A Porphyrin Based Potentiometric Sensor for Zn2+ Determination
Abstract
:Introduction
Experimental
Reagents

Protoporphyrin (IX) 3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphine-dipropionic acid disodium salt
Apparatus
Electrode preparation
Potential Measurements
- Internal reference electrode (SCE)
- Internal solution (0.1M Zn2+)
- Membrane
- Test solution
- External reference electrode (SCE)
Results and Discussion
Membrane Characteristics
| Electrode No. | Components in membranes (% w/w) (I) PVC NaTPB TEP DOP CN TBP DBP DBBP | Working Concentration range (M) | Slope mV/decade of activity | Response Time (s) | ||||||||
| 1. | 10 | 150 | - | - | - | - | - | - | - | 7.9 × 10-4 to 1.0 × 10-1 | 38.2 | 40 |
| 2. | 10 | 150 | 2 | - | - | - | - | - | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.0 | 10 |
| 3. | 10 | 150 | 2 | - | 150 | - | - | - | - | 1.7 × 10-4 to 1.0 × 10-1 | 33.0 | 15 |
| 4. | 10 | 150 | 2 | - | - | - | - | 150 | - | 7.9 × 10-5 to 1.0 × 10-1 | 35.0 | 20 |
| 5. | 10 | 150 | 2 | - | - | 150 | - | - | - | 3.1 × 10-5 to 1.0 × 10-1 | 34.0 | 22 |
| 6. | 10 | 150 | 2 | 165 | - | - | - | - | - | 3.9 × 10-4 to 1.0 × 10-1 | 40.0 | 32 |
| 7. | 10 | 150 | 2 | - | - | - | 170 | - | - | 7.0 × 10-4 to 1.0 × 10-1 | 39.0 | 35 |
Effect of Plasticizer
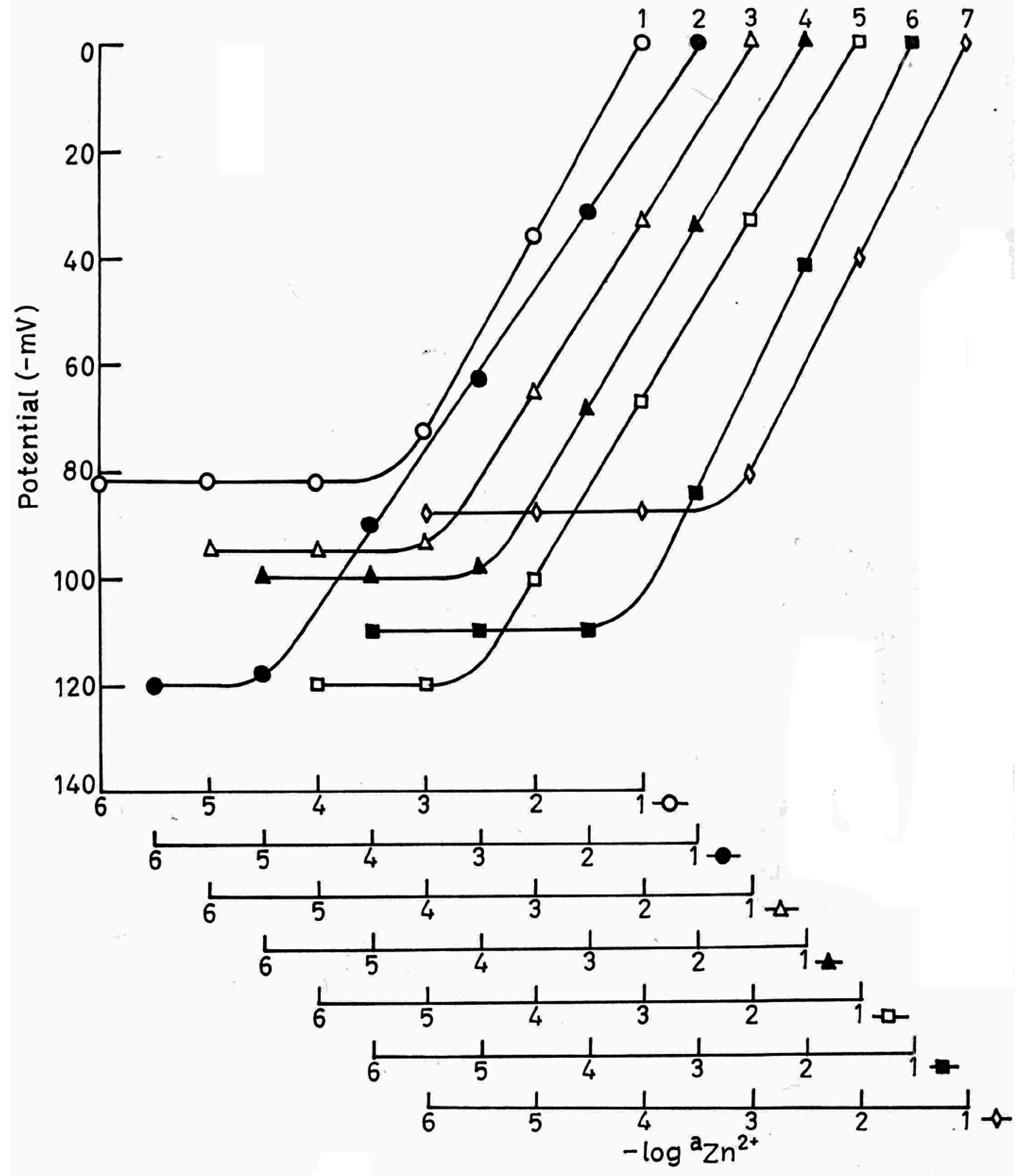
| Electrode No. | Components of the membranes (% w/w) (I) PVC NaTPB DBBP | Working concentration range (M) | Slope mV/decade of activity | Response Time (s) | |||
| A. | 8 | 150 | 2 | 200 | 5.3 × 10-5 to 1.0 × 10-1 | 28.2 | 15 |
| B. | 9.5 | 150 | 2 | 200 | 1.5 × 10-5 to 1.0 × 10-1 | 29.0 | 12 |
| C. | 10 | 150 | 2 | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.0 | 10 |
| D. | 11 | 150 | 2 | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.2 | 10 |
| E. | 12 | 150 | 2 | 200 | 1.3 × 10-5 to 1.0 × 10-1 | 30.0 | 10 |

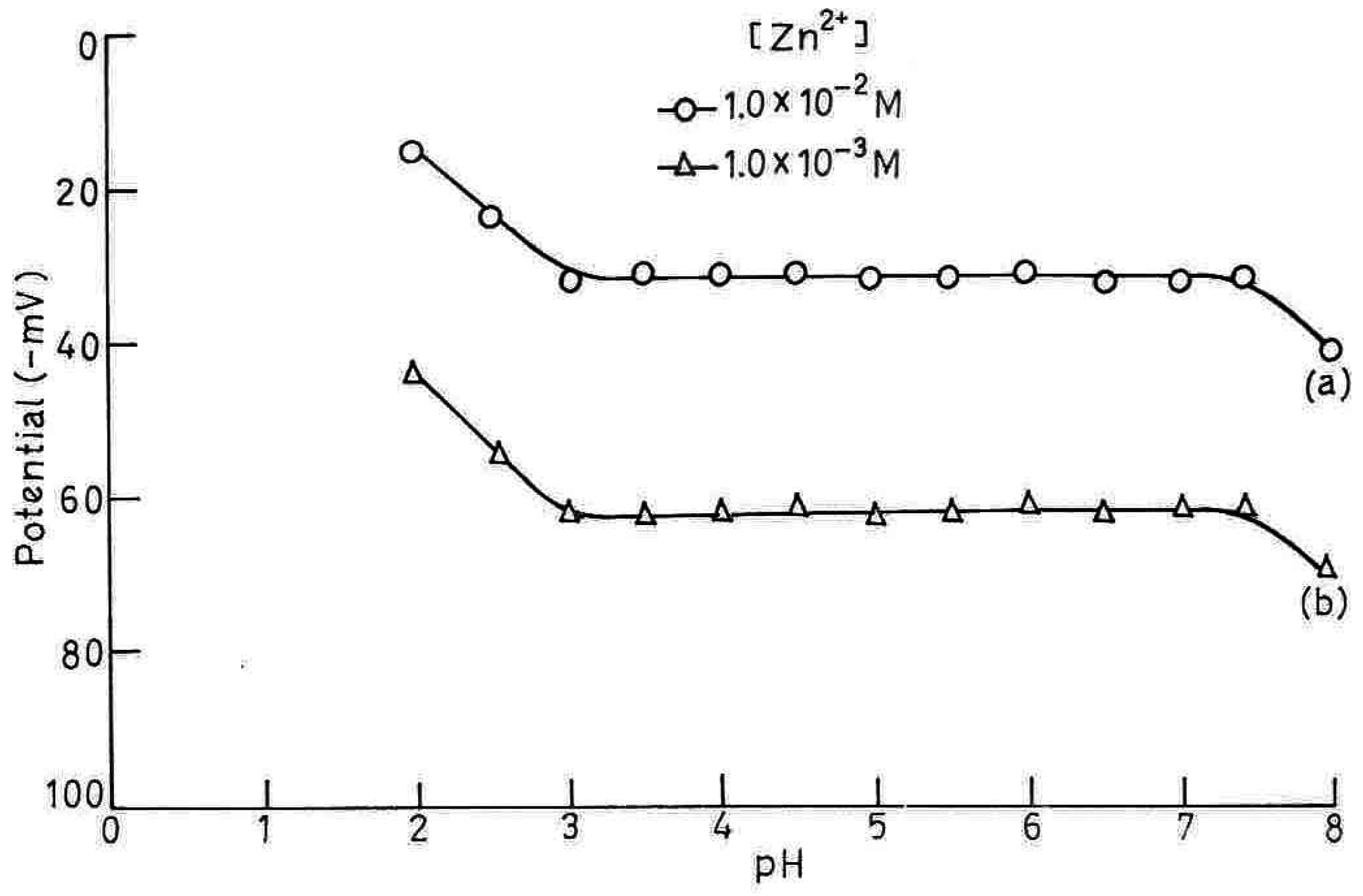
pH Dependence and Non-aqueous Effect
| Non- aqueous Content (% v/v) | Slope (mV/decade of activity) | Working Concentration Range (M) |
| 0 | 30.0 | 1.3 × 10-5 to 1.0 × 10-1 |
| Methanol | ||
| 30 | 30.2 | 1.3 × 10-5 to 1.0 × 10-1 |
| 40 | 29.3 | 1.5 × 10-5 to 1.0 × 10-1 |
| 45 | 25.2 | 2.5 × 10-4 to 1.0 × 10-1 |
| Ethanol | ||
| 30 | 29.6 | 1.5 × 10-5 to 1.0 × 10-1 |
| 40 | 29.0 | 1.6 × 10-5 to 1.0 × 10-1 |
| 45 | 24.5 | 3.6 × 10-4 to 1.0 × 10-1 |
| Acetone | ||
| 30 | 30.2 | 1.3 × 10-5 to 1.0 × 10-1 |
| 40 | 29.5 | 1.4 × 10-5 to 1.0 × 10-1 |
| 45 | 24.6 | 1.6 × 10-4 to 1.0 × 10-1 |
Potentiometric Selectivity
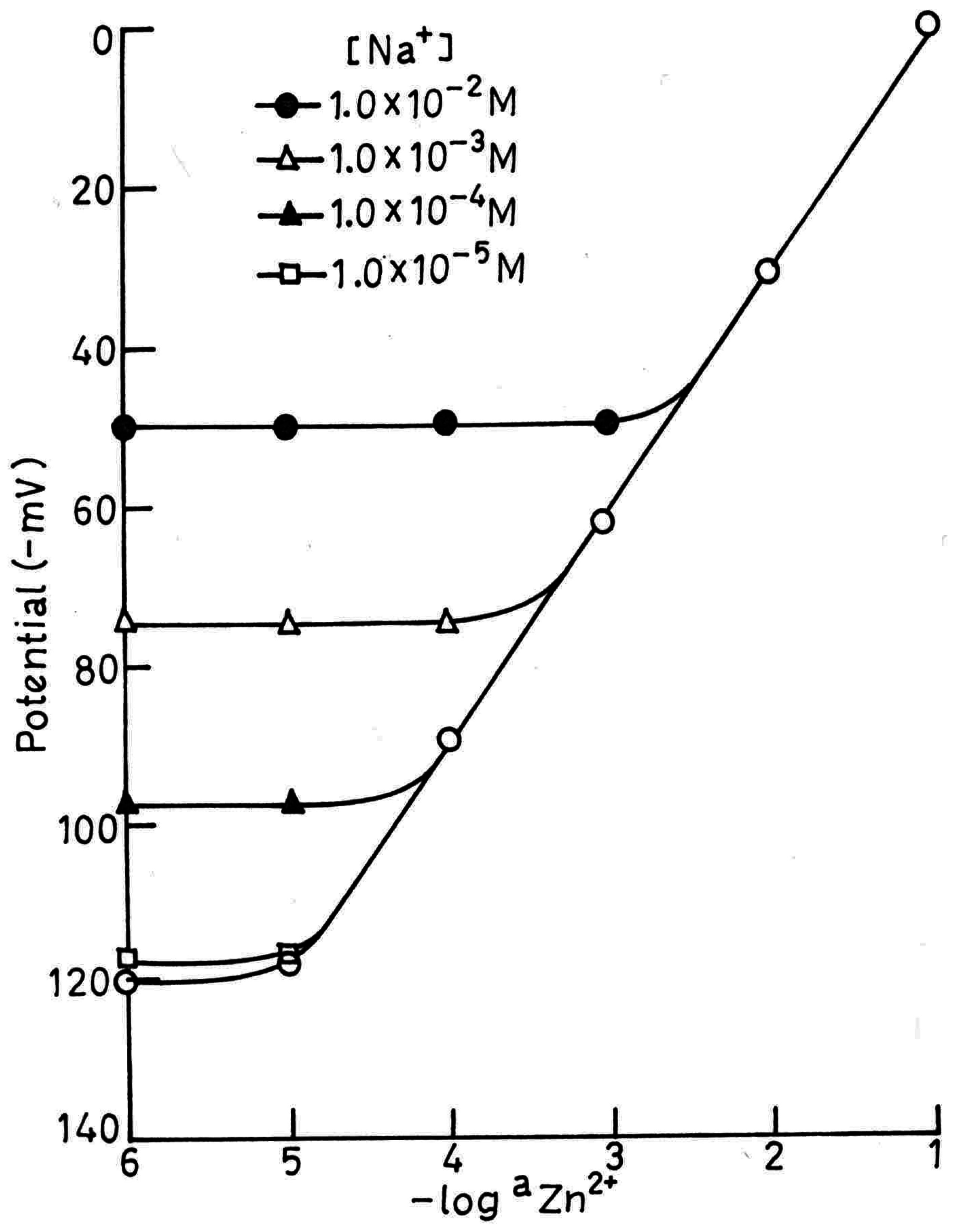
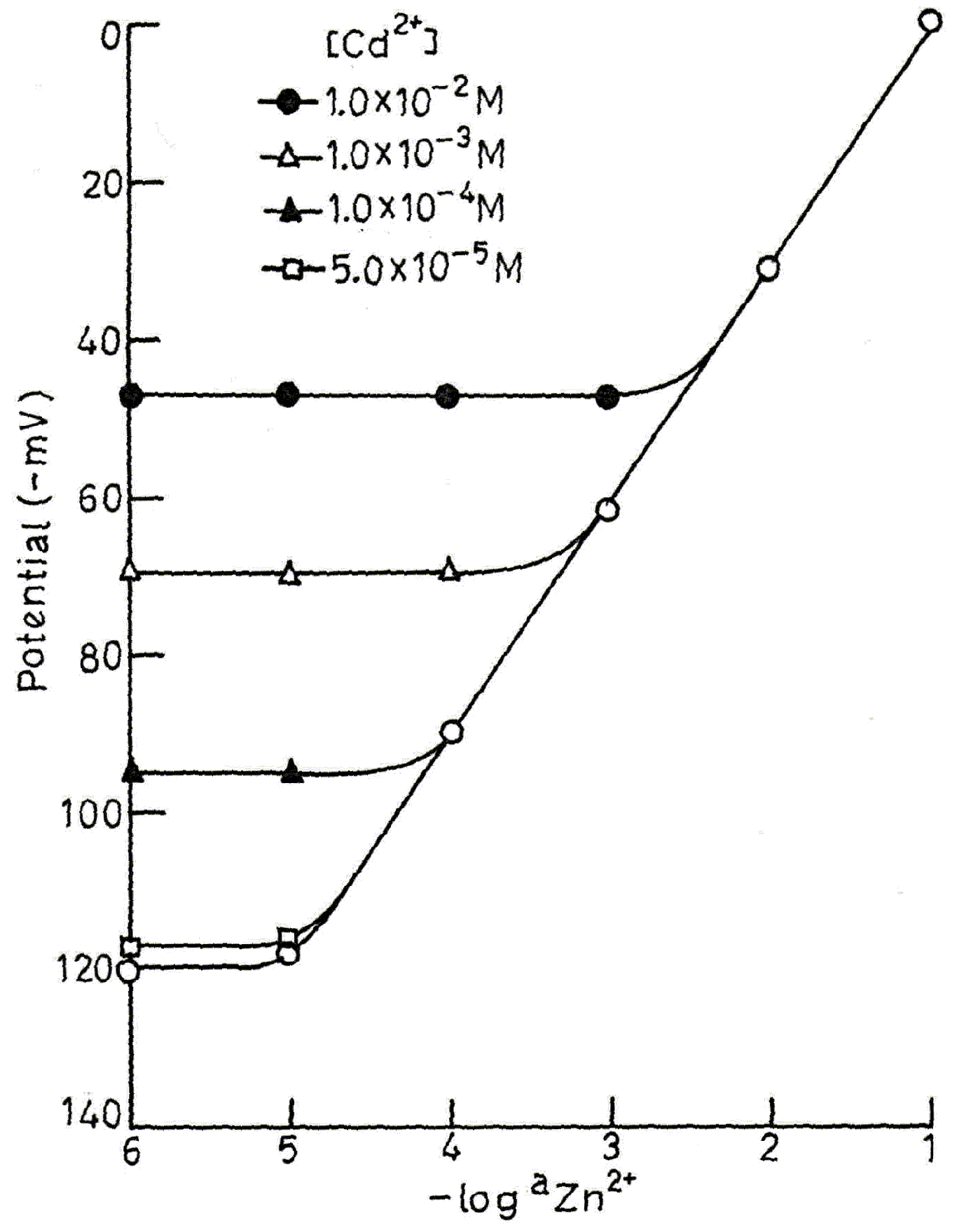
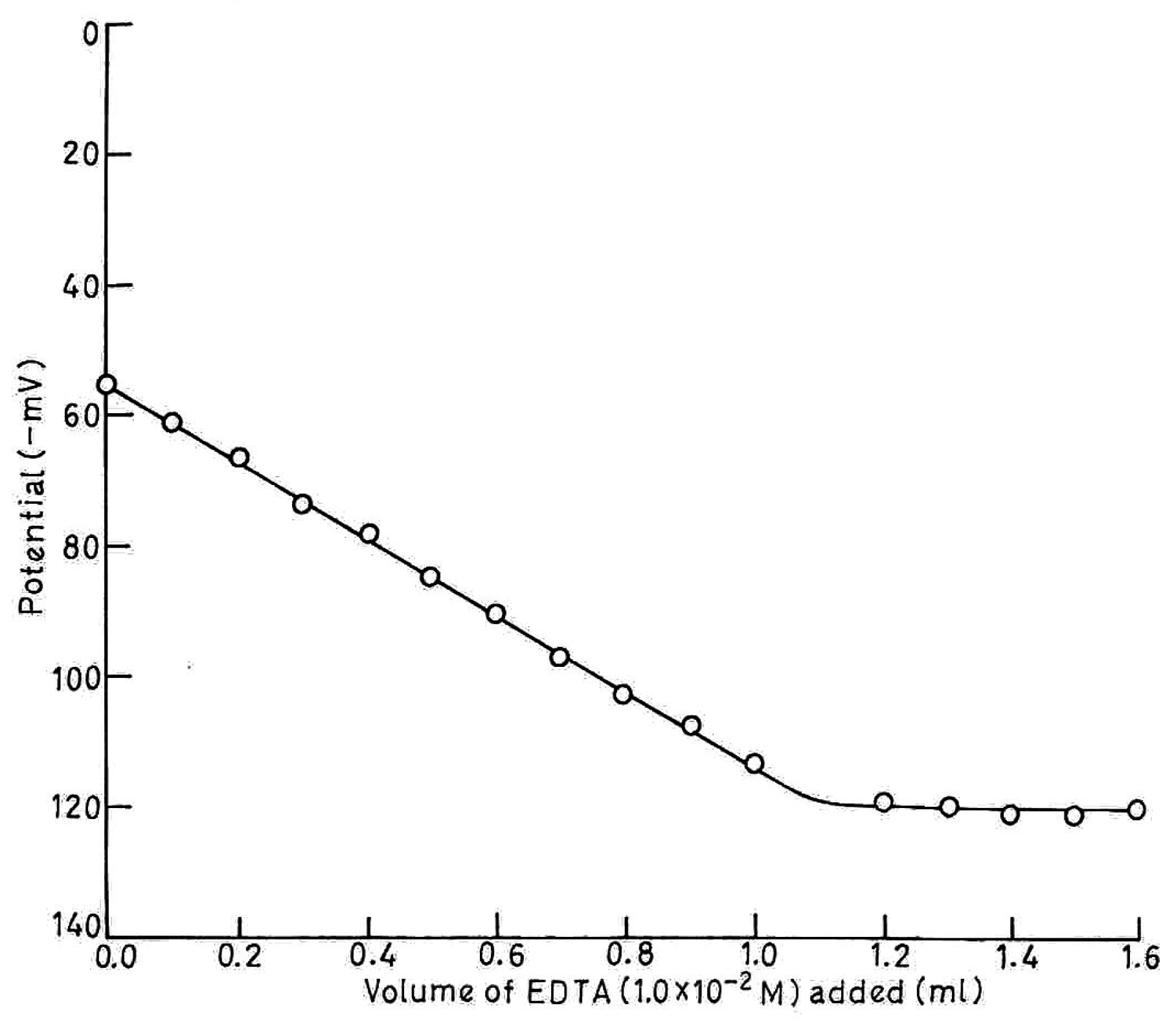
Analytical Applications
| Interfering ions (B) | Selectivity Coefficient | ||
| Fixed Interference Method | Matched Potential Method | ||
| With exponent | Without exponent | ||
| Li+ | 31.6 × 10-1 | 3.1 × 10-2 | 0.17 |
| Na+ | 25.1 | 2.5 × 10-1 | 0.65 |
| K+ | 25.1 × 10-1 | 2.5 × 10-2 | 0.20 |
| NH4+ | 31.6 × 10-1 | 3.1 × 10-2 | 0.16 |
| Cs+ | 28.1 × 10-1 | 2.8 × 10-2 | 0.18 |
| Tl+ | 17.7 × 10-1 | 1.7 × 10-2 | 0.10 |
| Ag+ | 44.6 × 10-1 | 4.4 × 10-2 | 0.20 |
| Ca2+ | 1.2 × 10-2 | 1.2 × 10-2 | 0.13 |
| Sr2+ | 6.3 × 10-2 | 6.3 × 10-2 | 0.25 |
| Cu2+ | 5.9 × 10-2 | 5.9 × 10-2 | 0.22 |
| Ni2+ | 5.6 × 10-2 | 5.6 × 10-2 | 0.20 |
| Cd2+ | 3.1 × 10-1 | 3.1 × 10-1 | 0.60 |
| Hg2+ | 6.3 × 10-2 | 6.3 × 10-2 | 0.24 |
| Co2+ | 4.4 × 10-2 | 4.4 × 10-2 | 0.20 |
| Pb2+ | 7.0 × 10-2 | 7.0 × 10-2 | 0.25 |
| Fe3+ | 1.7 × 10-2 | 7.9 × 10-2 | 0.25 |
| Al3+ | 1.9 × 10-3 | 8.9 × 10-2 | 0.31 |
| Cr3+ | 1.9 × 10-3 | 8.9 × 10-2 | 0.28 |
Conclusion
Acknowledgements
References
- Moore, J.W.; Ramamoorthy, S. Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment; Springer: New York, 1984; pp. 182–204. [Google Scholar]
- Gorton, L.; Fiedler, U. Zinc Sensitive Polymeric Membrane Electrode. Anal. Chim. Acta. 1977, 90, 233–236. [Google Scholar] [CrossRef]
- Linnersund, U.F.; Bhatti, K.M. Development of Polymeric Membranes for the Zinc-Ion Selective Electrodes. Anal. Chim. Acta. 1979, 111, 57–70. [Google Scholar] [CrossRef]
- Lebedeva, O.A.; Jansons, E. Cd and Zn-Selective Liquid Electrodes on the Basis of Quinoline-8-Carbodithioates. Latv. PSR Zinat. Akad. Vestis. Kim. Ser. 1987, 4, 483–485. [Google Scholar]
- Kojima, R.; Kamata, S. Zinc Selective Membrane Electrode Using Tetrabutyl Thiuram Disulfide Neutral Carrier. Anal. Sci. 1994, 10, 409–412. [Google Scholar] [CrossRef]
- Rocheleaw, M.J.; Purdy, W.C. Investigation of Materials for Making a Carbon Support Zinc-Selective Electrode. Talanta. 1990, 37, 307–311. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Vardhan, H.; Singh, M.; Rao, G.N.; Srivastava, S. A New Chelating Ion-Exchange Resin Based Sensor for Zinc Ions. Anal. Proc. 1995, 32, 173–178. [Google Scholar] [CrossRef]
- Obmetho, A.A.; Rakhmanko, E.M.; Lomako, V.L.; Starvobinets, G.L. Determination of Zinc in Alloys by a Ion-Selective Electrode. Zh. Anal. Khim. 1988, 43, 444–452. [Google Scholar]
- Srivastava, S.K.; Gupta, V.K.; Jain, S. PVC-based 2,2,2-Cryptand Sensor for Zinc Ions. Anal. Chem. 1996, 68, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Jain, A.K.; Singh, L.P.; Khurana, U. Zn2+ Sensor Based on Zn-bis(2,4,4-trimethylpentyl) dithiophosphinic acid Complex in PVC Matrix. Electrochim. Acta. 1998, 43, 2047–2052. [Google Scholar] [CrossRef]
- Gupta, V.K. A PVC based 12-Crown-4 Membrane Potentiometric Sensor for Zinc(II) Ions. Sens. Actuators B. 1999, 55, 195–200. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rouhani, S.; Ganjali, M.R.; Sharghi, H.; Eshghi, H. Zinc Selective Membrane Potentiometric Sensor Based on a Recently Synthesized Benzosubstituted Macrocyclic Diamide. Sens. Actuators B. 1999, 59, 30–34. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Alaghemand, M.; Shamsipur, M. Zinc-Selective Membrane Electrode Based on 5,6,14,15-dibenzo-1,4-dioxa-8,12-diazacyclopentadecane-5,14-diene. Anal. Lett. 2001, 34, 2169–2174. [Google Scholar]
- Gupta, V.K.; Chandra, S.; Chauhan, D.K.; Mangla, R. Membranes of 5,10,15,20-Tetrakis(4- Methoxyphenyl Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- Selective Sensors. Sensors. 2002, 2, 164–174. [Google Scholar] [CrossRef]
- Craggs, A.; Moody, G.J.; Thomas, J.D.R. Procedure for the Construction of All Solid-State PVC Membrane Electrode. J. chem. Educ. 1974, 51, 541–546. [Google Scholar] [CrossRef]
- Bakker, E.; Buhlman, P.; Pretsch, E. Carrier-Based Ion Selective Electrodes and Bulk Optodes.1.General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [PubMed]
- Christon, G. D. Analytical Chemistry; John Wiley Pte. Ltd.: Singapore, 1994; Chapter 4; p. 331. [Google Scholar]
- Sa’ez de Viteri, F.J.; Diamond, D. Determination and Application of Ion Selective Electrode Model Parameters Using Flow Injuction and Simplex Optimization. Analyst. 1994, 119, 749–758. [Google Scholar] [CrossRef]
- Gadzekpo, V.P.Y.; Christian, G.D. Determination of Selectivity Coefficients of Ions Selective Electrodes by a Matched Potential Method. Anal. Chim. Acta. 1984, 164, 279–282. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.net). Reproduction is permitted for noncommercial purposes.
Share and Cite
Gupta, V.K.; Chauhan, D.K.; Saini, V.K.; Agarwal, S.; Antonijevic, M.M.; Lang, H. A Porphyrin Based Potentiometric Sensor for Zn2+ Determination. Sensors 2003, 3, 223-235. https://doi.org/10.3390/s30700223
Gupta VK, Chauhan DK, Saini VK, Agarwal S, Antonijevic MM, Lang H. A Porphyrin Based Potentiometric Sensor for Zn2+ Determination. Sensors. 2003; 3(7):223-235. https://doi.org/10.3390/s30700223
Chicago/Turabian StyleGupta, V. K., D. K. Chauhan, V. K. Saini, Shiva Agarwal, M. M. Antonijevic, and H. Lang. 2003. "A Porphyrin Based Potentiometric Sensor for Zn2+ Determination" Sensors 3, no. 7: 223-235. https://doi.org/10.3390/s30700223




