Integration of a Capacitive EIS Sensor into a FIA System for pH and Penicillin Determination
Abstract
:Introduction
Experimental
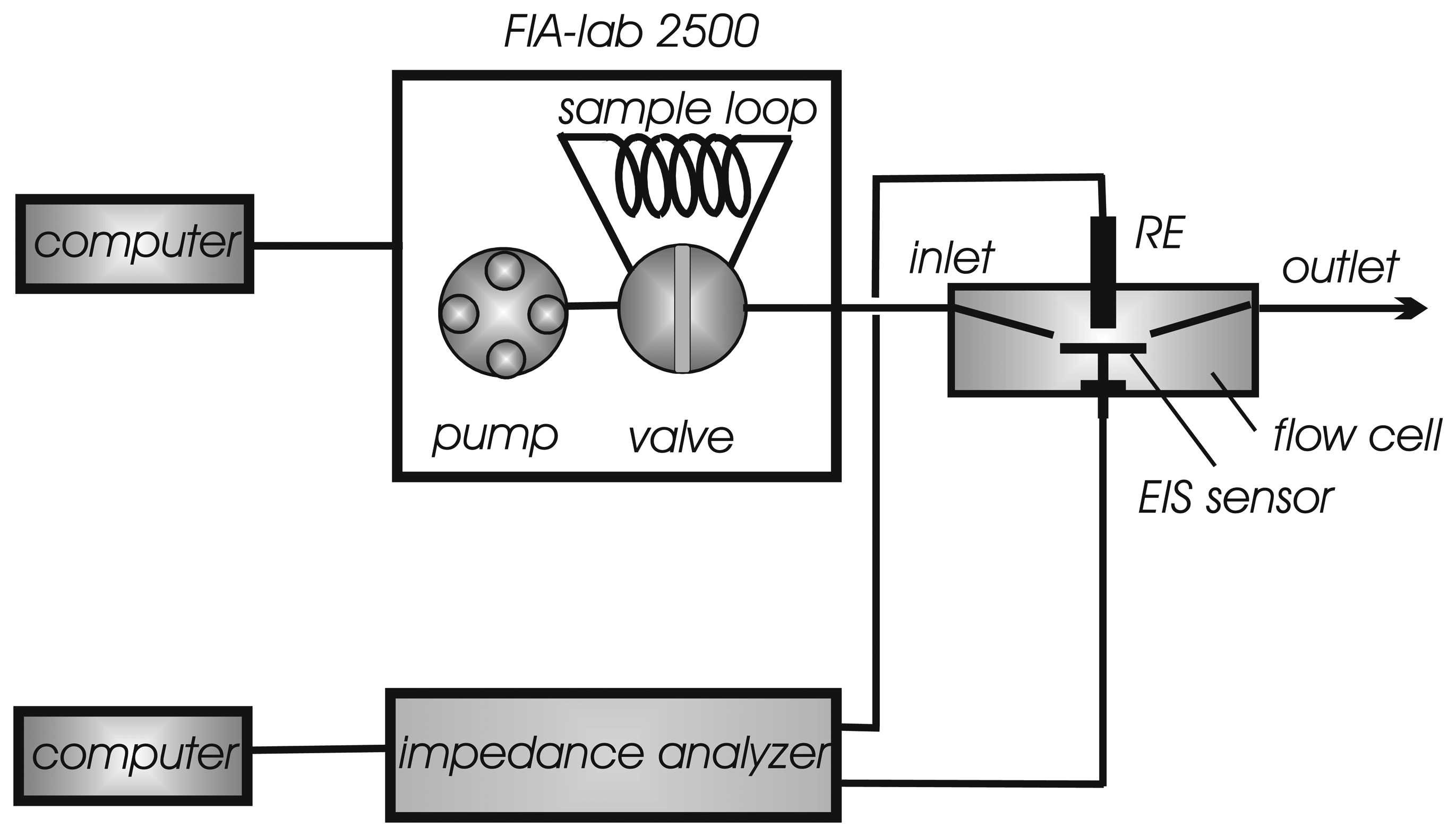
FIA system
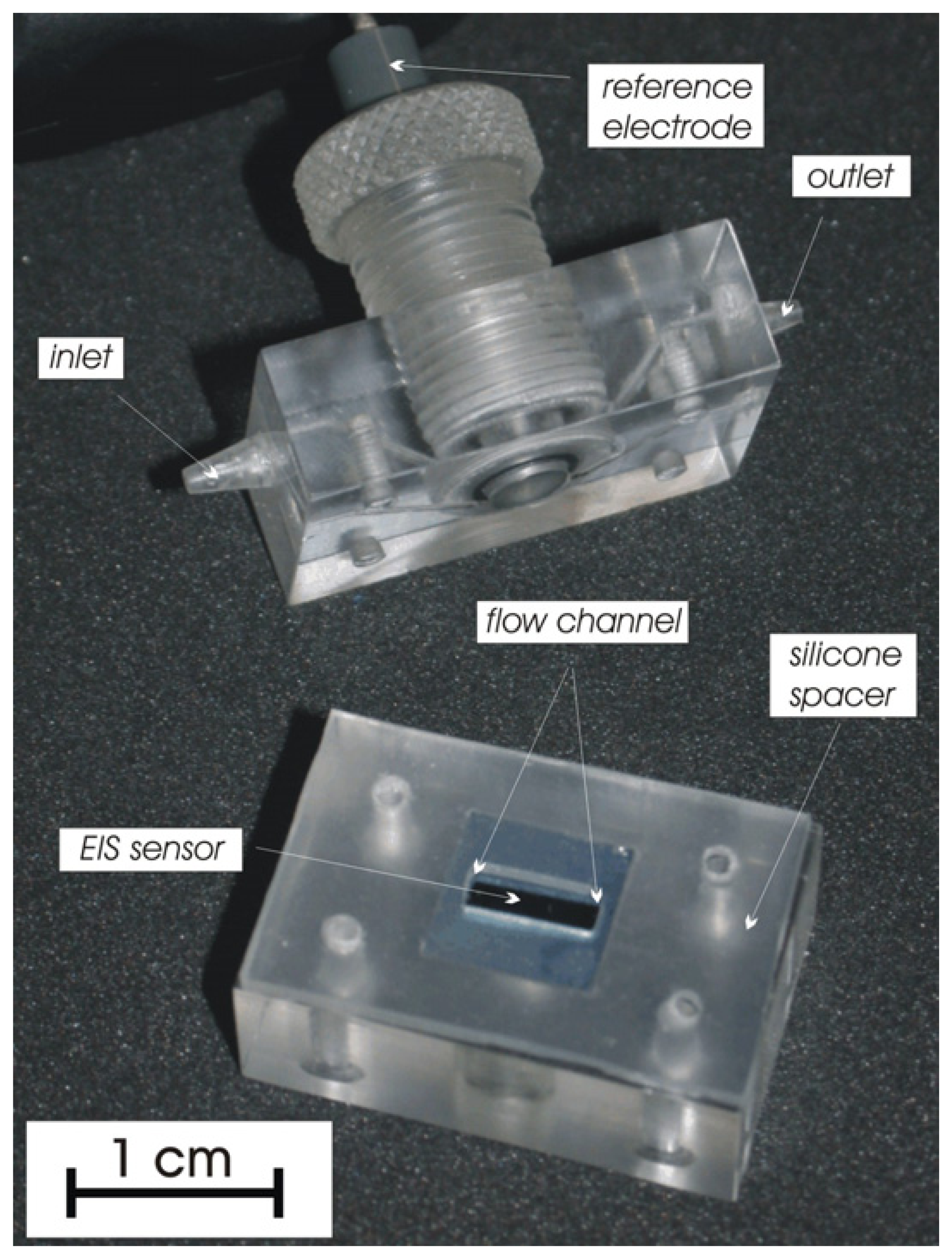
Flow-through cell
Sensor preparation
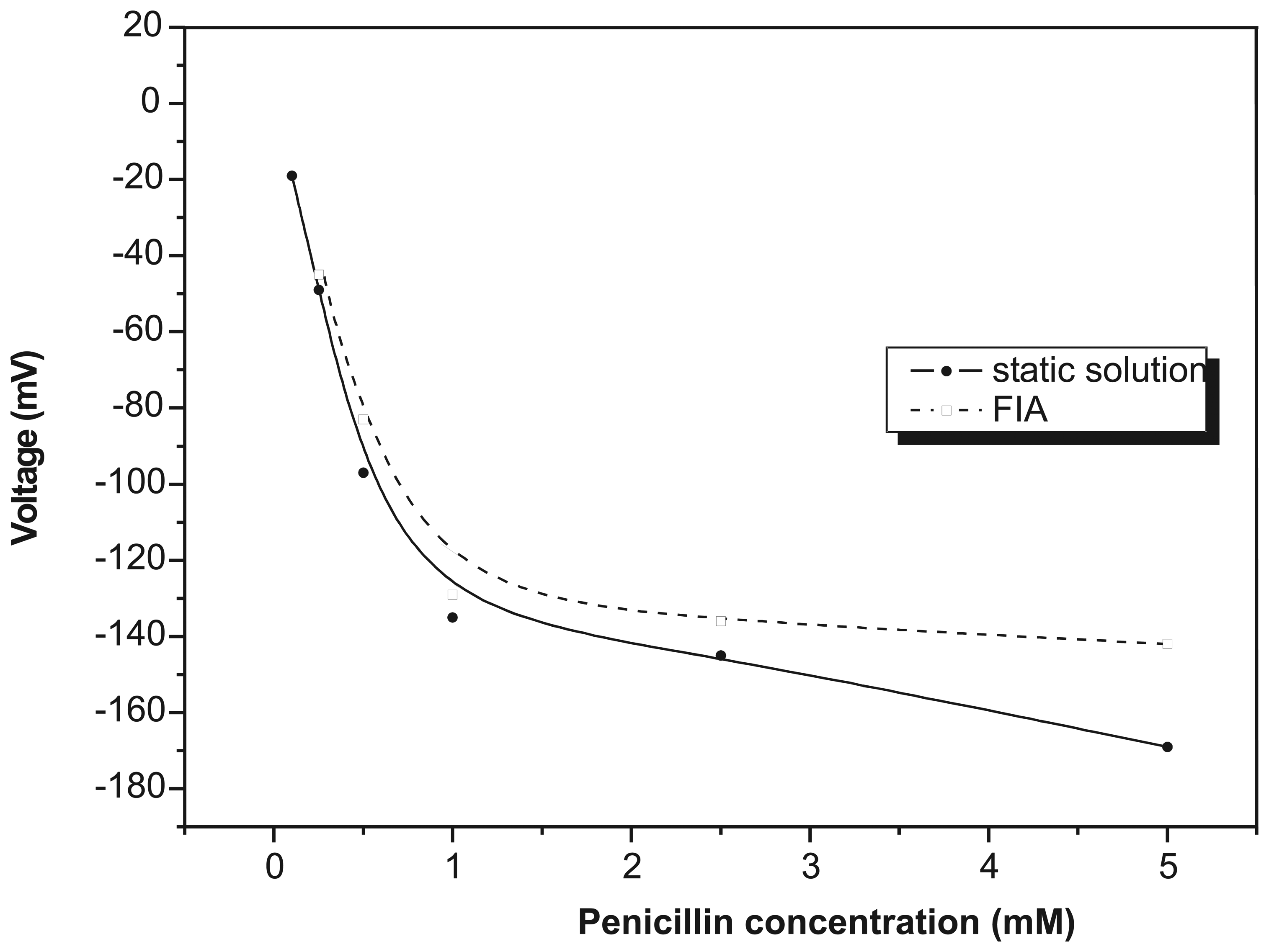
Results and discussion
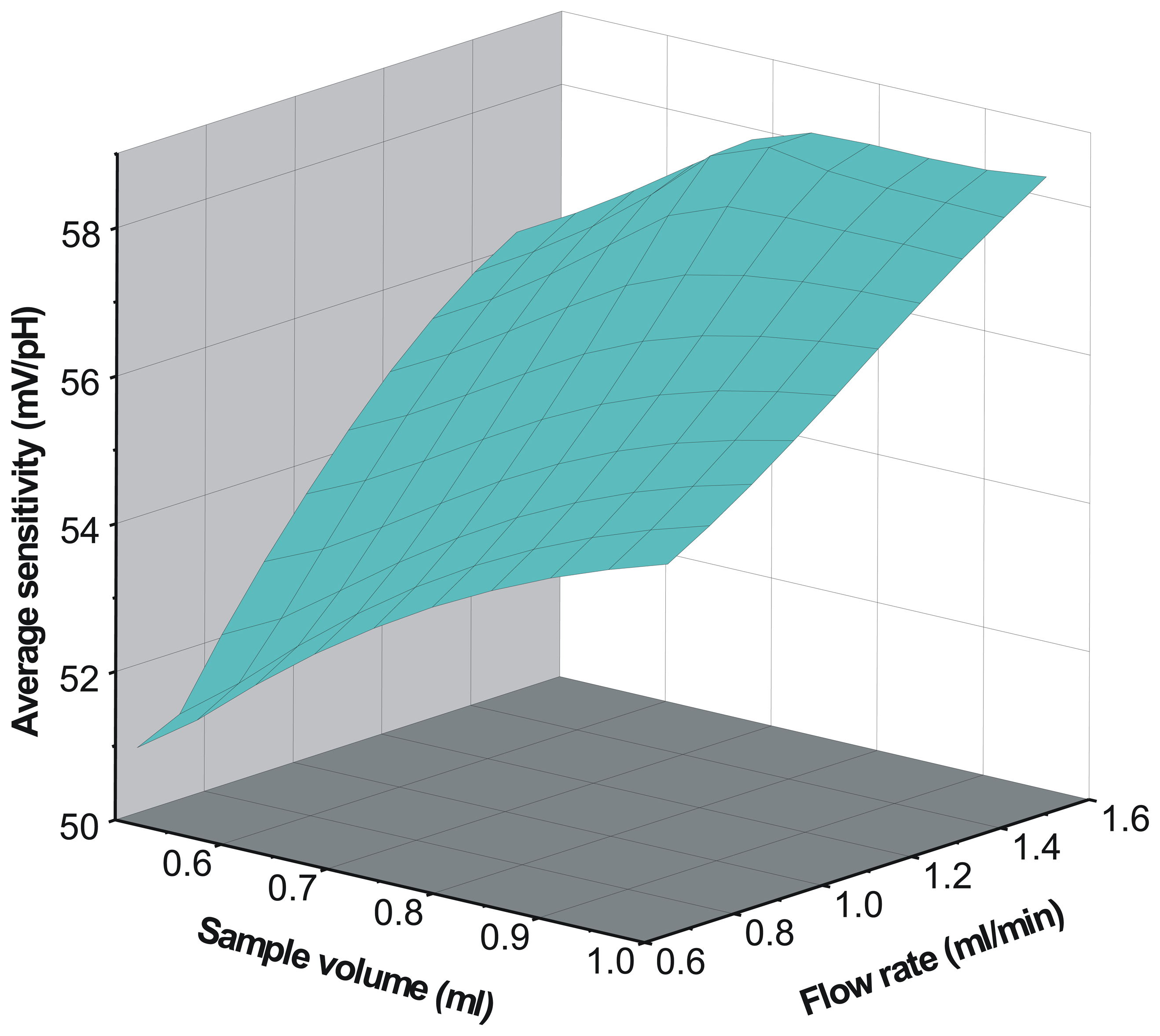
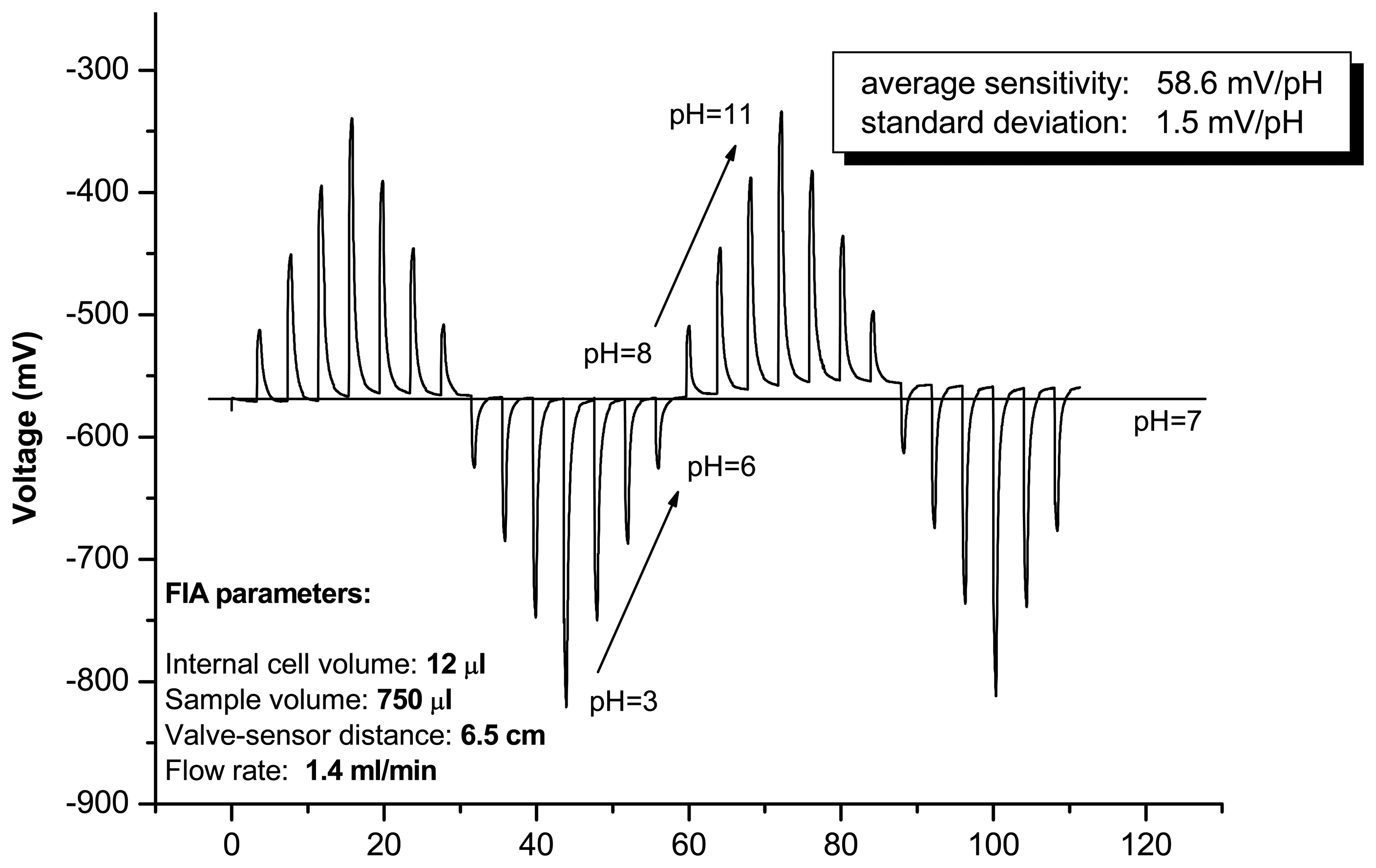
pH sensor
Penicillin measurement in FIA mode with EIS biosensor
Conclusions
Acknowledgments
References
- Ruzicka, J.; Hansen, E.H. Flow Injection Analysis; Wiley: New York, 1981. [Google Scholar]
- Karlberg, B.; Pacey, G.E. Flow Injection Analysis; Elsevier: Amsterdam, 1989. [Google Scholar]
- Hassan, S.S.M.; Marzouk, S.A.M.; Badawy, N.M. Solid state iridium oxide-titanium based sensor for flow injection pH measurements. Anal. Lett. 2002, 35, 1301. [Google Scholar]
- Dimitrakopoulos, L.T.; Dimitrakopoulos, T.; Alexander, P.W.; Logic, D.; Hibbert, D.B. A tungsten oxide wire electrode used as a pH sensor in flow injection potentiometry. Anal. Commun. 1998, 35, 395. [Google Scholar]
- Alegret, S.; Alonso, J.; Bartroli, J.; Domenech, J.; Jaffrezic-Renault, N.; Duvault-Herrera, Y. Flow-through pH-ISFET detector for flow-injection analysis. Anal. Chim. Acta 1989, 222, 373. [Google Scholar]
- Alegret, S.; Bartroli, J.; Jimenez-Jorquera, C.; del Valle, M.; Dominguez, C.; Esteve, J.; Bausells, J. Flow-throgh pH-ISFET + reference-ISE as integrated detector in automated FIA determinations. Sens. Actuators B 1992, 7, 555. [Google Scholar]
- Alegret, S.; Alonso, J.; Bartroli, J.; del Valle, M.; Jaffrezic-Renault, N.; Duvault-Herrera, Y. Flow-through pH-ISFET as detector in the determination of ammonia. Anal. Chim. Acta 1990, 231, 53. [Google Scholar]
- Ramsing, A.U.; Janata, J.; Ruzicka, J.; Levy, M. Miniaturisation in analytical chemistry - a combination of flow injection analysis and ion-sensitive field effect transistors for determination of pH, and potassium and calcium ions. Anal. Chim. Acta 1980, 118, 45. [Google Scholar]
- Kullick, T.; Bock, U.; Scheper, T.; Schügerl, K. Application of enzyme-field effect transistor sensor arrays as detectors in a flow-injection analysis system for simultaneous monitoring of medium components. Anal. Chim. Acta 1995, 300, 25. [Google Scholar]
- Cobben, P.L.H.M.; Egberink, R.J.M.; Bomer, J.; Haak, J.R.; Bergveld, P.; Reinhoudt, D.N. Detection of heavy metal ions by ISFETs in a flow injection analysis cell. Sens. Actuators B 1992, 6, 304. [Google Scholar]
- Hongbo, C.; Hansen, E.H.; Ruzicka, J. Evaluation of critical parameters for measurement of pH by flow injection analysis. Anal. Chim. Acta 1985, 169, 209. [Google Scholar]
- Van der Wal, P.D.; Sudhölter, E.J.R.; Reinhoudt, D.N. Design and properties of a flow-injection analysis cell using potassium-selective ion-sensitive field-effect transistors as detection elements. Anal. Chim. Acta 1991, 245, 159. [Google Scholar]
- Birrell, S.J.; Hummel, J.W. Real-time multi ISFET/FIA soil analysis system with automatic sample extraction. Computers and Electronics in Agriculture 2001, 32, 45. [Google Scholar]
- Van der Schoot, B.H.; van den Vlekkert, H.H.; de Rooij, N.F.; van den Berg, A.; Grisel, A. A flow injection analysis system glass-bonded ISFETs for the simultaneous detection of calcium and potassium ions and pH. Sens. Actuators B 1991, 4, 239. [Google Scholar]
- Jimenez, C.; Marques, I.; Bartroli, J. Continuous-flow system for on-line water monitoring using back-side contact ISFET-based sensors. Anal. Chem. 1996, 68, 3801. [Google Scholar]
- Schöning, M.J.; Thust, M.; Müller-Veggian, M.; Kordos, P.; Lüth, H. A novel silicon-based sensor array with capacitive EIS structure. Sens. Actuators B 1998, 47, 225. [Google Scholar]
- Poghossian, A.; Yoshinobu, T.; Simonis, A.; Ecken, H.; Lüth, H.; Schöning, M.J. Penicillin detection by means of field effect based sensors: ENFET, capacitive EIS sensor or LAPS? Sens. Actuators B 2001, 78, 237. [Google Scholar]
- Beyer, M.; Menzel, C.; Quack, R.; Scheper, T.; Schügerl, K.; Treichel, W.; Voigt, H.; Ullrich, M.; Ferretti, R. Development and application of a new enzyme sensor type based on the EIScapacitance structure for bioprocess control. Biosens. Bioelectron. 1994, 9, 17. [Google Scholar]
- Menzel, C.; Lerch, T.; Scheper, T.; Schügerl, K. Development of biosensors based on an electrolyte isolator semiconductor (EIS)-capacitor structure and their application for process monitoring. Part 1. Development of the biosensors and their characterisation. Anal. Chim. Acta 1995, 317, 259. [Google Scholar]
- Menzel, C.; Lerch, T.; Schneider, K.; Weidemann, R.; Tollnick, C.; Kretzmer, G.; Scheper, T.; Schügerl, K. Application of biosensors with an electrolyte isolator semiconductor capacitor (EISCAP) transducer for process monitoring. Process Biochem. 1998, 33, 175. [Google Scholar]

© 2004 by MDPI ( http://www.mdpi.org). Reproduction is permitted for non-commercial purposes.
Share and Cite
Rolka, D.; Poghossian, A.; Schöning, M.J. Integration of a Capacitive EIS Sensor into a FIA System for pH and Penicillin Determination. Sensors 2004, 4, 84-94. https://doi.org/10.3390/s40670084
Rolka D, Poghossian A, Schöning MJ. Integration of a Capacitive EIS Sensor into a FIA System for pH and Penicillin Determination. Sensors. 2004; 4(6):84-94. https://doi.org/10.3390/s40670084
Chicago/Turabian StyleRolka, David, Arshak Poghossian, and Michael J. Schöning. 2004. "Integration of a Capacitive EIS Sensor into a FIA System for pH and Penicillin Determination" Sensors 4, no. 6: 84-94. https://doi.org/10.3390/s40670084



