Iodide Selective Electrodes Based on Bis(2-mercaptobenzothiazolato) Mercury(II) and Bis(4-chlorothiophenolato) Mercury(II) Carriers
Abstract
:Introduction
Experimental
Reagents
Preparation of electrodes
Apparatus and potential measurement
Results and discussion
| Complex | Log K1 | Log K2 | Log K3 | Log K4 |
|---|---|---|---|---|
| HgI42− | 12.9 | 11.0 | 3.8 | 2.3 |
| HgBr42− | 9.0 | 8.3 | 1.4 | 1.3 |
| HgCl42− | 6.7 | 6.5 | 0.9 | 1.0 |
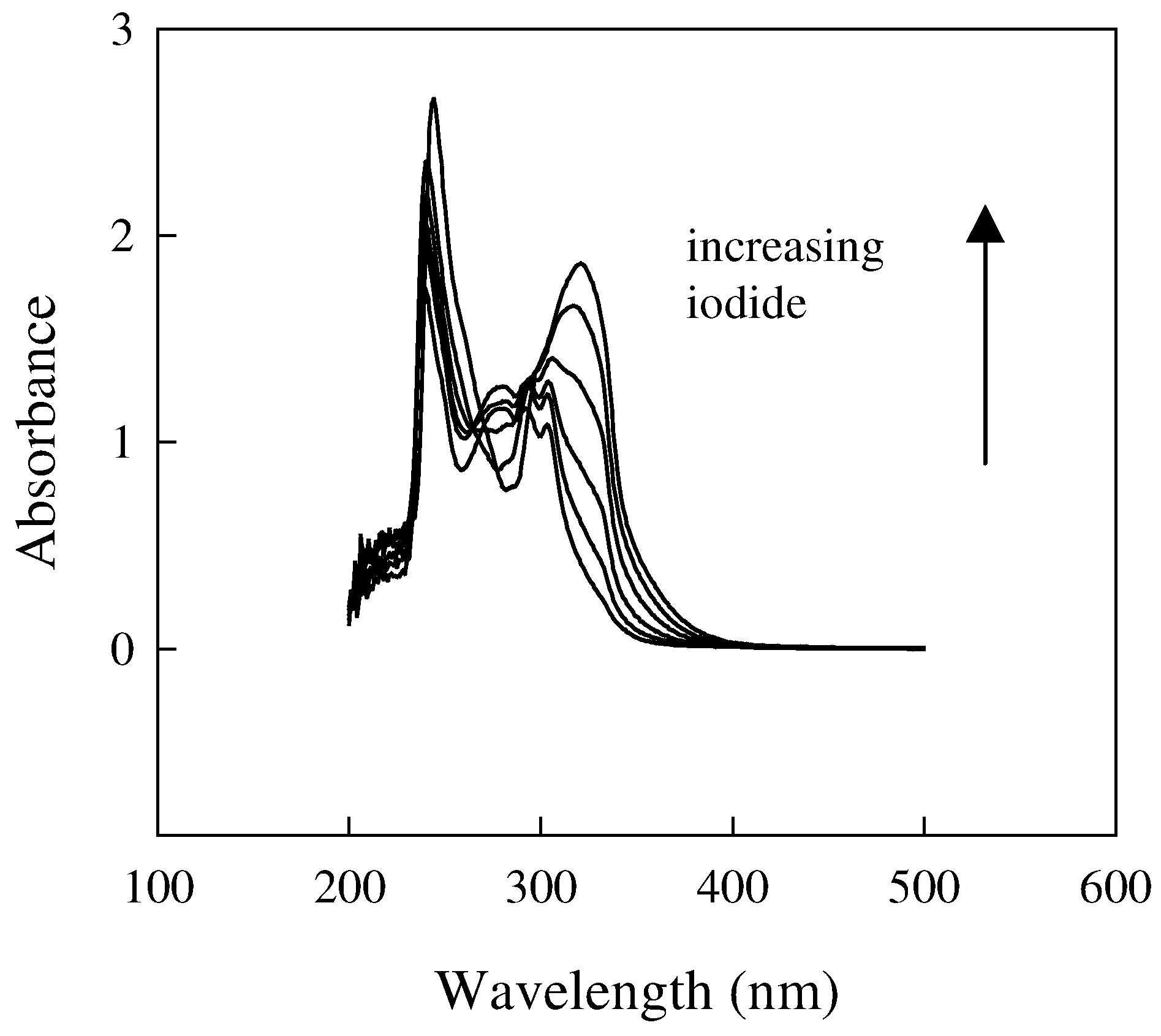
Influence of the membrane composition
| Properties | Values / Range | |
|---|---|---|
| Hg(MBT)2 | Hg(CTP)2 | |
| Optimized membrane composition (% by weight) | PVC (31.0), DBP (61.9), Hg(MBT)2 (5.0), MTOAC (2.1) MTOAC/Hg(MBT)2 mole ratio = 0.55 | PVC (30.9), DOP (61.8), Hg(CTP)2 (5.0), MTOAC (2.3) MTOAC / Hg(CTP)2 mole ratio = 0.56 |
| Electrode type | Coated-graphite (solid contact) electrode | |
| pH range | 3.5−11 for both electrodes | |
| Conditioning time | at least 18 h in 0.05 M KI | |
| Linear range (I−, M) | 1×10−6 -1×10−1 | 1×10−6 -1×10−1 |
| Slope (mV/decade) | −57.6 | −58.4 |
| Detection limit (M) | 4×10−7 | 6×10−7 |
| Standard deviation of slope (within electrode variation, mV/decade) | ± 0.8 | ± 1.4 |
| Standard deviation of slope (between electrode variation, RSD%) | ||
| during 15 days in: 0.05 M KI | ± 1.7 | |
| ambient air | ± 2.3 | |
| distilled water | ± 3.8 | |
| during 1 month in: 0.05 M KI | ± 2.5 | |
| ambient air | ± 3.5 | |
| distilled water | ± 4.3 | |
| Response time (s) | ≤ 10 | ≤ 10 |
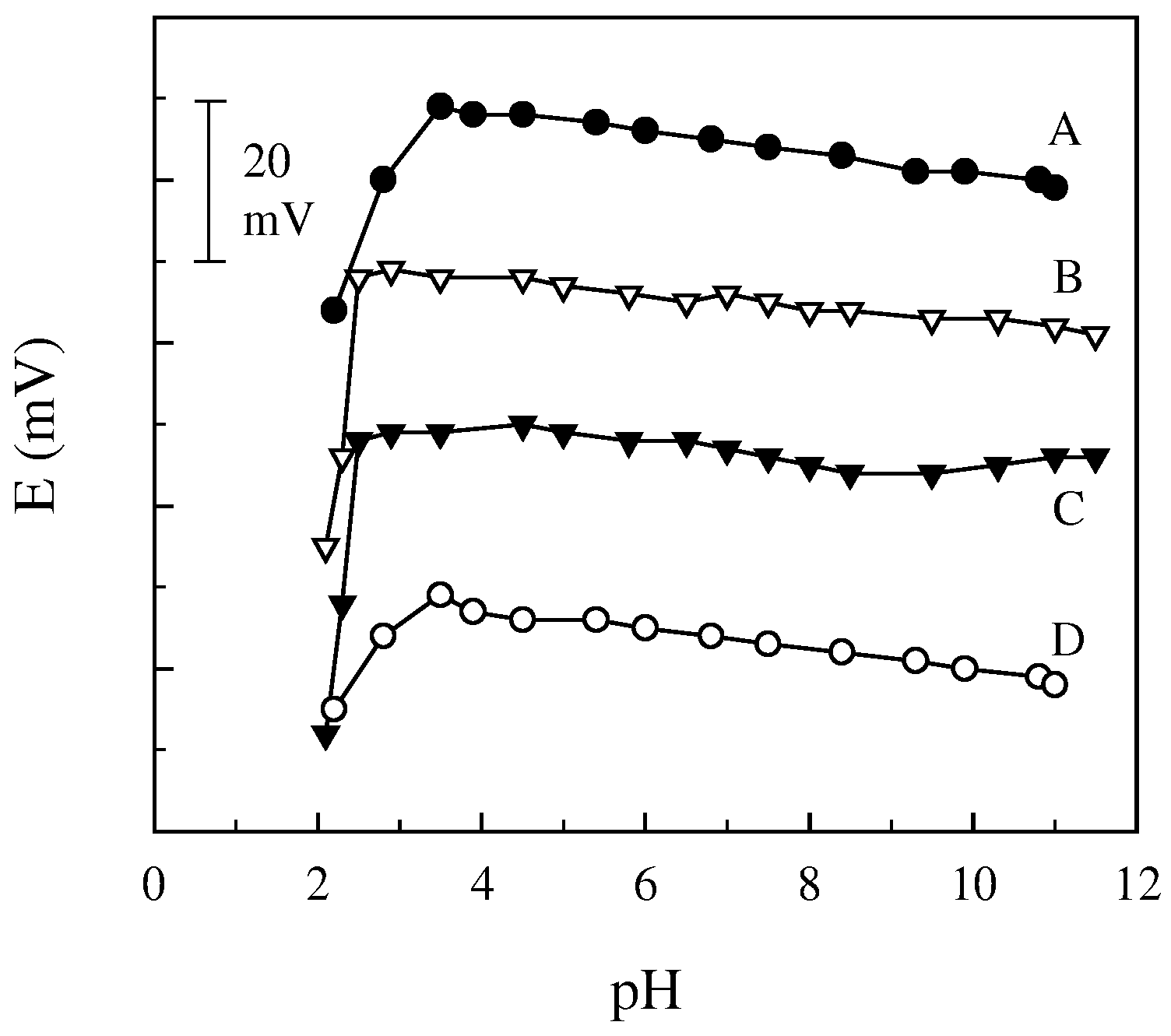
Response characteristics and selectivity of the electrodes


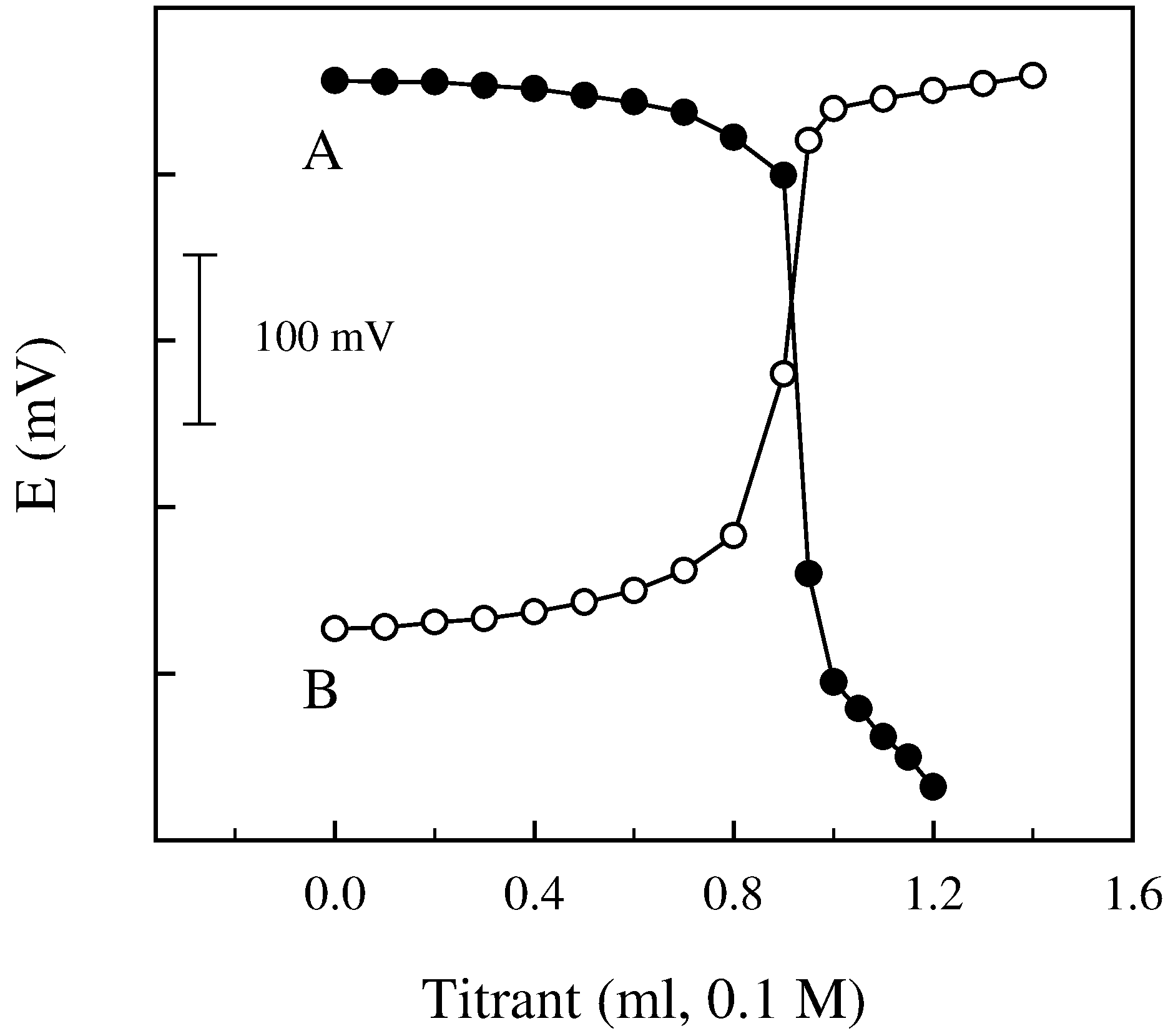
Analytical applications
| LogKSCN,i | ||||
|---|---|---|---|---|
| Hg(MBT)2 | Hg(CTP)2 | |||
| Ion | FIM | SSM | FIM | SSM |
| Perchlorate | −2.57 | −3.15 | −2.45 | −2.85 |
| Salicylate | −4.12 | −4.62 | −3.98 | −4.36 |
| Phosphate | −4.52 | −4.18 | −4.49 | −4.35 |
| Azide | −4.27 | −3.65 | −4.33 | −3.84 |
| Oxalate | −4.65 | −4.76 | −4.70 | −4.78 |
| Bromide | −3.05 | −2.42 | −3.15 | −2.49 |
| Chloride | −3.58 | −3.34 | −3.83 | −3.48 |
| Carbonate | −3.98 | −4.67 | −4.05 | −4.75 |
| Nitrate | −4.83 | −4.86 | −4.78 | −4.93 |
| Nitrite | −4.75 | −4.83 | −4.88 | −4.92 |
| Sulfate | −4.52 | −4.46 | −4.35 | −4.75 |
| Thiocyanate | −2.47 | −1.18 | −2.43 | −1.22 |
| Acetate | −4.78 | −4.72 | −4.53 | −4.86 |
| Fluoride | −3.62 | −3.75 | −3.75 | −3.96 |
Conclusions
| Sample | Iodide added | Iodide found Hg(MBT)2 | Iodide found Hg(CTP)2 |
|---|---|---|---|
| 1. (Tap Water) | − | ND | ND |
| 8.0×10−6 | (8.35 ± 0.38) ×10−6 | (8.41 ± 0.41) ×10−6 | |
| 1.6×10−5 | (1.49 ± 0.08) ×10−5 | (1.52 ± 0.0.07) ×10−5 | |
| 4.0×10−5 | (4.18 ± 0.18) ×10−5 | (4.20 ± 0.17) ×10−5 | |
| 8.0×10−5 | (8.28 ± 0.42) ×10−5 | (8.21 ± 0.40) ×10−5 | |
| 4.90×10−4 | (5.04 ± 0.06) ×10−4 | (5.06 ± 0.0.06) ×10−4 | |
| 4.76×10−3 | (4.77 ± 0.04) ×10−3 | (4.78 ± 0.0.09) ×10−3 | |
| 2. (Meglumine Compound) | (2.78 ± 0.14) ×10−3 * | (2.89 ± 0.16) ×10−3 | (2.66 ± 0.15) ×10−3 |
Acknowledgement
References
- Jakmunee, J.; Grudpan, K. Flow injection amperometry for the determination of iodate in iodized table salt. Anal. Chim. Acta 2001, 438, 299–304. [Google Scholar] [CrossRef]
- Shin, H. S.; Oh-Shin, Y. S.; Kim, J. H.; Ryu, J. K. Trace Level determination of Iodide, Iodine and Iodate. J. Chromatogr. A 1996, 732, 327–333. [Google Scholar] [CrossRef]
- Bichsel, Y.; Von-Gunten, U. Determination of Iodide and Iodate by Ion Chromatography With Post Column Reaction and UV/Visible Detection. Anal. Chem. 1999, 71, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, W.; Ahrer, W. Combination of Suppressed and non-Suppressed Ion Chromatography With Atmospheric Pressure Ionization Mass Spectrometry for the Determination of Anions. J. Chromatogr. A 1999, 850, 99–106. [Google Scholar] [CrossRef]
- Larsen, E. H.; Ludwigsen, M. B. Determination of Iodine in Food-Related Certified Reference Materials Using Wet Ashing and Detection by Inductively Coupled Plasma Mass Spectrometry. J. Anal. At. Spectom. 1997, 12, 435–440. [Google Scholar] [CrossRef]
- Hou, X.; Dahlgaard, H.; Rietz, B.; Jacobsen, U.; Nielsen, S. P.; Aarkrog, A. Determination of Chemical Species of Iodine in Seawater by Radiochemical Neuton Activation Analysis Combined With Ion-Exchange Preseparation. Anal. Chem. 1999, 71, 2745–2750. [Google Scholar] [CrossRef]
- Fujiwara, T.; Mohammadzai, H.; Inoue, H.; Kumamaru, T. Chemiluminescence Determination of Iodide and/or Iodine Using Luminol-Hexadecyltrimethylammonium Chloride Reversed Micelle System Following On-line Oxidation and Extraction. Analyst 2000, 125, 759–763. [Google Scholar] [CrossRef]
- Turner, J.A.; Abel, R.H.; Osteryoung, R. A. Normal Pulse Polarographic Analysis Based on Mercury Anodization. Sulfide and Iodide. Anal. Chem. 1975, 47, 1343–1347. [Google Scholar] [CrossRef]
- Prost, R.C. Cathodic Pulse Stripping Analysis of Iodide at the Parts-Per-Billion Level. Anal. Chem. 1977, 49, 1199–1205. [Google Scholar] [CrossRef]
- Svancara, I.; Konvalina, J.; Schachl, K.; Vytras, K. Stripping Voltammetric Determination of Iodide With Synergistic Accumulation at a Carbon Paste Electrode. Electroanalysis 1998, 10, 435–441. [Google Scholar] [CrossRef]
- Yeom, J.-S.; Won, M.-S.; Shim, Y.-B. Voltammetric Determination of Iodide Ion With Quinine Copper(II) Complex Modified Carbon Paste Electrode. J. Electroanal. Chem. 1999, 463, 16–23. [Google Scholar] [CrossRef]
- Håkedal, J. T.; Egeberg, P. K. Determination of Iodide in Brines by Membrane Permeation Flow Injection Analysis. Analyst 1997, 122, 1235–1237. [Google Scholar] [CrossRef]
- Yonehara, N.; Kozono, S.; Sakamoto, H. Flow Injection Spectrophotometric Determination of Trace Amounts of Iodide by its Catalytic Effect on the 4,4'-Bis(diethylamino)diphenylmethane-Chloramine T Reaction. Anal. Sci. 1991, 7, 229–234. [Google Scholar] [CrossRef]
- Daunert, S.; Wallace, S.; Florido, A.; Bachas, L. G. Anion-Selective Electrodes Based on Polymerized Porphurin Films. Anal. Chem. 1991, 63, 1676–1679. [Google Scholar] [CrossRef]
- Daunert, S.; Florido, A.; Bricker, J.; Dunaway, W.; Bachas, L. G.; Valiente, M. Iodide-Selective Electrodes Based on a Mercury Triisobutylphosphine Sulfide Complex. Electroanalysis 1993, 5, 839–843. [Google Scholar] [CrossRef]
- Bricker, J.; Daunert, S.; Bachas, L. G. Selective Electrodes for Silver and Anions Based on Polymeric Membranes Containing Complexes of Triisobutylphosphine Sulfide with Silver. Anal. Chem. 1991, 63, 1585–1589. [Google Scholar] [CrossRef]
- El Aamrani, F. Z.; Sastre, A.; Aguliar, M.; Beyer, L.; Florido, A. Iodide-Selective Electrodes Based on the Silver(I) Complex of a Novel N-thiocarbamoylimine-dithioether Derivative. Anal. Chim. Acta 1996, 329, 247–252. [Google Scholar] [CrossRef]
- El Aamrani, F. Z.; Garcia-Raurich, J.; Sastre, A.; Beyer, L.; Florido, A. PVC Membrane Based on Silver(I)-Thiourea Complexes. Anal. Chim. Acta 1999, 402, 129–135. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Ren, M. Selectivity of Liquid Membrane Electrode Based on Mercurated Polystyrene as Ion Exchanger to Anionic Surfactants and Soaps. Electroanalysis 1994, 6, 341–347. [Google Scholar] [CrossRef]
- Szczepaniak, W. Mercurated Polystyrene as a Sensor for Anionic Surfactants in Ion-Selective Polymeric Membrane Electrodes. Analyst 1990, 115, 1451–1455. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Yuan, R.; Ying, M.; Li, Z.-Q.; Chai, Y.-Q.; Cui, H.; Shen, G.-L.; Yu, R.-Q. A Highly Selective Iodide Electrode Based on Bis(benzoin)semi-ethylenediamine Complex of Mercury(II) as a Carrier. Fresenius' J. Anal. Chem. 1998, 360, 47–51. [Google Scholar] [CrossRef]
- Yuan, R.; Chai, Y.-Q.; Liu, D.; Gao, D.; Li, J.-Z.; Yu, R.-Q. Schiff Base Complexes of Cobalt(II) as Neutral Carriers for Highly Selective Iodide Electrodes. Anal. Chem. 1993, 65, 2572–2575. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Yuan, R.; Ying, M.; Song, Y.-Q.; Shen, G.-L.; Yu, R.-Q. Iodide Selective PVC Membrane Electrodes Based on Five Transitional Metal Chelates of Bis-furfural-semi-o-tolidine. Anal. Lett. 1997, 30, 1455–1464. [Google Scholar] [CrossRef]
- Ying, M.; Yuan, R.; Zhang, X.-M.; Song, Y.-Q.; Li, Z.-Q.; Shen, G.-L.; Yu, R.-Q. Highly Selective Iodide Poly(vinyl chloride) Membrane Electrode Based on Nickel(II) Tetraazaannulene Macrocyclic Complex. Analyst 1997, 122, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Lizondo-Sabater, J.; Martínez-Máñez, R.; Sancenón, F.; Seguí, M.-J.; Soto, J. Cobalt(II) and Nickel(II) Complexes of a Cyclam Derivative as Carriers in Iodide-Selective Electrodes. Anal. Chim. Acta 2002, 459, 229–234. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, T.; Li, B.; Sun, C. Fabrication of a Covalently Attached Multilayer Film Electrode Containing Cobalt Phthalocyanine and its Applications as a Potentiometric Sensor of Iodide Ion. Anal. Chim. Acta 2002, 455, 61–68. [Google Scholar] [CrossRef]
- Daunert, S.; Bachas, L. G. Anion-Selective Electrodes Based on Hydrophobic Vitamin B12 Derivative. Anal. Chem. 1989, 61, 499–503. [Google Scholar] [CrossRef]
- Shamsipur, M.; Sadeghi, S.; Naeimi, H.; Sharghi, H. Iodide Ion-Selective PVC Membrane Electrode Based on Recently Synthesized Salen-Mn(II) Complex. Polish J. Chem. 2000, 74, 231–238. [Google Scholar]
- Shamsipur, M.; Soleymanpour, A.; Akhond, M.; Sharghi, H.; Naseri, M. A. Iodide-Selective Carbon Paste Electrodes Based on Recently Synthesized Schiff Base Complexes of Fe(III). Anal. Chim. Acta 2001, 450, 37–44. [Google Scholar] [CrossRef]
- Alexander, P. W.; Dimitrakopoulos, T.; Hibbert, D. B. A Six Sensor Array of Coated-Wire Electrodes for Use in a Portable Flow Injection Analyzer. Electroanalysis 1998, 10, 707–712. [Google Scholar] [CrossRef]
- Rover, L., Jr.; Garcia, C. A. B.; de Oliveira-Neto, G.; Kubota, L. T.; Galembeck, F. Acetylsalicylic Acid Determination in Pharmaceutical Samples by FIA Potentiometry Using a Salicylate-Selective Tubular Electrode With an Ethylene-Vinylacetate Membrane. Anal. Chim. Acta 1998, 366, 103–109. [Google Scholar] [CrossRef]
- De Backer, B. L.; Nagels, L. J. Potentiometric Detection for Capillary Electrophoresis: Determination of Organic Acids. Anal. Chem. 1996, 68, 4441–4445. [Google Scholar] [CrossRef]
- Kappes, T.; Schnierle, P.; Hauser, P. C. Potentiometric Detection of Inorganic Anions and Cations in Capillary Electrophoresis With Coated Wire Ion Selective Electrodes. Anal. Chim. Acta 1997, 350, 141–147. [Google Scholar] [CrossRef]
- Schnierle, P.; Kappes, T.; Hauser, P. C. Capillary Electrophoresis Determination of Different Classes of Organic Ions by Potentiometric Detection With Coated-Wire Ion Selective Electrodes. Anal. Chem. 1998, 70, 3585–3589. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pedreno, C.; Ortuno, J. A.; Alvarez, J. Coated-Wire Ion-Selective Electrode for the Determination of Antimony(V). Anal. Chem. 1991, 63, 764–766. [Google Scholar] [CrossRef]
- Cha-Lun, H.; Ren, J. J.; Da-Feng, X. Studies of the Chloropheniramine Solid-State Ion-Selective Electrode. Talanta 1996, 43, 2061–2065. [Google Scholar]
- Yang, Y. H.; Bi, Y. M.; Liu, M.; Fu, J.; Xi, Z. W. The Properties of a Decylidene-bis(4-benzo-15-crown-5) Ether Membrane Rubidium(I) Electrode. Microchem. J. 1997, 55, 348–350. [Google Scholar]
- Amini, M. K.; Shahrokhian, S.; Tangestaninejad, S. PVC-Based Mn(III) Porphyrin Membrane-Coated Graphite Electrode for Determination of Histidine. Anal. Chem. 1999, 71, 2502–2505. [Google Scholar] [CrossRef] [PubMed]
- Amini, M. K.; Shahrokhian, S.; Tangestaninejad, S. Porphyrins as Carriers in Poly(vinyl chloride)-Based Membrane Potentiometric Sensors for Histamine. Analyst 1999, 124, 1319–1322. [Google Scholar] [CrossRef]
- Khalil, S. Ion-Selective Electrode for the Determination of Trazodone in Tablets. Analyst 1999, 124, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Amini, M. K.; Shahrokhian, S.; Tangestaninejad, S. Thiocyanate Selective Electrodes Based on Nickel and Iron Pphthalocyanines. Anal. Chim. Acta 1999, 402, 137–143. [Google Scholar] [CrossRef]
- Missel, P. J. Coated-Wire Ion-Sselective Electrode for Levobetaxolol. Langmuir 1999, 15, 7122–7124. [Google Scholar] [CrossRef]
- Xu, R.; Bloor, D. M. Preparation and Properties of Coated-Wire Ion Selective Electrodes for Anionic and Cationic Surfactant. Langmuir 2000, 16, 9555–9558. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Amini, M. K.; Kia, R.; Tangestaninejad, S. Salicylate-Selective Electrodes Based on Al(III) and Sn(IV) Salophens. Anal. Chem. 2000, 72, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Khaled, E. E.; Hassan, H. N. A.; Barsoum, B. N.; Vytras, K. Kinetic Catalytic Determination of Trace Nitrite Based on the Oxidation of Malachite Green With Bromate Monitored Potentiometrically Using Coated-Wire Electrodes. Electroanalysis 2001, 13, 3338–3341. [Google Scholar] [CrossRef]
- Ueyama, N.; Taniuchi, K.; Okamura, T.; Nakamura, A.; Maeda, H.; Emura, S. Effect of NH-S Hydrogen bond on the Nature of Hg-S Bonding in Bis[2-(acylamino)benzenethiolato] Mercury(II) and Bis[2,6-bis(acylamino)benzenethiolato] Mercury(II) Complexes. Inorg. Chem. 1996, 35, 1945–1951. [Google Scholar] [CrossRef]
- Bell, N. A.; Coles, S. J.; Constable, C. P.; Hibbs, D. E.; Hursthouse, M. B.; Mansor, R.; Raper, E.S.; Sammon, C. Complexes of Heterocyclic Thiones and Group 12 Metals, Part 5. Reactions of 1,3-Thiazolidine-2-thione and Benzo-1,3-thiazoline-2-thione With Mercury(II) Halides in a 2:1 Ratio: Crystal Structure of Bis(1,3-thiazolidine-2-thione) Mercury(II) Bromide and Bis(benzo-1,3-thiazolinato) Mercury(II). Inorg. Chim. Acta 2001, 323, 69–77. [Google Scholar]
- Skoog, D. A.; West, D. M.; Holler, F. J. Fundamentals of Analytical Chemistry, 6th Ed. ed; Saunders College Publishin: New York, 1991; p. 819. [Google Scholar]
- Sample Availability: Available from the authors.
© 2003 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Amini, M.K.; Ghaedi, M.; Rafi, A.; Habibi, M.H.; Zohory, M.M. Iodide Selective Electrodes Based on Bis(2-mercaptobenzothiazolato) Mercury(II) and Bis(4-chlorothiophenolato) Mercury(II) Carriers. Sensors 2003, 3, 509-523. https://doi.org/10.3390/s31100509
Amini MK, Ghaedi M, Rafi A, Habibi MH, Zohory MM. Iodide Selective Electrodes Based on Bis(2-mercaptobenzothiazolato) Mercury(II) and Bis(4-chlorothiophenolato) Mercury(II) Carriers. Sensors. 2003; 3(11):509-523. https://doi.org/10.3390/s31100509
Chicago/Turabian StyleAmini, Mohammad K., Mehrorang Ghaedi, Ali Rafi, Mohammad. H. Habibi, and Morteza M. Zohory. 2003. "Iodide Selective Electrodes Based on Bis(2-mercaptobenzothiazolato) Mercury(II) and Bis(4-chlorothiophenolato) Mercury(II) Carriers" Sensors 3, no. 11: 509-523. https://doi.org/10.3390/s31100509




