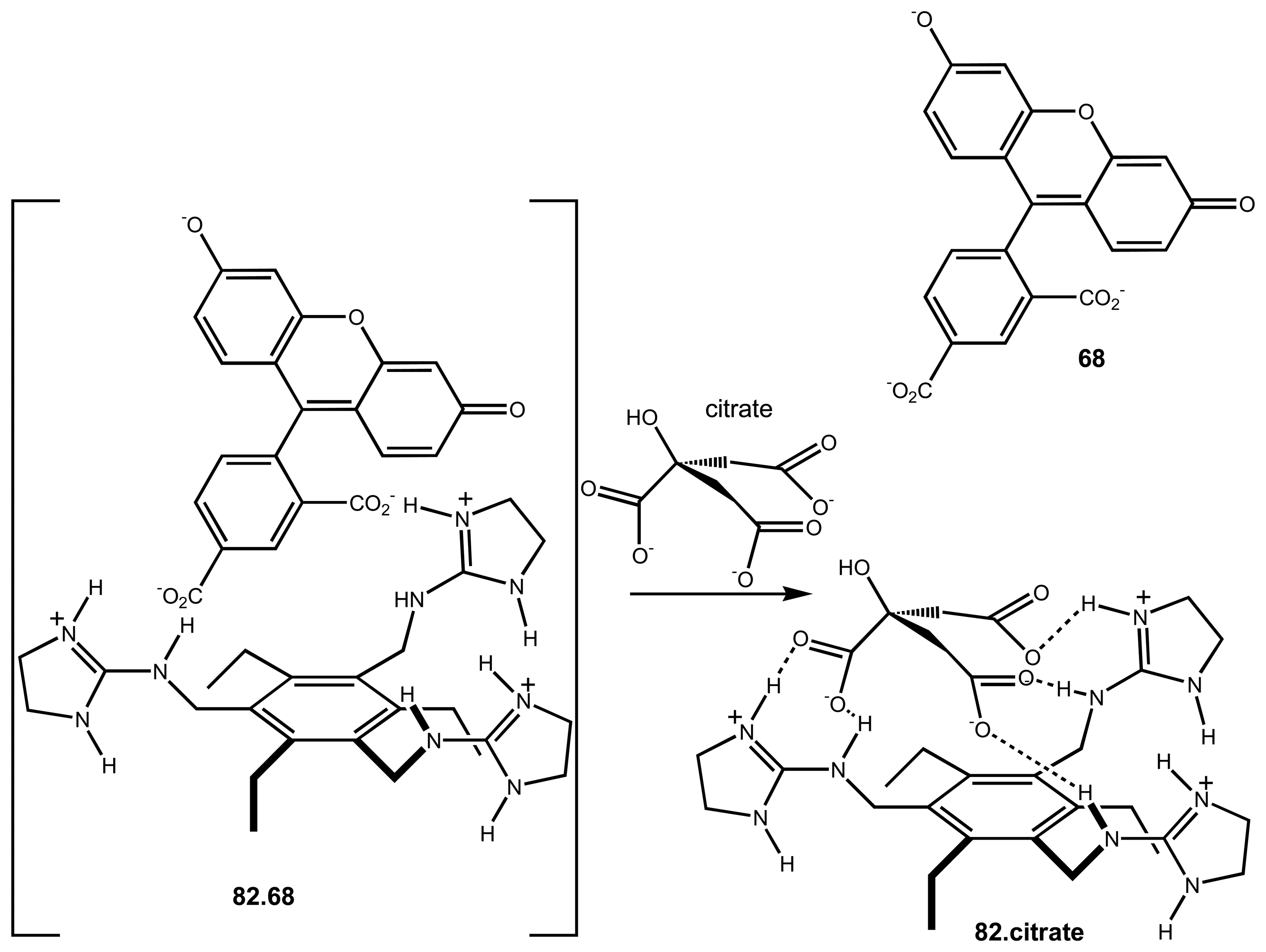
| 24 | 82 | Citrate | Fluorescence or Absorbance | displacement assay using 68 | 77 |
| 25 | Cu(II)-83 | Citrate | Fluorescence | baverage samples | 78, 118 |
| 26 | 85 | Tartrate | Colourimetric | addition of alizarin; also binds with malate | 55, 108 |
| 27 | 86 | Tartrate | Colourimetric | addition of bromopyrogallol or pyrocatechol violet for greater affinity over malate | 108 |
| 28 | 87 | Citrate | Fluorescence/ Absorbance Colourimetric | displacement assay using 68. | 109 |
| addition of xylenol orange or methylthymol blue | 110 | ||||
| 29 | 88 | Citrate | Naked eye/ Fluorescence | displacement assay using 68 no interference from malate or tartrate | 12 |
| 30 | 89-91 | CO32- | Potentiometric/ISE | 89a also binds salicylate | 9 |
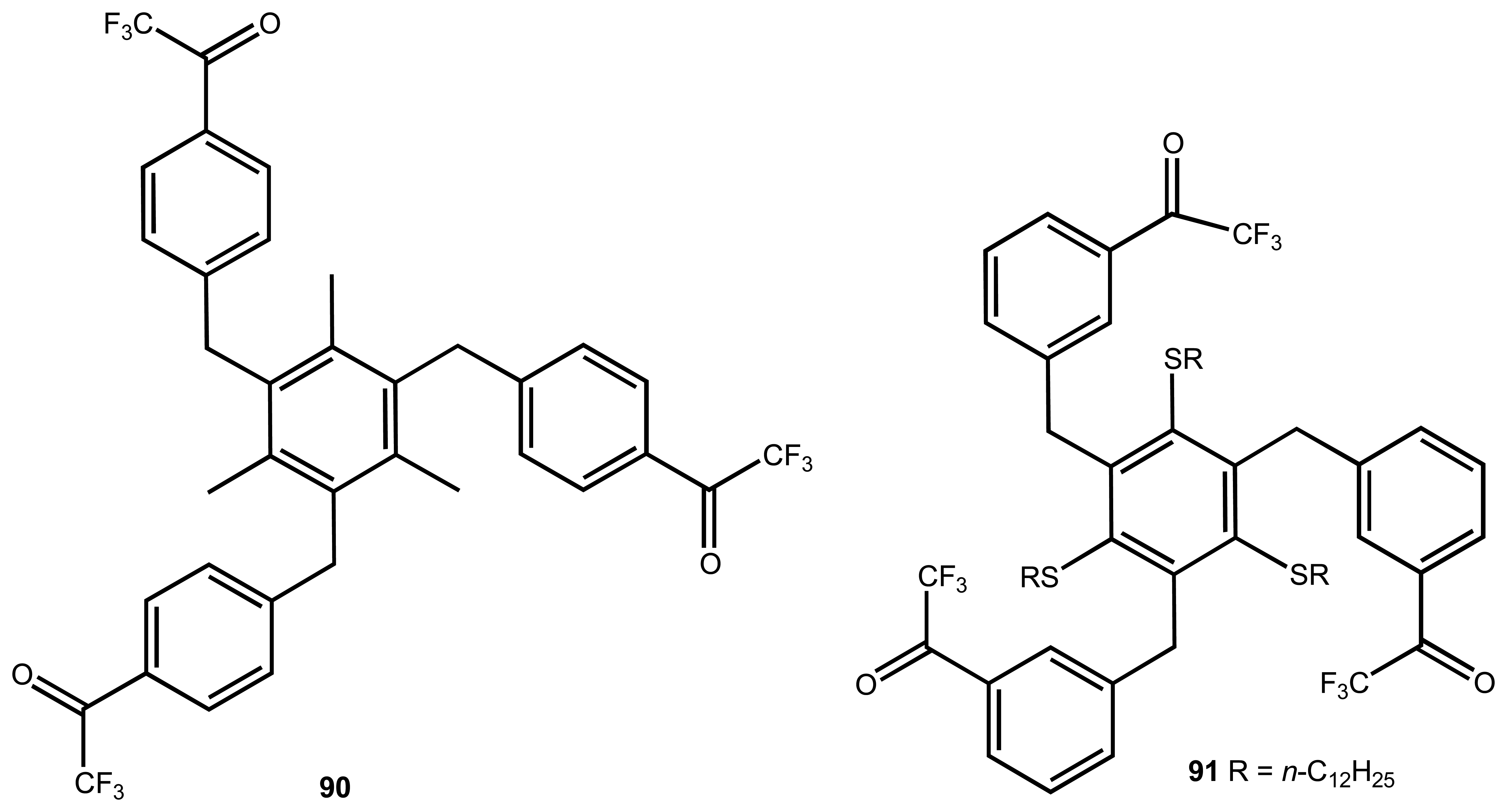
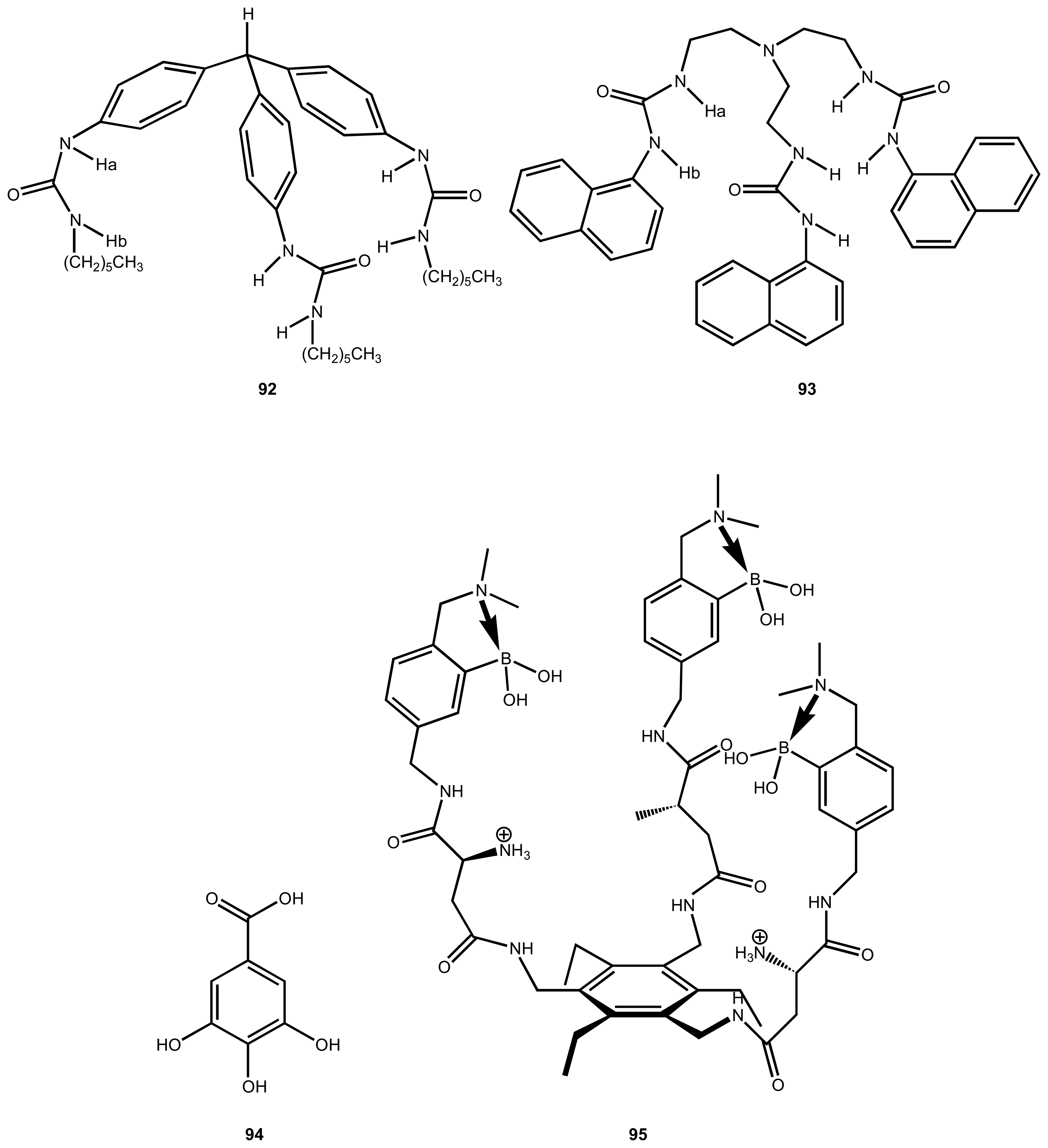
| 31 | 92, 93 | Carboxylate | Luminescence | - | 18 |
| 32 | 94 | gallate | Colourimetric | displacement assay using pyrocatechol violet | 114 |
| 33 | 95 | Heparin | Colourimetric | addition of pyrocatechol violet | 115 |
| 34 | 96-99 | ATP, ADP and AMP | Calorimetric | - | 116 |
| 35 | Zn(II)-100 | tryptophan | Potentiometric/ Fluorimetric | also binds phenylalanine | 119 |
| 36 | Zn(II)-101 | aromatic carboxylates | Fluorescence | aliphatic carboxylates and Cl-, NO3-, ClO4- did not interfere | 120 |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kuswandi, B.; N/a, N.; Verboom, W.; Reinhoudt, D.N. Tripodal Receptors for Cation and Anion Sensors. Sensors 2006, 6, 978-1017. https://doi.org/10.3390/s6080978
Kuswandi B, N/a N, Verboom W, Reinhoudt DN. Tripodal Receptors for Cation and Anion Sensors. Sensors. 2006; 6(8):978-1017. https://doi.org/10.3390/s6080978
Chicago/Turabian StyleKuswandi, Bambang, Nuriman N/a, Willem Verboom, and David N. Reinhoudt. 2006. "Tripodal Receptors for Cation and Anion Sensors" Sensors 6, no. 8: 978-1017. https://doi.org/10.3390/s6080978




