A poly(pyrrole-Cobalt(II)deuteroporphyrin) electrode for the potentiometric determination of nitrite
Abstract
:Introduction
Experimental part
Reagents
Apparatus
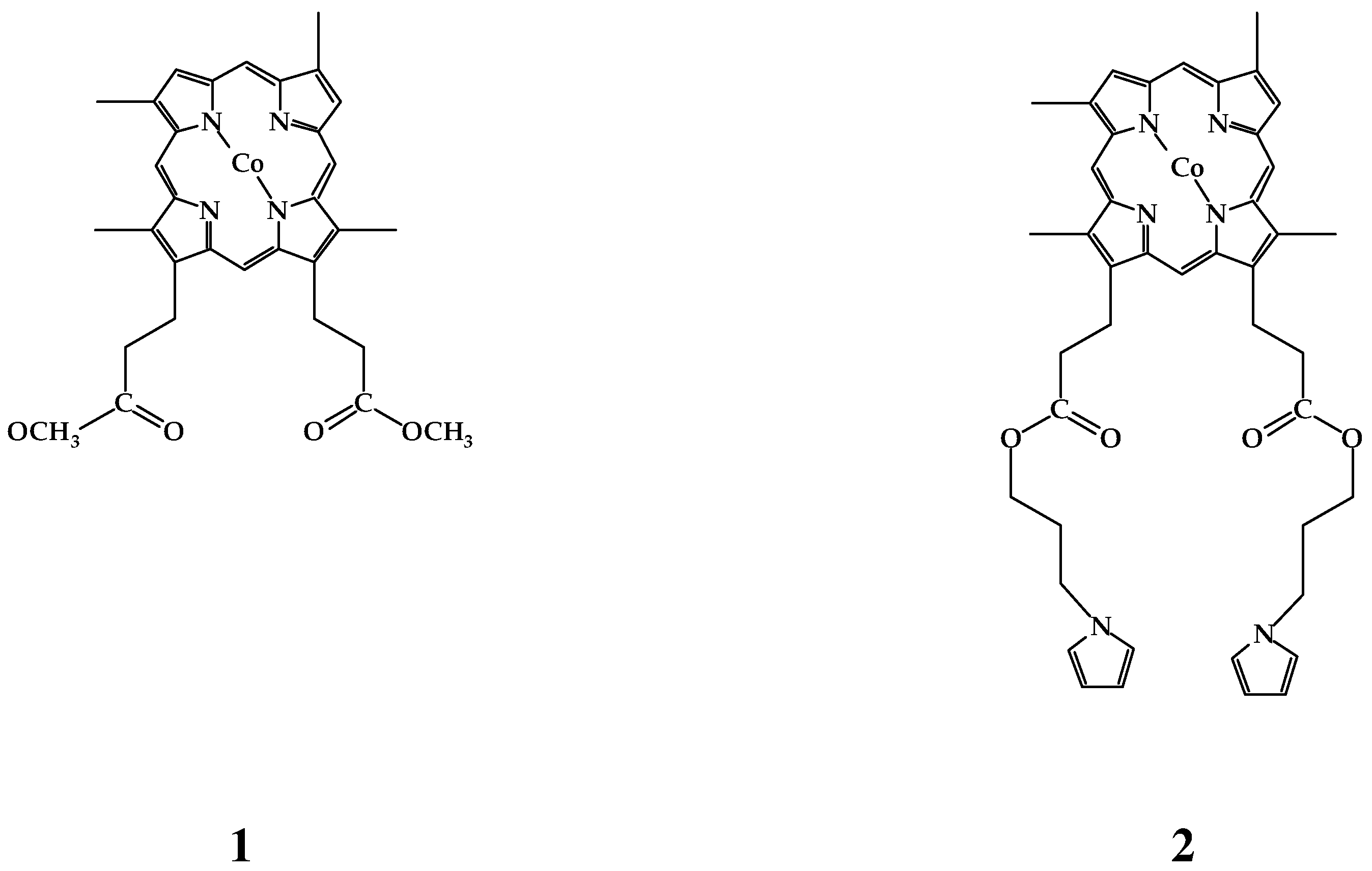
Result and discussion
Electroactivity of the complexes 1 and 2
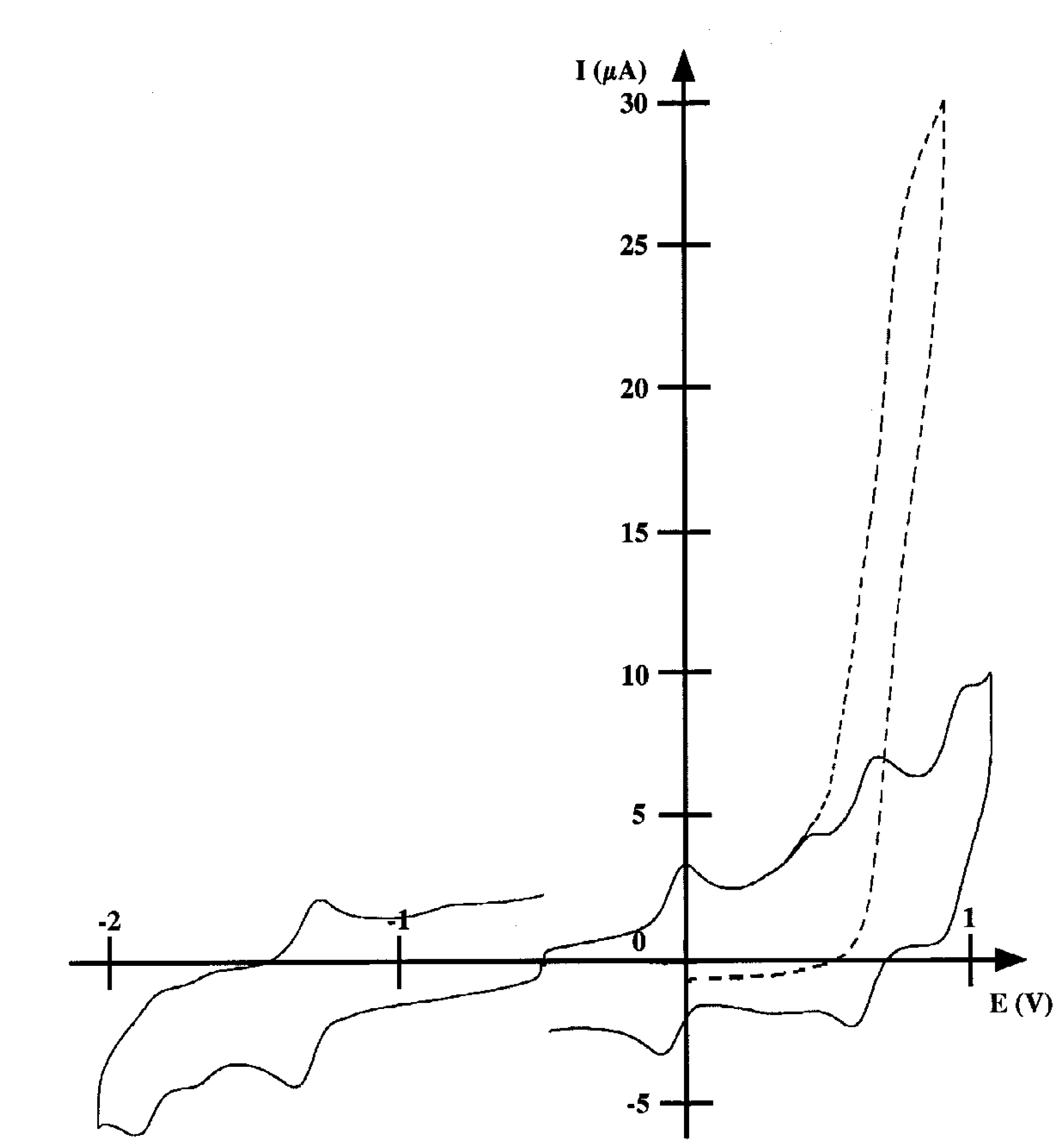
Electropolymerization of 2 and redox behavior of the resulting poly(pyrrole-cobalt(II) deuteroporphyrin)
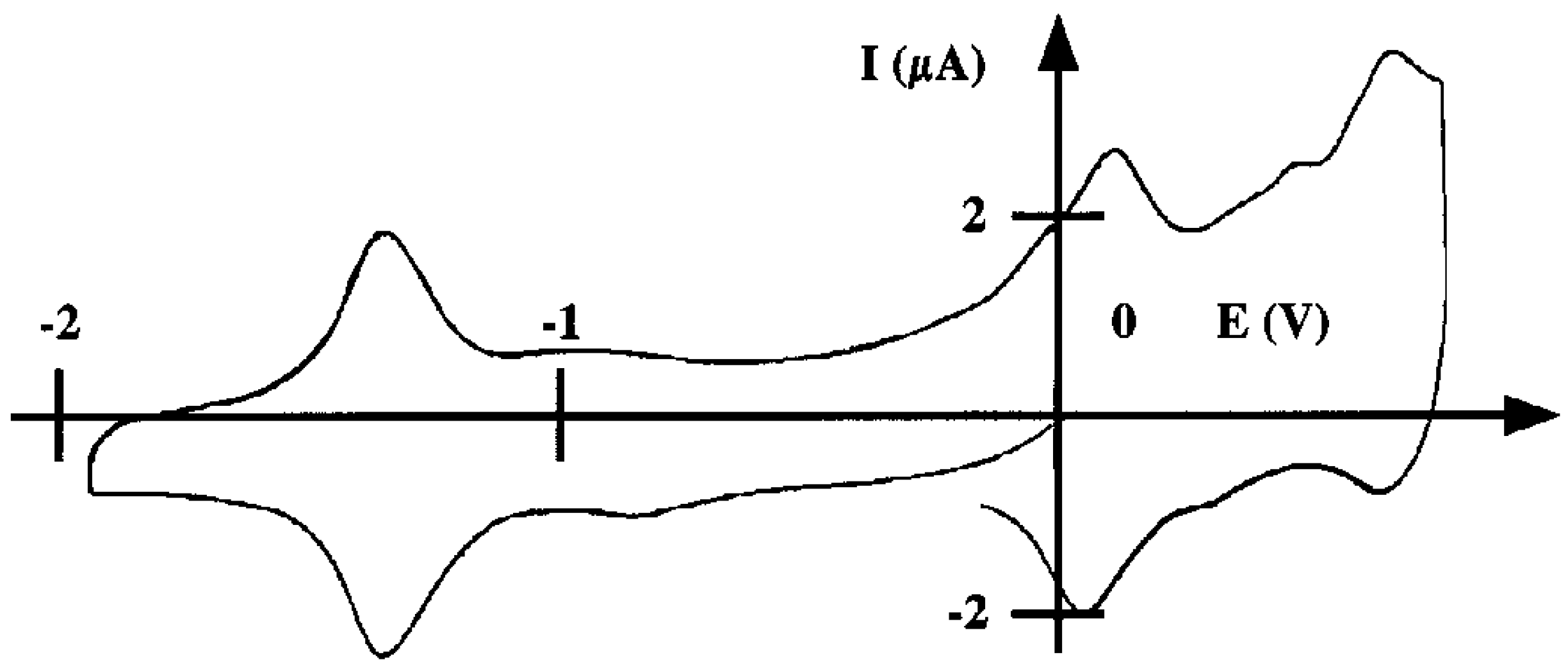
Capabilities of the poly 2 electrode as sensor for nitrite ions

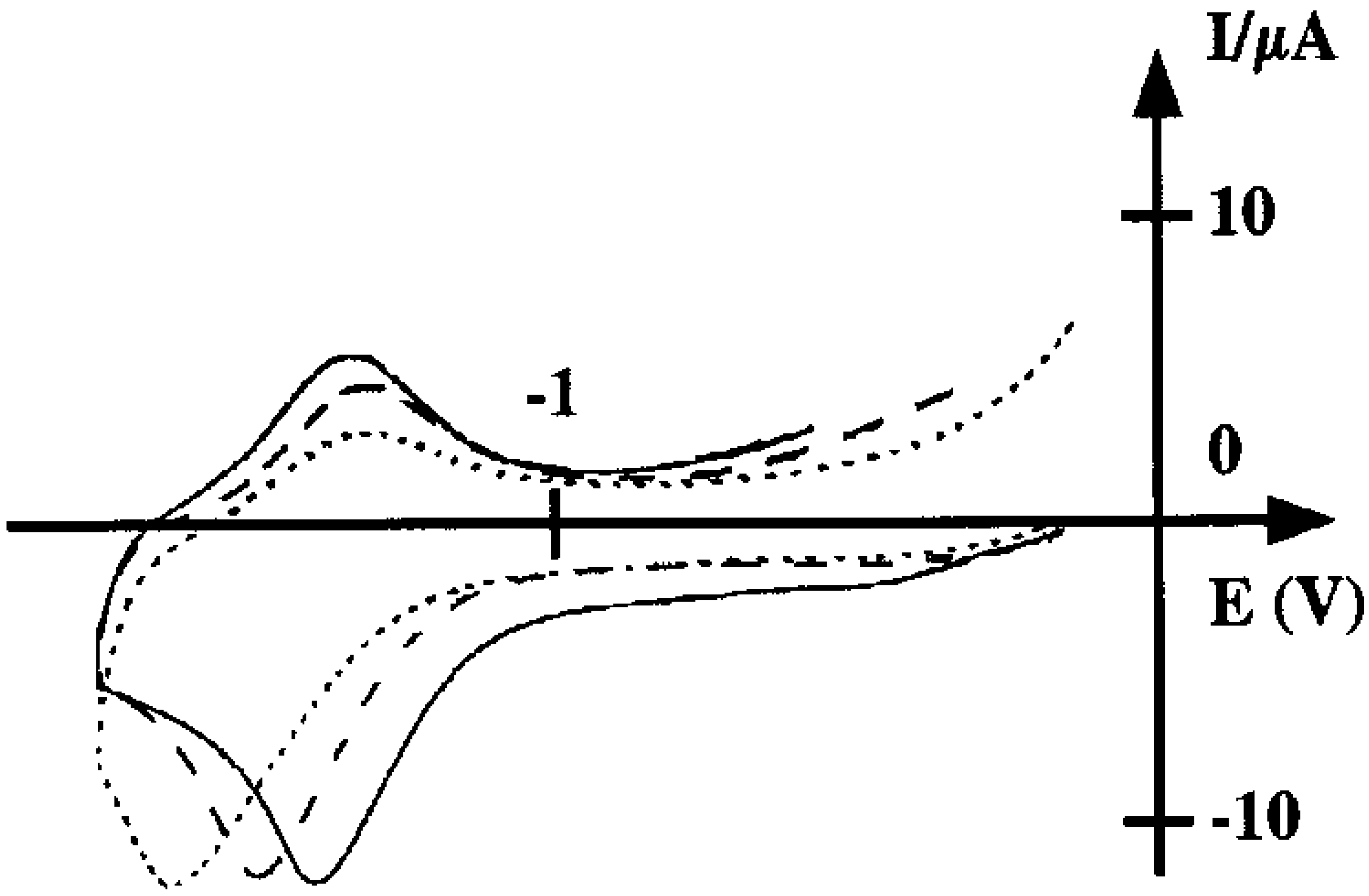
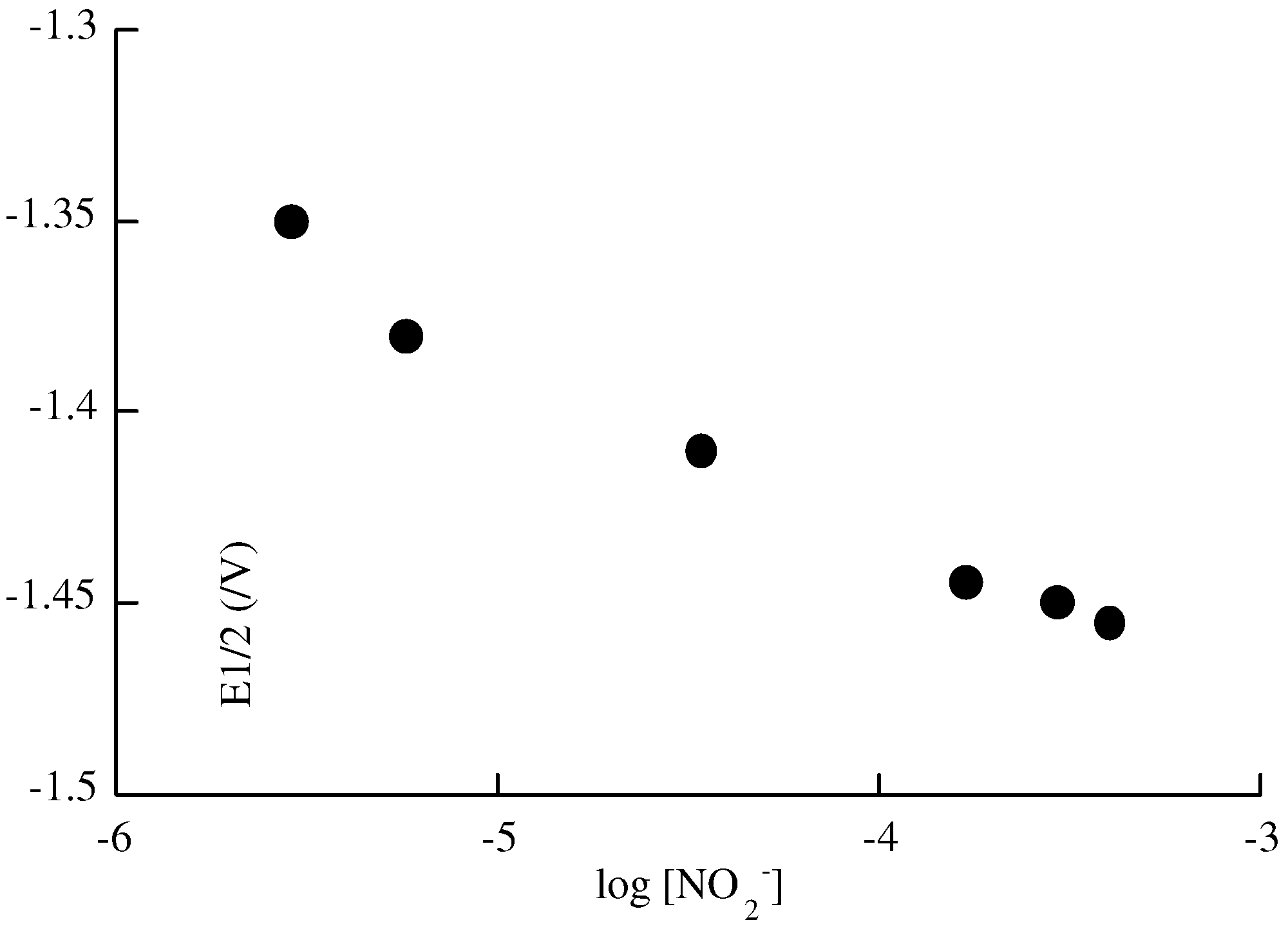
Conclusion
Acknowlegment
References
- Biegsaga, M.; Pyrzynska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta, 2000; 51, 209–224. [Google Scholar]
- Kadish, K.M.; Van Caemelbecke, E.; Royal, G. The porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, 2000; Vol. 8, Chapter 55; p. 1. [Google Scholar]
- Younathan, J.N.; Wood, K.S.; Meyer, T.J. Electrocatalytic Reduction of Nitrite and Nitrosyl by Iron(III) Protoporphyrin XI Dimethyl Ester Immobilized in Electropolymerized Film. Inorg. Chem. 1992, 31, 3280–3285. [Google Scholar] [CrossRef]
- Deronzier, A.; Devaux, R.; Limosin, D.; Latour, M. Poly(pyrrole-metallotetraphenylporphyrin)- modified electrodes. J. Electroanal. Chem. 1992, 324, 325–337. [Google Scholar] [CrossRef]
- Bedioui, F.; Devynck, J.; Bied-Charreton, C. Immobilization of Metalloporphyrins in Electropolymerized Films: Design and Applications. Acc. Chem. Res. 1995, 28, 30–36. [Google Scholar] [CrossRef]
- Carvalho de Medeiros, A.M.; Cosnier, S.; Deronzier, A.; Moutet, J.-C. Synthesis and Characterization of a New Series of Nickel(II) meso-Tetrakis(polyfluorophenyl)porphyrins Functionalized by Pyrrole Groups and Their Electropolyerized Films. Inorg. Chem. 1996, 35, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S.; Walter, A.; Montforts, F.-P. A Poly(pyrrole-copper(II) deuteroporphyrin) Modified Electrode. J. Porphyrins and Phtalocyanines 1998, 2, 39–43. [Google Scholar] [CrossRef]
- Cosnier, S.; Gondran, C.; Wessel, R.; Montforts, F.-P.; Wedel, M. Poly(pyrrole- metallodeuteroporphyrin)electrodes: towards electrochemical biomimetic devices. J. Electroanal. Chem. 2000, 488, 83–91. [Google Scholar] [CrossRef]
- Diab, N.; Schuhmann, W. Electropolymerized manganese porphyrin/polypyrrole films as catalytic surfaces for oxidation of nitric oxide. Electrochim. Acta 2001, 47, 265–273. [Google Scholar] [CrossRef]
- Griveau, S.; Bedioui, F. Electrocatalytic Oxidation of 2-Mercaptoethanol by Electropolymerized Cobalt Porphyrin Film on Vitreous Carbon Electrodes. Electroanalysis 2001, 13, 253–256. [Google Scholar] [CrossRef]
- Cosnier, S.; Gondran, C.; Gorgy, K.; Wessel, R.; Montforts, F.-P.; Wedel, M. Electrogeneration and characterization of poly(pyrrole-nickel(II)chorine)electrode. Electrochem. Com. 2002, 4, 426–430. [Google Scholar] [CrossRef]
- Chen, G.N.; Zhao, Z.F.; Wang, X.L.; Duan, J.P.; Chen, H.Q. Electrochemical behavior of tryptophan and its derivatives at a glassy carbon electrode modified with hemin. Anal. Chim. Acta 2002, 452, 245–254. [Google Scholar]
- Campo Dall'Orto, V.; Danilowicz, C.; Hurst, J.; Lo Balbo, A.; Rezzano, I. Studies of the Interaction Between Metalloporphyrin Films and Phenols in a Preconcentration Type Sensor. Electroanalysis 1998, 10, 127–131. [Google Scholar] [CrossRef]
- Okada, T.; Gokita, M.; Yuasa, M.; Sekine, I. Oxygen Reduction Characteristics of Heat Treated Catalysts Based on Cobalt Porphyrin Ion Complexes. J. Electrochem. Soc. 1998, 45, 815–822. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Su, Y.O. Electrocatalysis of Nitric Oxide Reduction by Water-Soluble Cobalt Porphyrin. Spectral and Electrochemical Studies. Inorg. Chem. 1994, 33, 5847–5854. [Google Scholar] [CrossRef]
- Dobson, D.J.; Saini, S. Porphyrin-Modified Electrodes as Biomimetic Sensors of the Determination of Organohalide Pollutants in Aqueous Samples. Anal. Chem. 1997, 69, 3532–3538. [Google Scholar] [CrossRef] [PubMed]
- Vilchez-Aguado, F.; Gutierrez-Granados, S.; Sucar-Succar, S.; Bied-Charreton, C.; Bedioui, F. Electrocatalysis of the reduction of trichoroacetic acid by polypyrrole-cobalt porphyrin modified electrodes. New J. Chem. 1997, 21, 1009–1013. [Google Scholar]
- Malinowska, E.; Meyerhoff, M.E. Role of axial ligation on potentiometric response of Co(III) tetraphenylporphyrin-doped polymeric membranes to nitrite ions. Anal. Chim. Acta 1995, 300, 33–43. [Google Scholar] [CrossRef]
- Wedel, M.; Walter, A.; Montforts, F.-P. Synthesis of Metalloporphyrins and Metallocholrins for Immobilization on Electrode Surfaces. Eur. J. Org. Chem. 2001, 1681–1687. [Google Scholar] [CrossRef]
- D'Souza, F.; Villard, A.; Van Caemelbecke, E.; Franzen, M.; Boschi, T.; Tagliatesta, P.; Kadish, K.M. Electrochemical and Spectrochemical Behaviour of Cobalt(III), Cobalt(II), and Cobalt(I) Complexes of meso-Tetraphenylporphyrinate Bearing Bromides on the • -Pyrrole Positions. Inorg. Chem. 1993, 32, 4042–4048. [Google Scholar] [CrossRef]
- Zheng, G.D.; Yan, Y.; Gao, S.; Tong, S.L.; Gao, D.; Zhen, K.J. The reactive Mechanism of alkyl halides with carbon dioxide catalyzed by 5,10,15,20,-Tetraphenyl porphyrin cobalt (CoTPP). Electrochim. Acta 1996, 41, 177–182. [Google Scholar] [CrossRef]
- Araullo-McAdams, C.; Kadish, K.M. Electrochemistry, Spectroscopy, and Reactivity of (meso- Tetrakis(1-methylpyridinium-4-yl)porphyrinato)cobalt(III,II,I) in Nonaqueous Media. Inorg. Chem. 1990, 29, 2749–2757. [Google Scholar] [CrossRef]
- Araki, K.; Angnes, L.; Azevedo, C.M.N.; Toma, H.E. Electrochemistry of tetraruthenated cobalt porphyrin and its use in modified electrodes as sensors of reducing analytes. J. Electroanal. Chem. 1995, 397, 205–210. [Google Scholar] [CrossRef]
- Deronzier, A.; Moutet, J.-C. Polypyrrole films containing metal complexes: syntheses and applications. Coord. Chem. Rev. 1996, 147, 339–371. [Google Scholar] [CrossRef]
- Cauquis, G.; Cosnier, S.; Deronzier, A.; Galland, B.; Limosin, D.; Moutet, J.-C.; Bizot, J.; Deprez, D.; Pulicani, J.-P. Poly(pyrrole-manganese porphyrin): a catalytic electrode material as a model system for olefin epoxidation and drug metabolism with molecular oxygen. J. Electroanal. Chem. 1993, 352, 181–195. [Google Scholar] [CrossRef]
- Thamae, M.; Nyokong, T. Cobalt(II) porphyrazin catalysed reduction of nitrite. J. Electroanal. Chem. 1999, 470, 126–135. [Google Scholar] [CrossRef]
- Malinowska, E.; Niedziolka, J.; Meyerhoff, M.E. Potentiometric and spectroscopic characterization of anion selective electrodes based on metal(III) porphyrin ionophores in polyurethane membranes. Anal. Chim. Acta 2001, 432, 67–78. [Google Scholar] [CrossRef]
- Ann Walker, F.; Beroiz, D.; Kadish, K.M. Electronic Effects in Transition Metal Porphyrins. 2. The Sensitivity of Redox and Ligand Addition Reactions in Para-Substituted Tetraphenylporphyrin Complexes of Cobalt(II). J. of Am. Chem. Soc. 1976, 98, 3484–3489. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cosnier, S.; Gondran, C.; Wessel, R.; Montforts, F.-P.; Wedel, M. A poly(pyrrole-Cobalt(II)deuteroporphyrin) electrode for the potentiometric determination of nitrite. Sensors 2003, 3, 213-222. https://doi.org/10.3390/s30700213
Cosnier S, Gondran C, Wessel R, Montforts F-P, Wedel M. A poly(pyrrole-Cobalt(II)deuteroporphyrin) electrode for the potentiometric determination of nitrite. Sensors. 2003; 3(7):213-222. https://doi.org/10.3390/s30700213
Chicago/Turabian StyleCosnier, Serge, Chantal Gondran, Rudolf Wessel, Franz-Peter Montforts, and Michael Wedel. 2003. "A poly(pyrrole-Cobalt(II)deuteroporphyrin) electrode for the potentiometric determination of nitrite" Sensors 3, no. 7: 213-222. https://doi.org/10.3390/s30700213



