A H2O2 Biosensor Based on Immobilization of HorseradishPeroxidase in a Gelatine Network Matrix
Abstract
:Introduction
2. Experimental
2.1. Reagents
2.2. Apparatus and measurements
2.3. Preparation of biosensor
3. Results and Discussion
3.1. Electrochemical studies
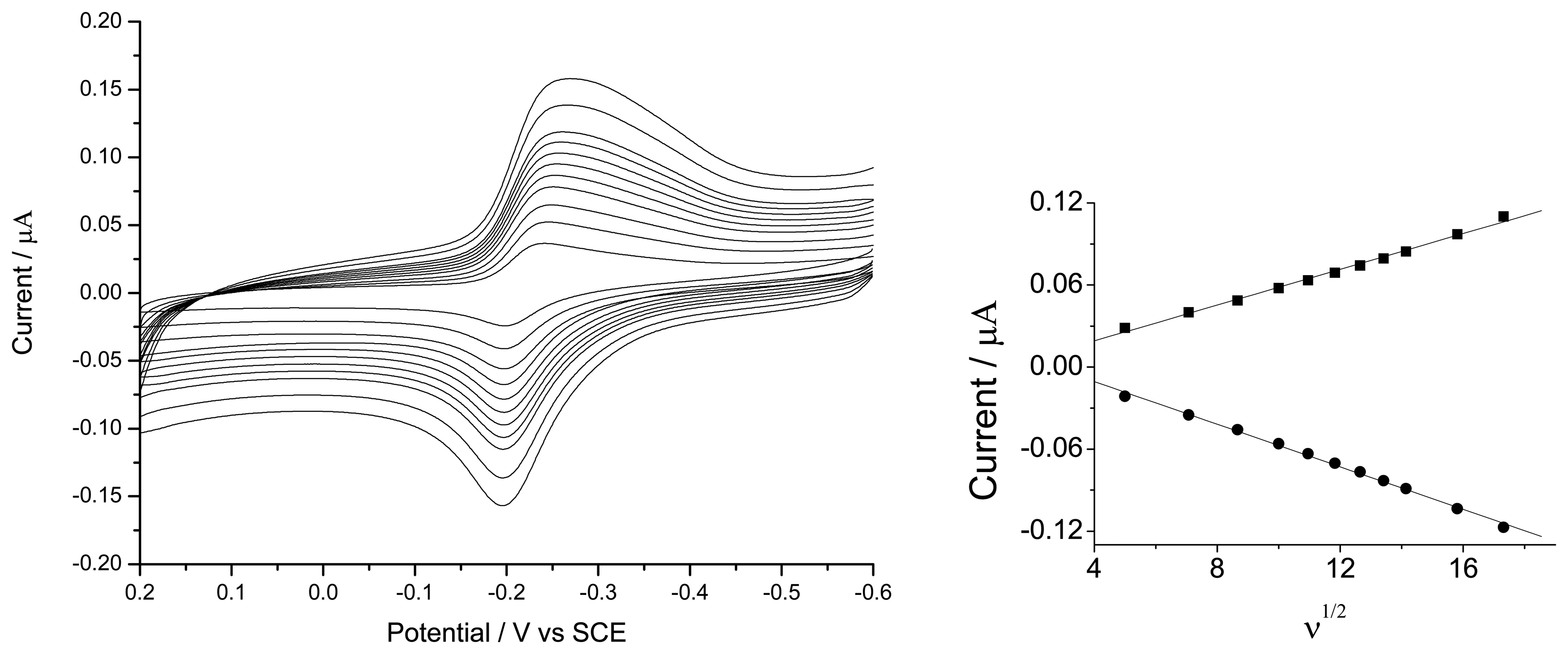
3.1.1. Electrochemical Characteristics of the Coimmobilization of HRP in Gelatine Membrance Modified Au Electrode
3.1.2. Electrochemical Response to Hydrogen Peroxide
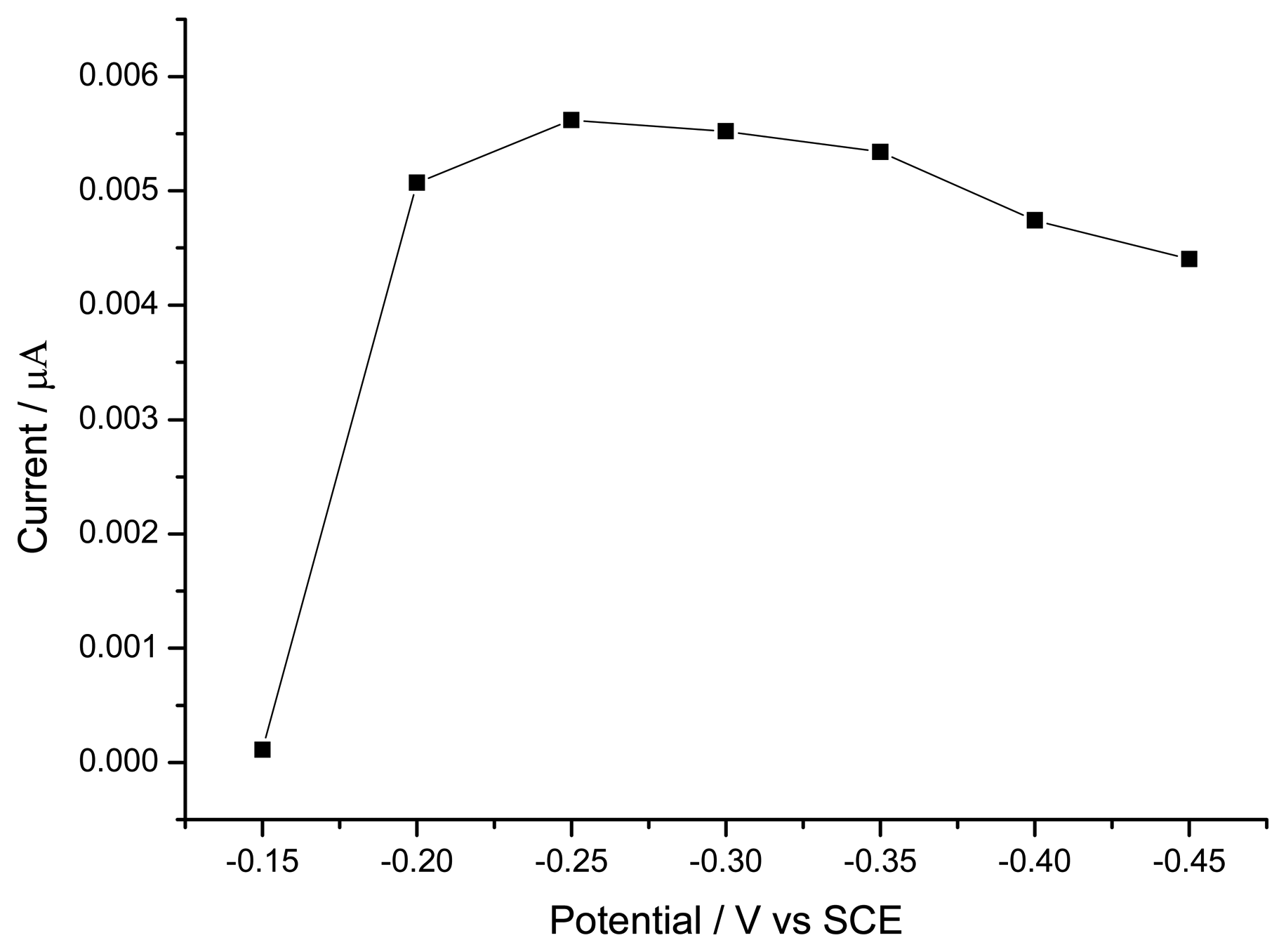
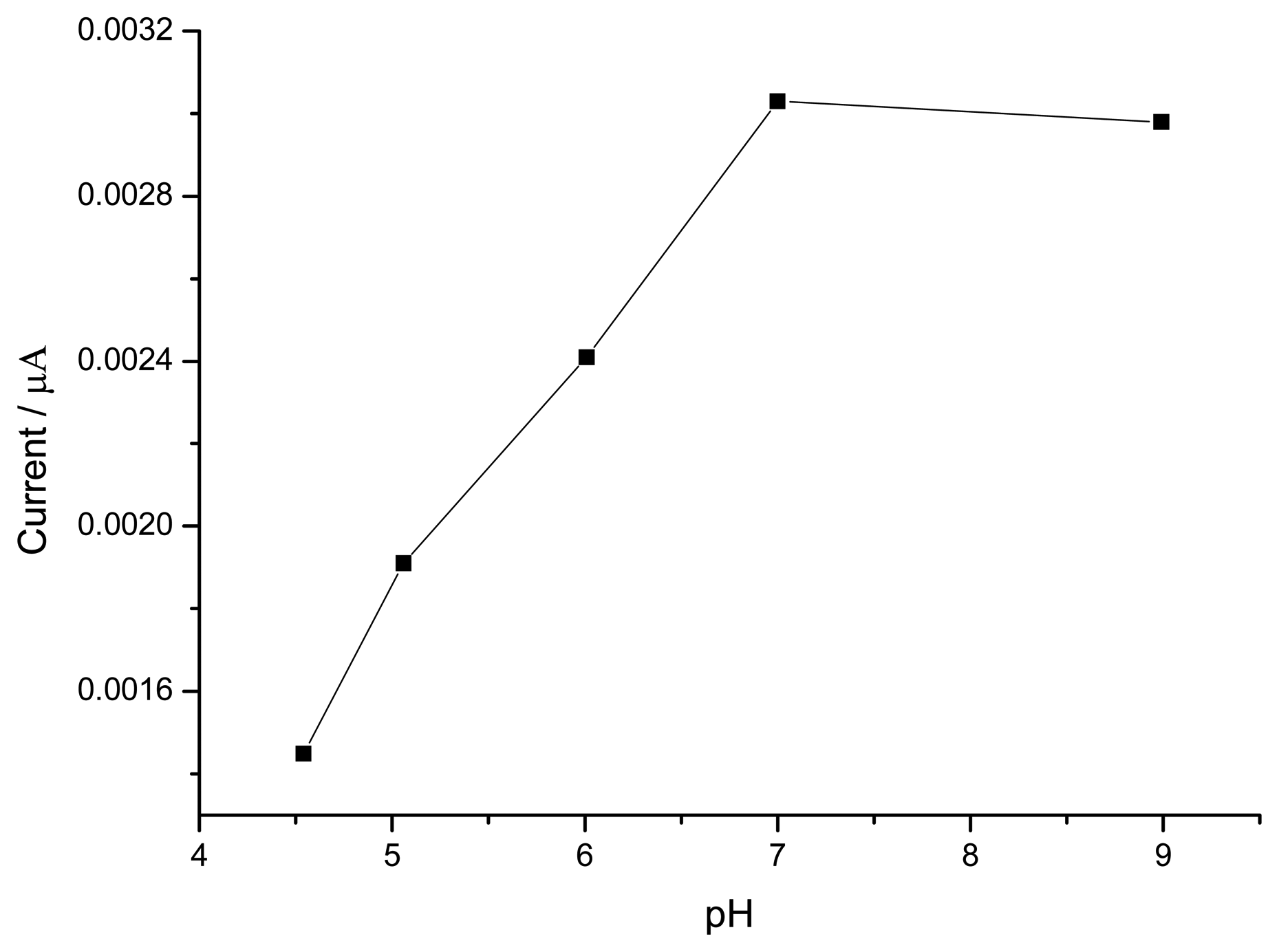
3.1.3. Optimization of Hydrogen Peroxide Monitoring
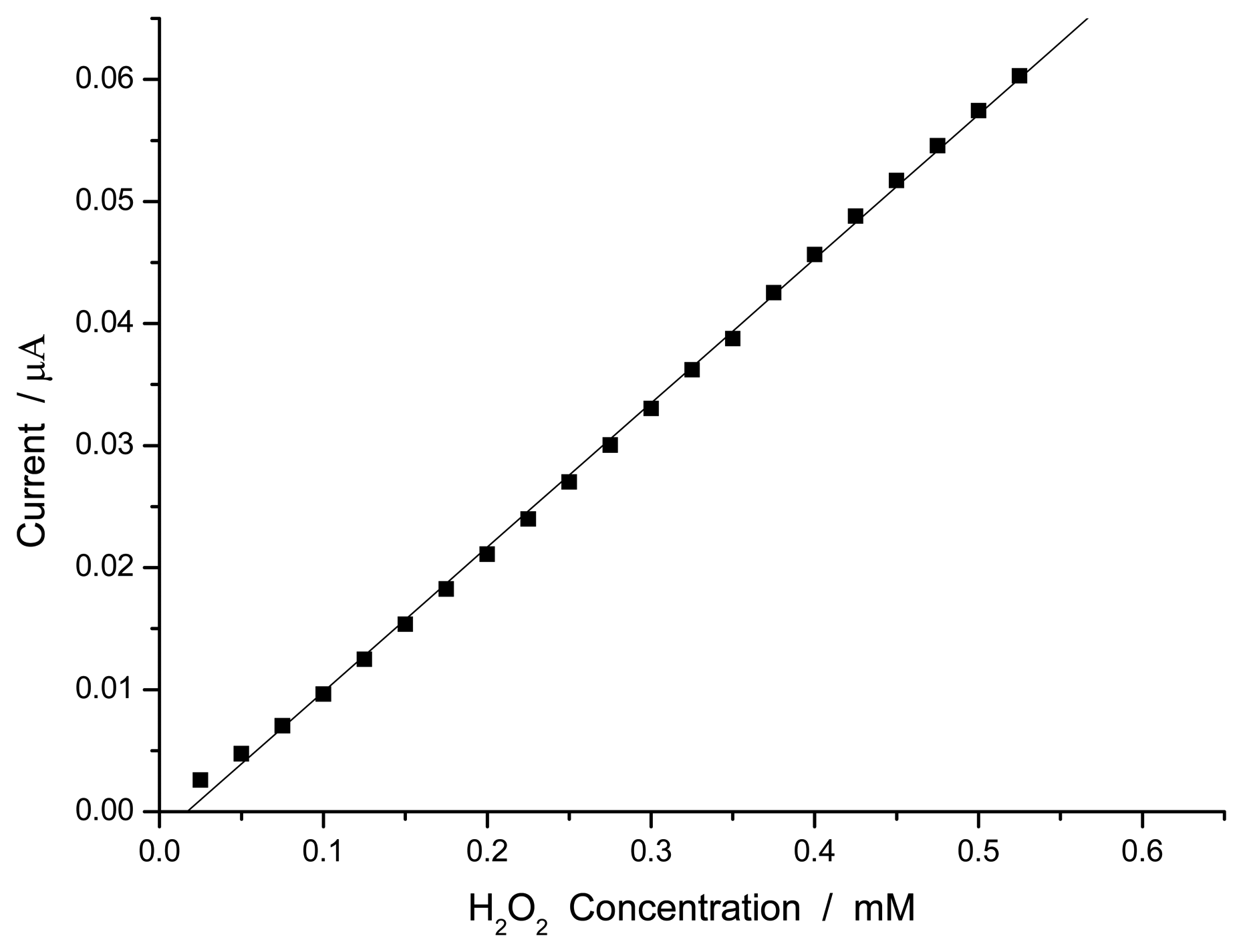
3.1.4. Steady-State Amperometric Response to Hydrogen Peroxide
Conclusions
Acknowledgments
References
- Amalvy, J.I.; Percy, M.J.; Armes, S.P. Langmuir 2001, 17, 4770.
- Aoki, K.; Kaneko, H. J. Electroanal. Chem. 1988, 247, 17.
- Arvand, M.; Sohrabnezhad, S.; Mousavi, M.F.; Shamsipur, M.; Zanjanchi, M.A. Anal. Chim. Acta 2003, 491, 193.
- Miao, Y.; Tan, S.N. Analyst 2000, 125, 1591.
- Zhou, G.; Wang, G.; Xu, J.; Chen, H. Sensor & Actuators B 2002, 81, 334.
- Jouenne, T.; Tresse, O.; Junter, G.A. FEMS Microbiol. Lett. 1994, 119, 237.
- Martinsen, A.; Storro, I.; Skjak-Brack, G. Biotechnol. Bioeng. 1992, 39, 186.
- Tziboula, A.; Horne, D.S. Colloids Surface B 1999, 12, 299.
- John, S.A.; Ramaraj, R. J. Electroanal. Chem. 2004, 561, 119.
- Kong, Y.T.; Boopathi, M.; Shim, Y.B. Biosens. Bioelectron. 2003, 19, 227.
- Tang, J.L.; Wang, B.Q.; Wu, Z.Y.; Han, X.J.; Dong, S.J.; Wang, E.K. Biosens. Bioelectron. 2003, 18, 867.
- Chen, X.; Zhang, J.; Wang, B.; Cheng, G.; Dong, S. Anal. Chim. Acta 2001, 434, 255.
- Segtnan, V.H.; Kvaal, K.; Rukke, E.O.; Schüller, R.B.; Isaksson, T. Food Hydrocolloid 2003, 17, 585.
- Dias, S.L.P.; Fujiwara, S.T.; Gushikem, Y.; Bruns, R.E. J. Electroanal. Chem. 2002, 531, 141.
- Zaitseva, G.; Gushike, Y.; Ribeiro, E.S.; Rosatto, S.S. Electrochim. Acta 2002, 47, 1469.
- Maehly, A.C. Plant peroxidases: Methods in Enzymology; Vol. 11, Academic Press: New York, 1995; p. p.807. [Google Scholar]


© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yao, H.; Li, N.; Wei, Y.-L.; Zhu, J.-J. A H2O2 Biosensor Based on Immobilization of HorseradishPeroxidase in a Gelatine Network Matrix. Sensors 2005, 5, 277-283. https://doi.org/10.3390/s5040277
Yao H, Li N, Wei Y-L, Zhu J-J. A H2O2 Biosensor Based on Immobilization of HorseradishPeroxidase in a Gelatine Network Matrix. Sensors. 2005; 5(4):277-283. https://doi.org/10.3390/s5040277
Chicago/Turabian StyleYao, Hui, Nan Li, Yan-Li Wei, and Jun-Jie Zhu. 2005. "A H2O2 Biosensor Based on Immobilization of HorseradishPeroxidase in a Gelatine Network Matrix" Sensors 5, no. 4: 277-283. https://doi.org/10.3390/s5040277



