Brain Tissue Oxygen: In Vivo Monitoring with Carbon Paste Electrodes
Abstract
:Introduction
Experimental
Reagents
Working Electrode Preparation
Voltammetric Techniques
Surgical Procedures
Experimental Conditions
Instrumentation and Software
Results and Discussion
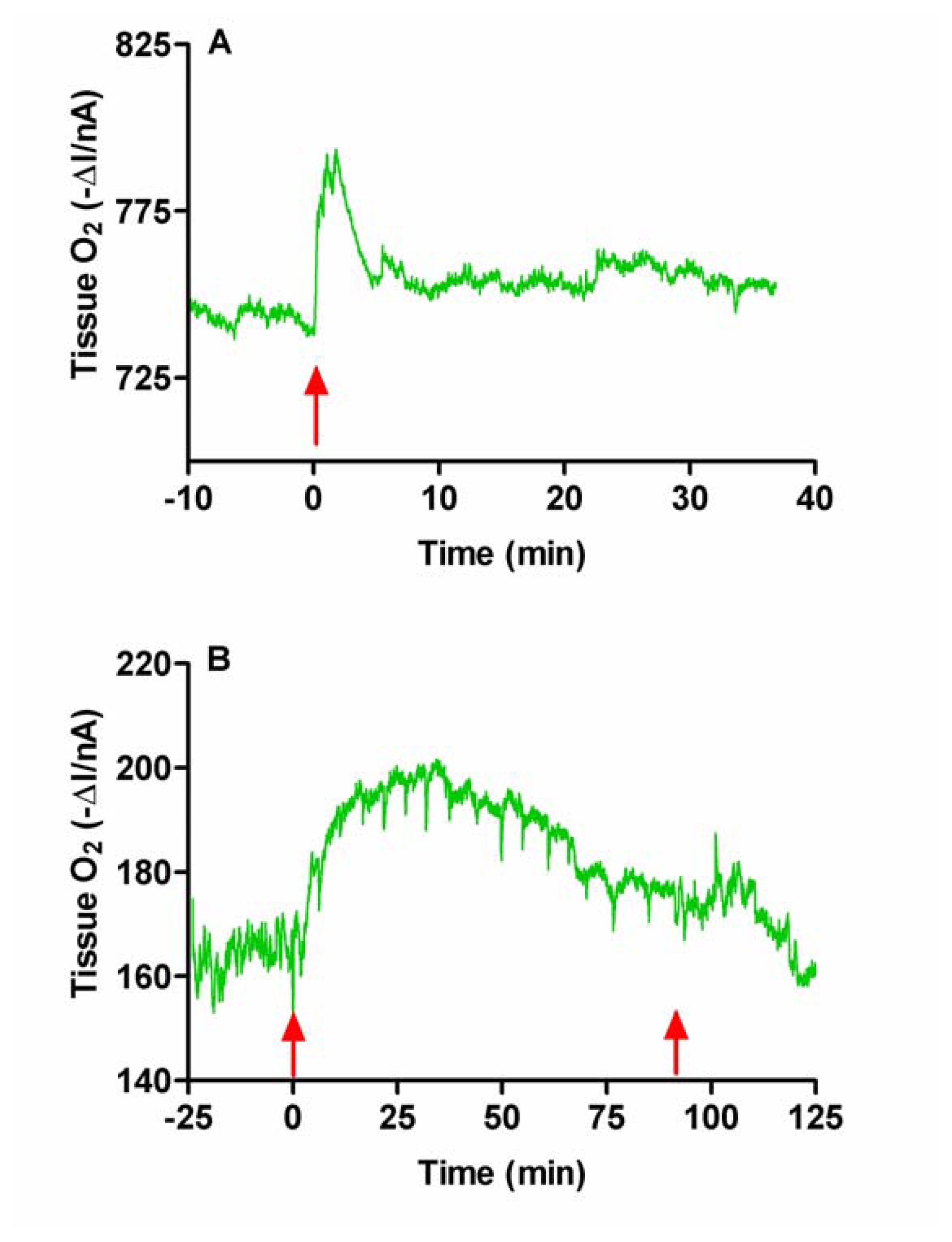
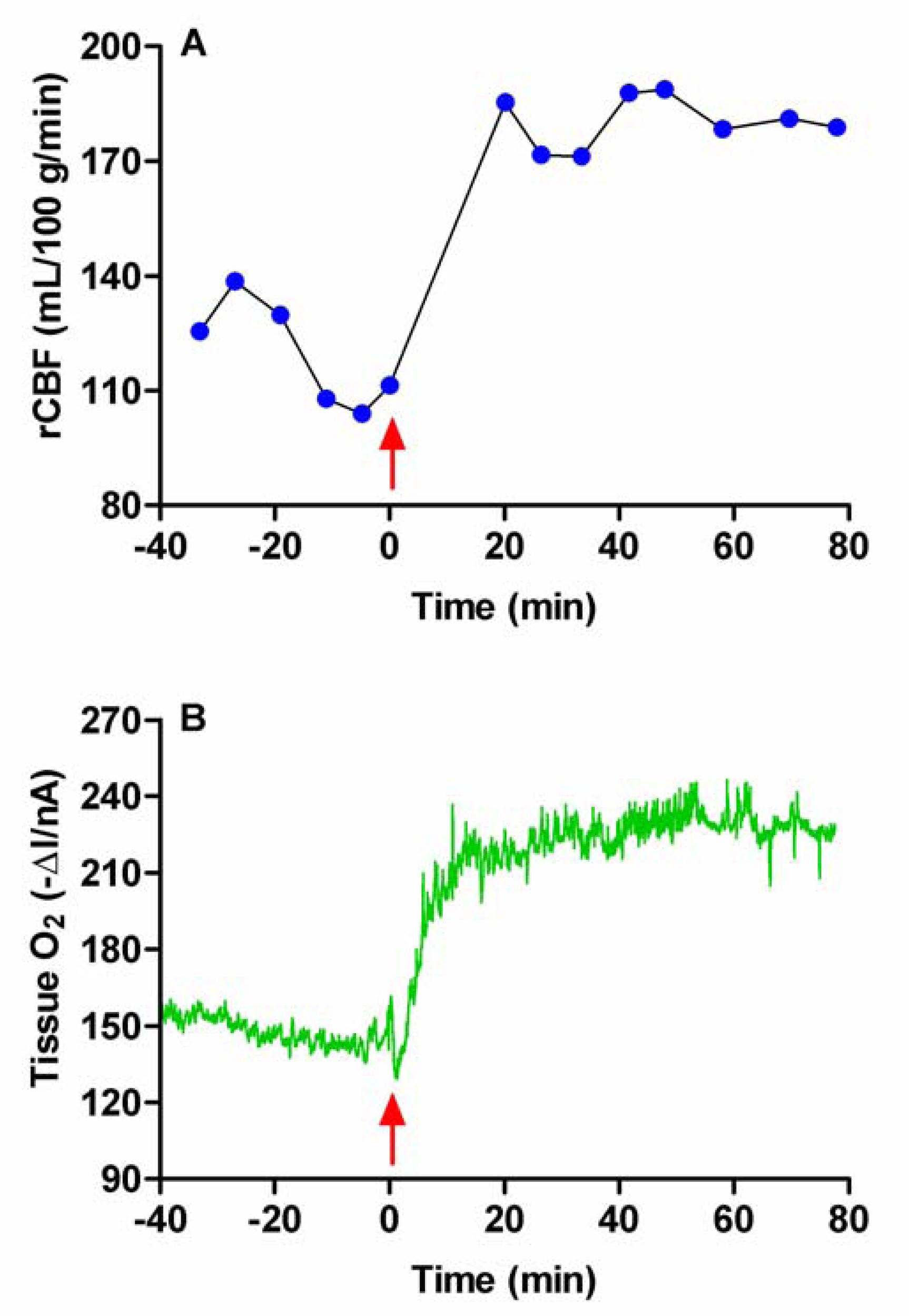
Hypoxia and Hyperoxia
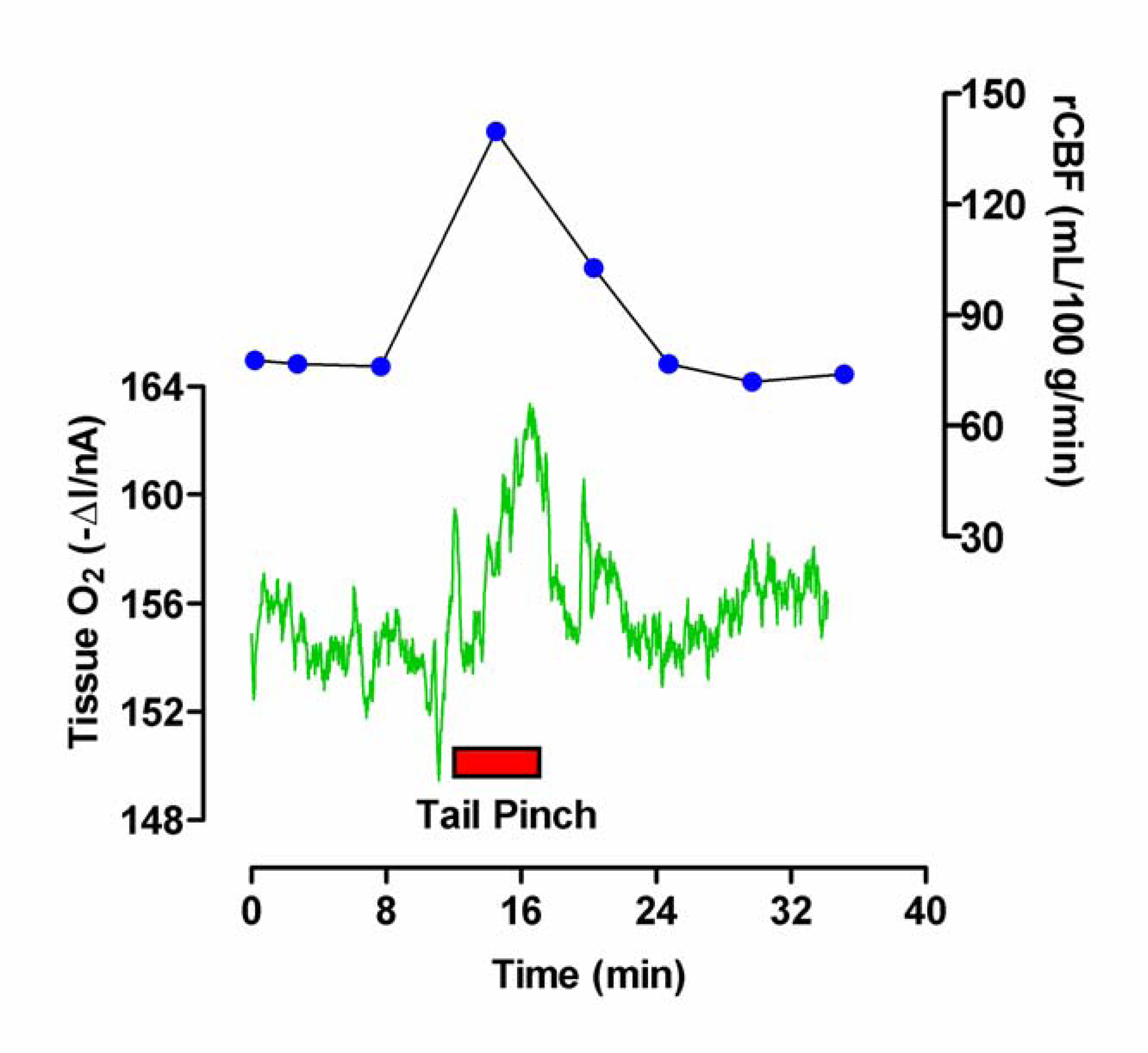
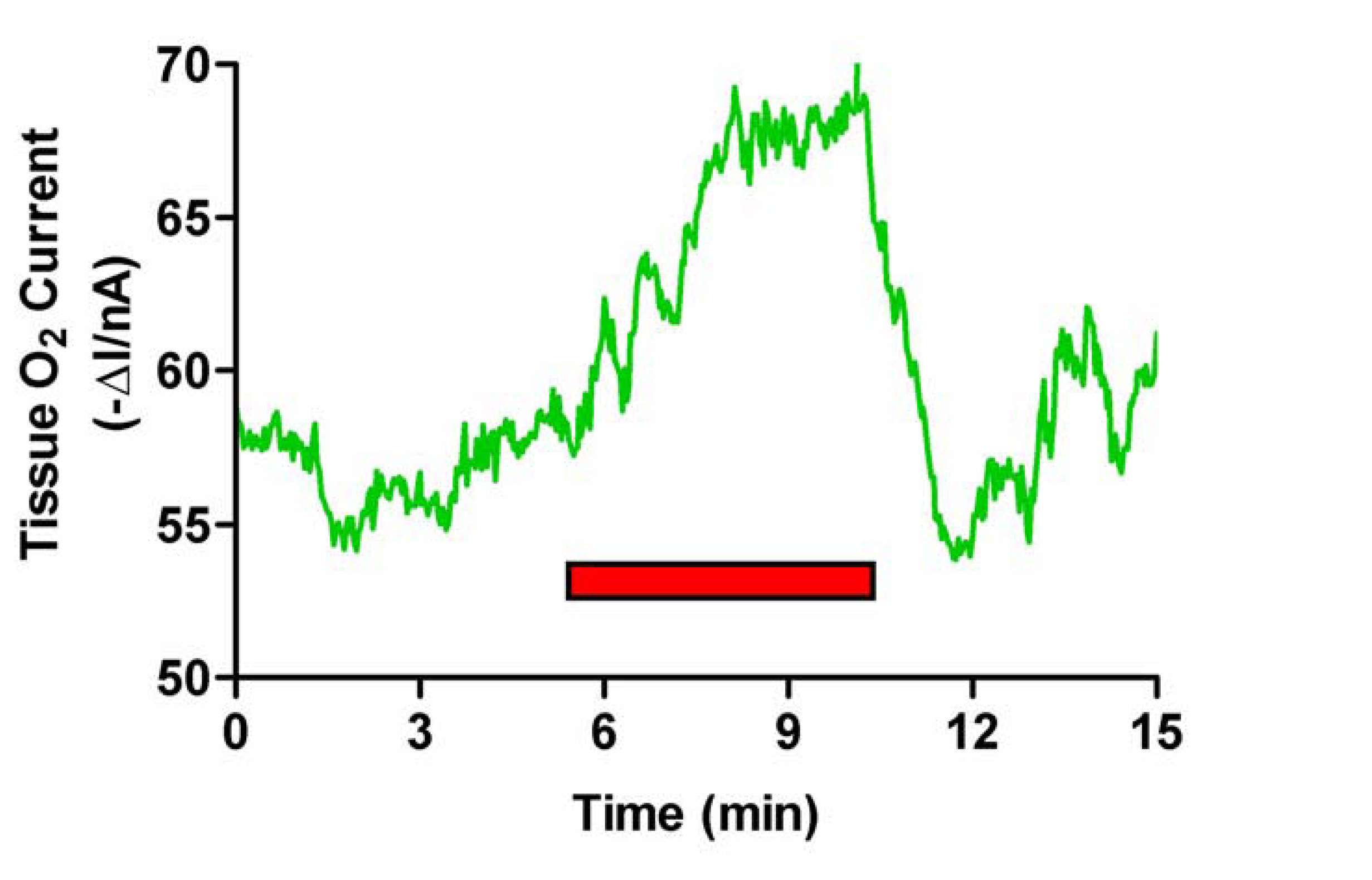
Neuronal Activation
Saline Injection and Chloral Hydrate Anaesthesia
The Effect of Acetazolamide
Conclusions
Acknowledgments
References
- Clark, L.C., Jr.; Misrahy, G.; Fox, R.P. Chronically implanted polarographic electrodes. J. Appl. Physiol. 1958, 13, 85–91. [Google Scholar]
- Clark, L.C., Jr.; Lyons, C. Studies of a glassy carbon electrode for brain polarography with observations on the effect of carbonic anhydrase inhibition. Ala. J. Med. Sci. 1965, 2, 353–359. [Google Scholar]
- Clark, L.C., Jr.; Clark, E.W. Epicardial oxygen measured with a pyrolytic graphite electrode. Ala. J. Med. Sci. 1964, 1, 142–148. [Google Scholar]
- Lowry, J.P.; Miele, M.; O'Neill, R.D.; Boutelle, M.G.; Fillenz, M. An amperometric glucose-oxidase/poly(o-phenylenediamine) biosensor for monitoring brain extracellular glucose: in vivo characterisation in the striatum of freely-moving rats. J. Neurosci. Methods 1998, 79, 65–74. [Google Scholar]
- Luebbers, D.W. Oxygen electrodes and optodes and their application in vivo. Adv. Exp. Med. Biol. 1996, 388, 13–34. [Google Scholar]
- Dittmar, A.; Mangin, S.; Ruban, C.; Newman, W.H.; Bowman, H.F.; Dupuis, V.; Delhomme, G.; Shram, N.F.; Cespuglio, R.; Jaffrezic-Renault, N.; Roussel, P.; Barbier, D.; Martelet, C. In vivo and in vitro evaluation of specially designed old and carbon fiber oxygen microelectrodes for living tissues. Sensor. Actuator. B - Chem. 1997, 44, 316–320. [Google Scholar]
- Jedlinska, B.; Mellstroem, A.; Hartmann, M.; Joensson, K. Comparison of tissue oxygen-tension measurements by different devices. An experimental study in pigs. Scand. J. Clin. Lab. Invest. 1998, 58, 63–71. [Google Scholar]
- McCreery, D.B.; Agnew, W.F.; Bullara, L.A.; Yuen, T.G. Partial pressure of oxygen in brain and peripheral nerve during damaging electrical stimulation. J. Biomed. Eng. 1990, 12, 309–315. [Google Scholar]
- Kennedy, R.T.; Jones, S.R.; Wightman, R.M. Simultaneous measurement of oxygen and dopamine: Coupling of oxygen consumption and neurotransmission. Neuroscience 1992, 47, 603–612. [Google Scholar]
- Nair, P.K.; Buerk, D.G.; Halsey, J.H., Jr. Comparison of oxygen metabolism and tissue pO2 in cortex and hippocampus. Stroke 1987, 18, 616–622. [Google Scholar]
- Baumgartl, H.; Heinrich, U.; Lubbers, D.W. Oxygen supply of the blood-free perfused guinea-pig brain in normo- and hypothermia measured by the local distribution of oxygen pressure. Pflugers Arch. 1989, 414, 228–234. [Google Scholar]
- Murr, R.; Berger, S.; Schuerer, L.; Peter, K.; Baethmann, A. A novel, remote-controlled suspension device for brain tissue PO2 measurements with multiwire surface electrodes. Pflugers Arch. 1994, 426, 348–350. [Google Scholar]
- Luebbers, D.W.; Baumgaertl, H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997, 51, 372–380. [Google Scholar]
- Zauner, A.; Bullock, R.; Di, X.; Young, H.F. Brain oxygen, CO2, pH, and temperature monitoring: Evaluation in the feline brain. Neurosurgery 1995, 37, 1168–1176. [Google Scholar]
- Kayama, T.; Yoshimoto, T.; Fujimoto, S.; Sakurai, Y. Intratumoral oxygen pressure in malignant brain tumor. J. Neurosurg. 1991, 74, 66–69. [Google Scholar]
- Hitchman, M.L. Measurement of Dissolved Oxygen; John Wiley: New York, 1978. [Google Scholar]
- Paliteiro, C.; Hamnett, A.; Goodenough, J.B. The electroreduction of oxygen at pyrolytic graphite. J. Electroanal. Chem. 1987, 233, 147–159. [Google Scholar]
- Zimmerman, J.B.; Wightman, R.M. Simultaneous electrochemical measurements of oxygen and dopamine in vivo. Anal. Chem. 1991, 63, 24–28. [Google Scholar]
- Lowry, J.P.; Boutelle, M.G.; O'Neill, R.D.; Fillenz, M. Characterization of carbon paste electrodes in vitro for simultaneous amperometric measurement of changes in oxygen and ascorbic acid concentrations in vivo. Analyst 1996, 121, 761–766. [Google Scholar]
- Venton, B.J.; Michael, D.J.; Wightman, R.M. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J. Neurochem. 2003, 84, 373–381. [Google Scholar]
- O'Neill, R.D.; Fillenz, M. Detection of homovanillic acid in vivo using microcomputer-controlled voltammetry: simultaneous monitoring of rat motor activity and striatal dopamine release. Neuroscience 1985, 14, 753–763. [Google Scholar]
- Zimmerman, J.B.; Kennedy, R.T.; Wightman, R.M. Evoked neuronal activity accompanied by transmitter release increases oxygen concentration in rat striatum in vivo but not in vitro. J. Cereb. Blood Flow Metab. 1992, 12, 629–637. [Google Scholar]
- Silver, I.A. Some observations on the cerebral cortex with an ultra-micro, membrane covered, oxygen electrode. Med. Electron. Biol. Engng 1965, 3, 377–387. [Google Scholar]
- O'Neill, R.D.; Grunewald, R.A.; Fillenz, M.; Albery, W.J. Linear sweep voltammetry with carbon paste electrodes in the rat striatum. Neuroscience 1982, 7, 1945–1954. [Google Scholar]
- Lowry, J.P.; Boutelle, M.G.; Fillenz, M. Measurement of brain tissue oxygen at a carbon paste electrode can serve as an index of increases in regional cerebral blood flow. J. Neurosci. Methods 1997, 71, 177–182. [Google Scholar]
- Lowry, J.P.; Fillenz, M. Real-time monitoring of brain energy metabolism in vivo using microelectrochemical sensors: The effects of anesthesia. Bioelectrochemistry 2001, 54, 39–47. [Google Scholar]
- O'Neill, R.D. Sensor-tissue interactions in neurochemical analysis with carbon paste electrodes in vivo. Analyst 1993, 118, 433–438. [Google Scholar]
- Kane, D.A.; O'Neill, R.D. Major differences in the behaviour of carbon paste and carbon fibre electrodes in a protein-lipid matrix: implications for voltammetry in vivo. Analyst 1998, 123, 2899–2903. [Google Scholar]
- Bourdillon, C.; Thomas, V.; Thomas, D. Electrochemical study of D-glucose oxidase autoinactivation. Enzyme Microb. Technol. 1982, 4, 175–180. [Google Scholar]
- Zhang, Y.N.; Wilson, G.S. In vitro and in vivo evaluation of oxygen effects on a glucose oxidase based implantable glucose sensor. Anal. Chim. Acta 1993, 281, 513–520. [Google Scholar]
- Wolfensohn, S.; Lloyd, M. Handbook of Laboratory Animal Management and Welfare; Oxford University Press: Oxford, 1994. [Google Scholar]
- Lowry, J.P.; Fillenz, M. Evidence for uncoupling of oxygen and glucose utilization during neuronal activation in rat striatum. J. Physiol. (London) 1997, 498, 497–501. [Google Scholar]
- Fray, A.E.; Forsyth, R.J.; Boutelle, M.G.; Fillenz, M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: A microdialysis study. J. Physiol. (Lond) 1996, 496, 49–57. [Google Scholar]
- Morton, D.B.; Griffiths, P.H.M. Guidelines on the recognition of pain and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar]
- Fillenz, M.; Lowry, J.P. Studies of the source of glucose in the extracellular compartment of the rat brain. Dev. Neurosci. 1998, 20, 365–368. [Google Scholar]
- Antelman, S.M.; Szechtman, H.; Chin, P.; Fisher, A.E. Tail pinch-induced eating, gnawing and licking behavior in rats: dependence on the nigrostriatal dopamine system. Brain Res. 1975, 99, 319–337. [Google Scholar]
- Fellows, L.K.; Boutelle, M.G. Rapid changes in extracellular glucose levels and blood flow in the striatum of the freely moving rat. Brain Res. 1993, 604, 225–231. [Google Scholar]
- Ohata, M.; Fredericks, W.R.; Sundaram, U.; Rapoport, S.I. Effects of immobilization stress on regional cerebral blood flow in the conscious rat. J. Cereb. Blood Flow Metab. 1981, 1, 187–194. [Google Scholar]
- Ormonde, D.E.; O'Neill, R.D. The oxidation of ascorbic acid at carbon paste electrodes. Modified response following contact with surfactant, lipid and brain tissue. J. Electroanal. Chem. 1990, 279, 109–121. [Google Scholar]
- Feng, Z.C.; Roberts, E.L.; Sick, T.J.; Rosenthal, M. Depth profile of local oxygen tension and blood flow in rat cerebral cortex, white matter and hippocampus. Brain Res. 1988, 445, 280–288. [Google Scholar]
- Leniger-Follert, E.; Lübbers, D.W. Behavior of microflow and local Po2 of the brain cortex during and after electrical stimulation. Pflügers Arch. 1976, 366, 39–44. [Google Scholar]
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar]
- Kawamoto, T.; Hobara, T.; Kobayashi, H.; Iwamoto, S.; Saki, T.; Takano, T.; Miyazaki, Y. The metabolite ratio as a function of chloral hydrate dose and intracellular redox state in the perfused rat liver. Pharmacol. Toxicol. 1987, 60, 325–329. [Google Scholar]
- Tao, R.; Auerbach, S.B. Anesthetics block morphine-induced increases in serotonin release in rat CNS. Synapse 1994, 18, 307–314. [Google Scholar]
- Lovinger, D.M.; Zimmerman, S.A.; Levitin, M.; Jones, M.V.; Harrison, N.L. Trichloroethanol potentiates synaptic transmission mediated by γ-aminobutyric acidA receptors in hippocampal neurons. J. Pharmacol. Exp. Ther 1993, 264, 1097–1103. [Google Scholar]
- Fillenz, M.; Lowry, J.P. The relation between local cerebral blood flow and extracellular glucose concentration in rat striatum. Exp. Physiol. 1998, 83, 233–238. [Google Scholar]
- Vahabzadeh, A.; Fillenz, M. Comparison of stress-induced changes in noradrenergic and serotonergic neurons in the rat hippocampus using microdialysis. Eur. J. Neurosci. 1994, 6, 1205–1212. [Google Scholar]
- Chen, H.T.; Kandasamy, S.B. Effect of chloral hydrate on in vivo KCl-induced striatal dopamine release in the rat. Neurochem. Res. 1996, 21, 695–700. [Google Scholar]
- Hamilton, M.E.; Mele, A.; Pert, A. Striatal extracellular dopamine in conscious vs. anesthetized rats: Effects of chloral hydrate anesthetic on responses to drugs of different classes. Brain Res. 1992, 597, 1–7. [Google Scholar]
- Shiraishi, M.; Kamiyama, Y.; Huettemeier, P.C.; Benveniste, H. Extracellular glutamate and dopamine measured by microdialysis in the rat striatum during blockade of synaptic transmission in anesthetized and awake rats. Brain Res. 1997, 759, 221–227. [Google Scholar]
- Clemens, J.A.; Phebus, L.A. Changes in brain chemistry produced by dopaminergic agents: in vivo electrochemical monitoring reveals opposite changes in anaesthetized vs unanaesthetized rats. Brain Res. 1983, 267, 183–186. [Google Scholar]
- Petrinec, J.; Guadalupe, T.; Fumero, B.; Viejo, E.; Gonzalez-Mora, J. L.; Mas, M. Effects of different anaesthetics on striatal dopaminergic activity as assessed by in vivo voltammetry. In Monitoring Molecules in Neuroscience; Gonzalez-Mora, J. L., Borges, R., Mas, M., Eds.; University of La Laguna: Tenerife, 1996; pp. 293–294. [Google Scholar]
- Silver, I.A.; Erecinska, M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J. Neurosci. 1994, 14, 5068–5076. [Google Scholar]
- Netchiporouk, L.I.; Shram, N.F.; Jaffrezic-Renault, N.; Martelet, C.; Cespuglio, R. In vivo brain glucose measurements: differential normal pulse voltammetry with enzyme-modified carbon fiber microelectrodes. Anal. Chem. 1996, 68, 4358–4364. [Google Scholar]
- Shram, N.F.; Netchiporouk, L.I.; Martelet, C.; Jaffrezic-Renault, N.; Cespuglio, R. Brain glucose: Voltammetric determination in normal and hyperglycaemic rats using a glucose microsensor. NeuroReport 1997, 8, 1109–1112. [Google Scholar]
- Hu, Y.B.; Wilson, G.S. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J. Neurochem. 1997, 68, 1745–1752. [Google Scholar]
- Shram, N.F.; Netchiporouk, L.I.; Martelet, C.; Jaffrezic-Renault, N.; Bonnet, C.; Cespuglio, R. In vivo voltammetric detection of rat brain lactate with carbon fiber microelectrodes coated with lactate oxidase. Anal. Chem. 1998, 70, 2618–2622. [Google Scholar]
- Ikegami, Y.; Maeda, M.; Yokota, A.; Hayashida, Y. Cerebral extracellular lactate concentration and blood flow during chemical stimulation of the nucleus tractus solitarii in anesthetized rats. Brain Res. 1997, 758, 33–38. [Google Scholar]
- Garguilo, M.G.; Michael, A.C. Amperometric microsensors for monitoring choline in the extracellular fluid of brain. J. Neurosci. Methods 1996, 70, 73–82. [Google Scholar]
- Garguilo, M.G.; Michael, A.C. An enzyme-modified microelectrode that detects choline injected locally into brain tissue. J. Am. Chem. Soc. 1993, 115, 12218–12219. [Google Scholar]
- Garguilo, M.G.; Michael, A.C. Optimization of amperometric microsensors for monitoring choline in the extracellular fluid of brain tissue. Anal. Chim. Acta 1995, 307, 291–299. [Google Scholar]
- Garguilo, M.G.; Michael, A.C. Quantitation of choline in the extracellular fluid of brain tissue with amperometric microsensors. Anal. Chem. 1994, 66, 2621–2629. [Google Scholar]
- Hu, Y.; Mitchell, K.M.; Albahadily, F.N.; Michaelis, E.K.; Wilson, G.S. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994, 659, 117–125. [Google Scholar]
- Walker, M.C.; Galley, P.T.; Errington, M.L.; Shorvon, S.D.; Jefferys, J.G.R. Ascorbate and glutamate release in the rat hippocampus after perforant path stimulation: A “dialysis electrode” study. J. Neurochem. 1995, 65, 725–731. [Google Scholar]
- Asai, S.; Iribe, Y.; Kohno, T.; Ishikawa, K. Real time monitoring of biphasic glutamate release using dialysis electrode in rat acute brain ischemia. NeuroReport 1996, 7, 1092–1096. [Google Scholar]
- Kulagina, N.V.; Shankar, L.; Michael, A.C. Monitoring glutamate and ascorbate in the extracellular space of brain tissue with electrochemical microsensors. Anal. Chem 2000, 71, 5093–5100. [Google Scholar]
- Dixon, B.M.; Lowry, J.P.; O'Neill, R.D. Oxygen dependence of an enzyme/polymer biosensor for monitoring brain glucose in vivo. Journal of Neuroscience Methods 2002, 119, 135–142. [Google Scholar]

© 2005 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Bolger, F.B.; Lowry, J.P. Brain Tissue Oxygen: In Vivo Monitoring with Carbon Paste Electrodes. Sensors 2005, 5, 473-487. https://doi.org/10.3390/s5110473
Bolger FB, Lowry JP. Brain Tissue Oxygen: In Vivo Monitoring with Carbon Paste Electrodes. Sensors. 2005; 5(11):473-487. https://doi.org/10.3390/s5110473
Chicago/Turabian StyleBolger, Fiachra B., and John P. Lowry. 2005. "Brain Tissue Oxygen: In Vivo Monitoring with Carbon Paste Electrodes" Sensors 5, no. 11: 473-487. https://doi.org/10.3390/s5110473



