Introduction
Entero-gastric reflux is the return of the duodenal contents back into stomach and sometimes even up to esophagus. More and more experimental and clinical evidences suggest that entero-gastric reflux may play an important role in dyspepsia, gastric ulcer, gastritis, gastric cancer, esophageal mucosal injury, or other pathological changes in digestive tract [
1].
It is necessary to find a useful way for the clinic to monitor entero-gastric reflux in situ and continuously for a 24-hour or longer period. Traditional analytical methods need time-consuming sample preparation, careful separation and spectrophotometric procedure and are not suitable for in vivo clinical analysis. Because of its remote and fast sensing, fiber-optic sensors developed recently makes it possible to meet the demand.
This kind of fiber optic sensor needs to be made portable and lightweight. And the sensing system should avoid the cumbersome and expensive spectrophotometers or optical power meters. Moreover the electric supply should ensure the sensor to operate for long-operational detection
in situ. P.Bechi first reported the fiber optic sensor for ambulatory entero-gastric reflux analysis and it has been applied in clinical analysis [
2].
Using the fiber optic sensor, Francesco Baldini et al. examined the relationship between pH of bile samples and its absorption by the in vitro analysis [
3]. However, pH values may influence the entero-gastric fiberoptic sensor in two aspects: pH effect on biological samples in clinical analysis and on standard bilirubin solution as a calibrator in design of the sensor. There has been no description concerned with the latter.
In this paper we design an improved fiber-optic sensor for analysis of entero-gastric reflux and study the pH effect in design of the sensor. In the study, bilirubin standard solutions with pH ranging from 1.24 to 7.76 are used to set up linear regression equations expressing the relationship between bilirubin absorbance and its concentration. The results are compared with that obtained by the in vitro analysis of bilirubin present in bile. The appropriate pH range for the sensor is proposed. The discussions about pH effect on bilirubin estimation of the kind of sensors [
3,
4,
5,
6] are given and possible reasons of their discrepancies are presented.
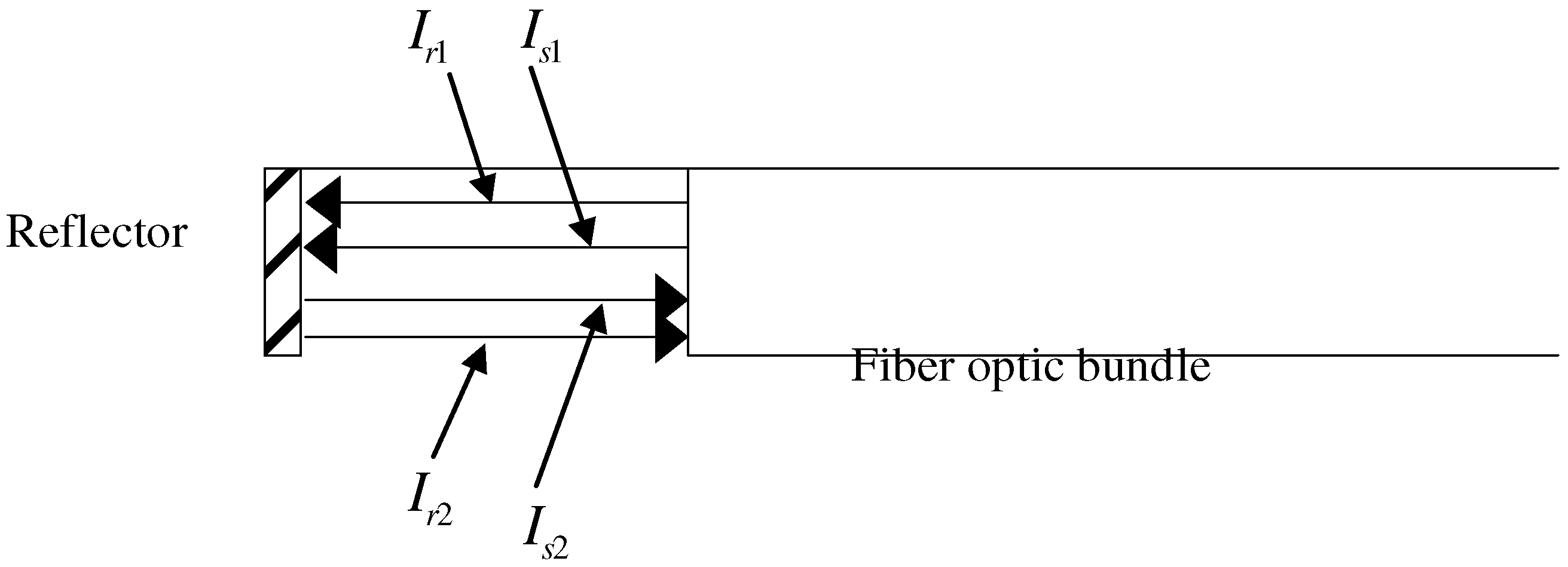
Principle and Experimental Set-up of the Sensor
Figure 1.
The principle of the double-beam compensation. The sensing tip is dipped into sample solution containing an absorbing substance.
Figure 1.
The principle of the double-beam compensation. The sensing tip is dipped into sample solution containing an absorbing substance.
The fiber optic sensor performs on the principle of double-beam compensation [
7] as illustrated in
Figure 1. The reference and signal lights (
Ir1 and
Is1 respectively) transmit out of the fiber-optic bundle and go through the bilirubin solution. The intensities of reflected lights (
Ir2 and
Is2) transmitting back into the bundle are found to be as follows:
where
![Sensors 02 00447 i003]()
(Lambert-Beer law) ,
L is the optic length,
c is the concentration of an absorbing substance and
ε is the molar absorptive coefficient. As for a certain fixed sensor,
α ,
k and
ε can be regarded as constants.
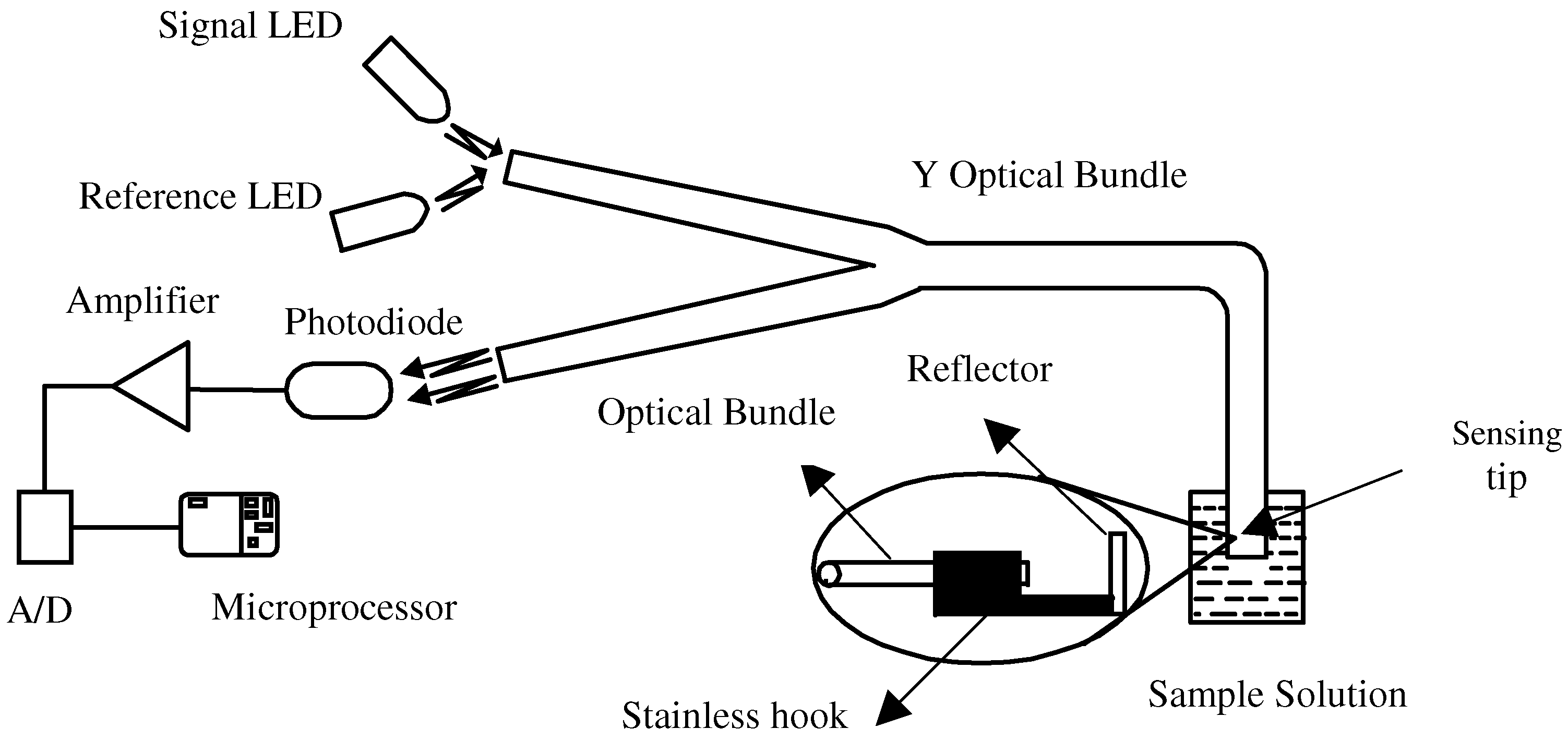
Figure 2 outlines the optical sensing system used for analytical research. Two super bright LEDs (470 nm as signal light and 595 nm as reference light) are utilized as optical sources. A special band-pass filter is positioned before the signal LED. The lights emitting from the LEDs are coupled to one of the arms of the Y-model fiber-optic bundle with core/cladding diameter 300/350
μm . The beams transmit through the bundle to the distal end and then are reflected from a mirror. The reflected beams, traveling into and then out of the other arm of the bifurcated bundle, are converted into electric signals by a photodiode receiver. After A/D conversion, the signals are sampled and stored by a microprocessor and will be analyzed later.
Figure 2.
Experimental set-up of the sensing system.
Figure 2.
Experimental set-up of the sensing system.
Results and Discussion
Two of the most concerns in sensors are their sensitivity and stability. The calibration curves of bilirubin standard solution at pH 1.26 and 7.46 respectively are constructed in
Figure 3. The absorbance/concentration curves exhibit a reasonable linear dynamic range up to 10 mg/dl (1.71×10
−4 mol /
l) with a correlation coefficient of 0.9998. The linear range seems to be better than that reported by P.Becchi [
2] and comparable to that by the spectrophotometric analysis [
6]. The stability is checked by dipping the sensing tip into distilled water and measuring its absorbance every hour for 8 hours. It is found that the mean relative error is less than 2%. The stability can fulfill the clinical analysis.
Figure 3.
Absorbance A vs. concentration of the bilirubin standard solution.
Figure 3.
Absorbance A vs. concentration of the bilirubin standard solution.
As is well known, gastric fluid is normally acidic with pH range 1-2. When pH increases over 4, the pepsin then loses its activity and digestive system may be damaged. But as for the patients with duodenogastroesophageal diseases, pH of gastric juice mixed with duodenal reflux or medications may fluctuate from acid about at pH 1.0 to alkaline at about 7.0. So it is necessary to study pH effect on concentration estimation when the fiber-optic sensors well designed are applied in clinical analysis.
For bilirubin (mono- and di-) glucuronide is not available in the market, unconjugated bilirubin (Bu) or bilirubin conjugate-ditaurobilirubin (DTB) is widely used as calibrators in clinical analysis of bilirubin. In the same way they are applied in design of the fiber optic sensors as calibrators. The relationship between pH and bilirubin absorption of the calibrator is associated with the accuracy of the fiberoptic sensor for clinical analysis.
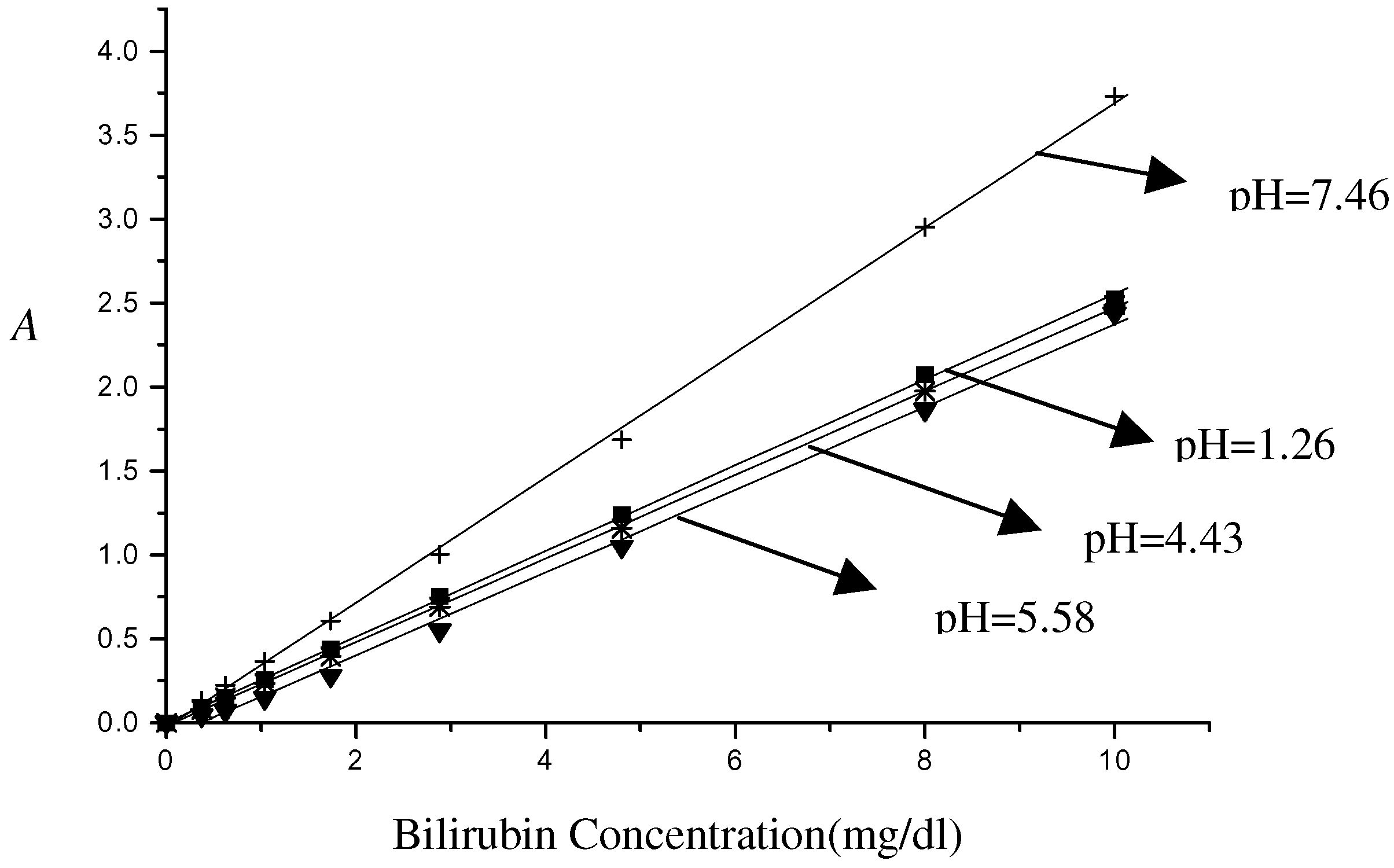
Figure 4 and
Figure 5 show the pH dependence on bilirubin calibrator absorbance by the fiberoptic sensor. Interestingly, a very weak pH dependence is apparent in the acid environment. But at pH>7 the calibration curve strays obviously from those obtained in acid solutions. The maximal relative error of concentration evaluation is about 15% as for 3 mg/dl in acid solution. As pH rises above 7, the estimation error can be up to 36%. Similar results are found in
Figure 5, in which the absorbance is versus different concentrations of bilirubin at 10,8,4 and 0.8 mg/dl in the pH range 1-7.5. As expected, the curves fluctuate little at pH <7 while drastic increase appears in the presence of alkaline pH.
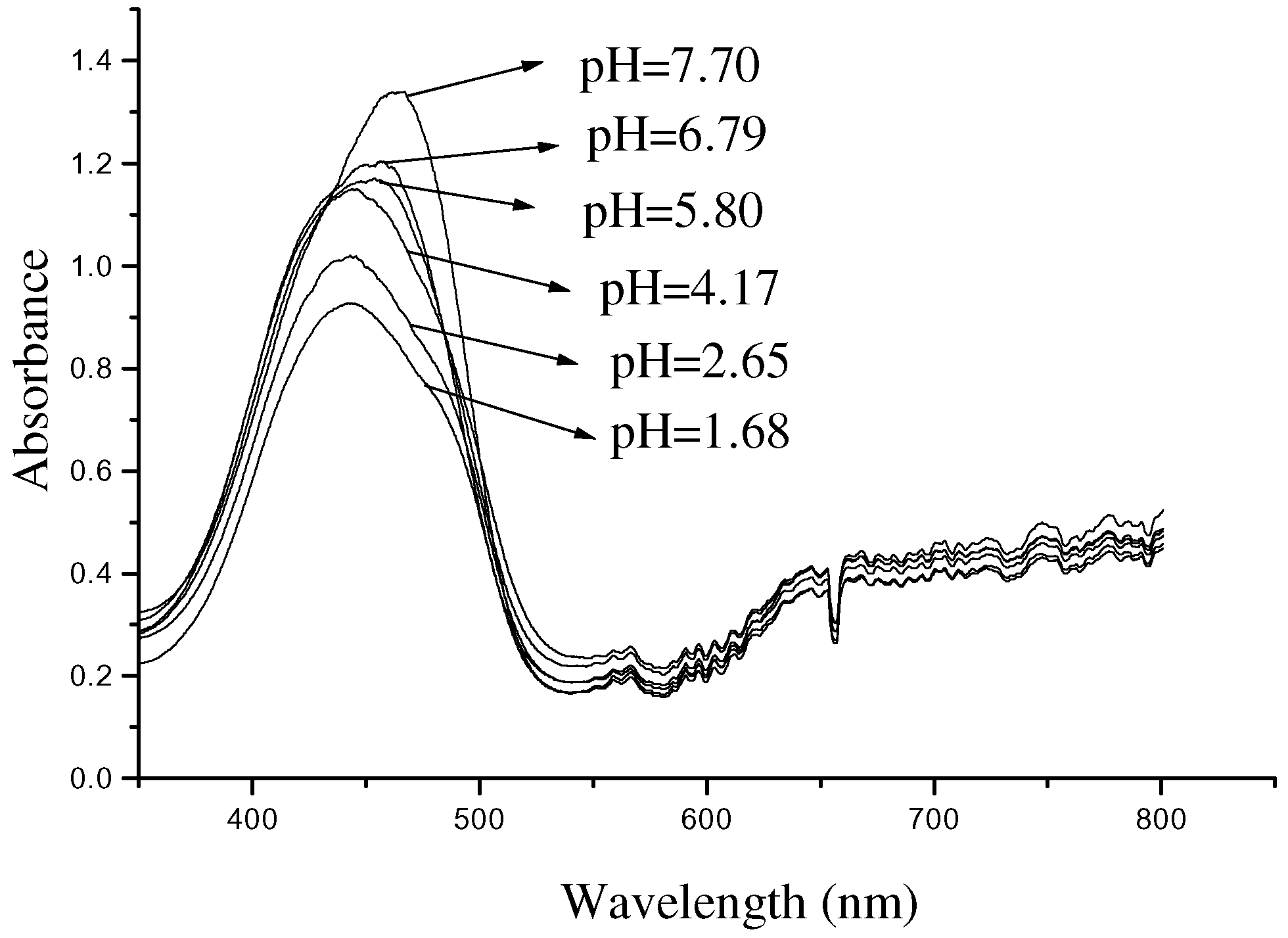
The relation between pH and the absorbance might be attributed to the N-B transformation of albumin conformation [
8]. This can be found in the absorption spectra (
Figure 6) by a fiber optic spectrophotometer (Ocean Optics PS 2000). In
Figure 6 a red shift of about 25 nm for the absorption peak is observed over the 1.68-7.70 pH range, and the shoulders become narrower or relative narrower as pH increases.
Figure 4.
Bilirubin absorbance /concentration curves at different pH.
Figure 4.
Bilirubin absorbance /concentration curves at different pH.
Figure 5.
Absorbance of bilirubin with concentrations at 0.8, 4, 8 and 10 mg/dl in the range of pH 1-8. The absorbance A is deduced from the regression lines obtained.
Figure 5.
Absorbance of bilirubin with concentrations at 0.8, 4, 8 and 10 mg/dl in the range of pH 1-8. The absorbance A is deduced from the regression lines obtained.
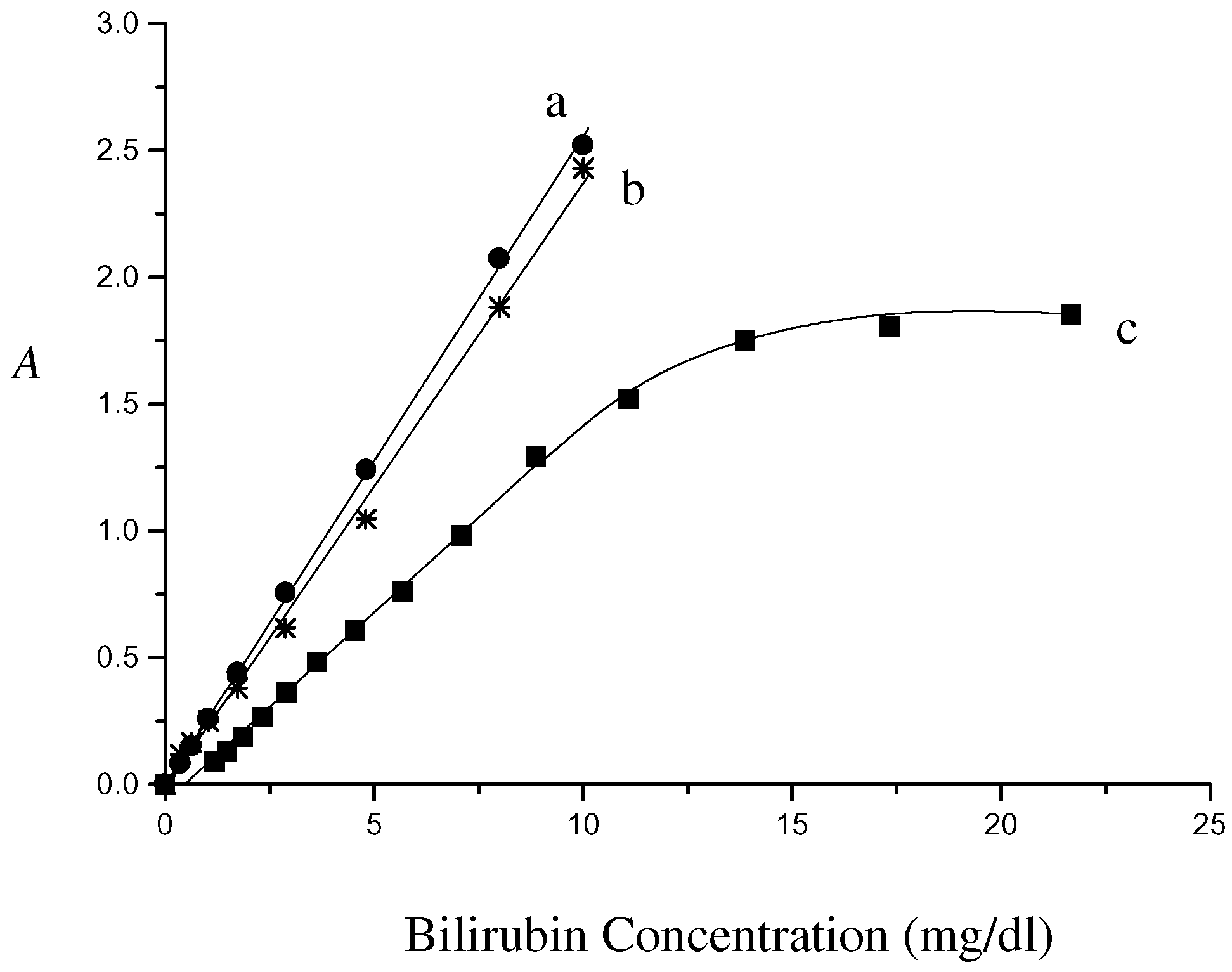
In vitro experimental results for the fiber optic sensor are shown in
Figure 7. The linear regression lines at pH 1.26 and 6.21 are herein plotted together. Signal linearity with up to concentration of 10 mg/dl is achieved for the bile sample detection. The linear response is consistent with the dynamic linear range obtained by the standard bilirubin solution. Above the limit of 10 mg/dl the curve saturates obviously. Considering pH effect on standard calibration, it is easy to find that the line at pH 6.21 is preferably used as the calibration curve in design of the fiber optic sensor. However the calibration (curve b) compared with sampling response (curve c) is still not very ideal. The estimation error as to absorbance 1.0 comes up to 33%.
Figure 6.
Absorbance spectra of bilirubin with different pH values.
Figure 6.
Absorbance spectra of bilirubin with different pH values.
Figure 7.
Comparison of the fiberoptic sensor response in bilirubin standard solution at pH 1.26 (curve a) and 6.21 (curve b) to that in bile mixture with HCl solution at pH 1.16 (curve c).
Figure 7.
Comparison of the fiberoptic sensor response in bilirubin standard solution at pH 1.26 (curve a) and 6.21 (curve b) to that in bile mixture with HCl solution at pH 1.16 (curve c).
There are a few reports concerning the relationship between pH and bilirubin absorbance detected by the commercial fiber optic sensor, Bilitec 2000. And they seem to have no consistency. It was reported by Werner K.H., et al [
4] that there is about 30% underestimation of bilirubin absorbance in acid compared to alkaline environment. And G.Champion et al. [
5] suggested that an underestimation of up to 30 % for pH<3.5 occurred when using the Bilitec 2000. However, based on results of the analysis of bile by Bilitec 2000, Francesco Baldini, et al. [
3] concluded that the maximum error in evaluation of bilirubin concentration was only about 13% instead of 30%. Gong Jun, et al. [
6] also provided some important information about pH and bile sample sensing by Bilitec 2000.
Our results suggest that in acid solution pH has less effect on bilirubin absorbance than in alkaline environment. And even in acidic solution the relative error of concentration estimation corresponding to bilirubin absorbance 1.0 is more than 33%.
The discrepancies discussed above may be explained by the complexity of compounds in biological samples. Bile sample is composed of conjugated bilirubin (diglucuronide and mono glucuronide), unconjugated bilirubin, cholalic acid, phospholipid, and other organic or inorganic components. Their complicated interaction with each other and the respective spectra of colorful constitutes make optic behavior of the bile sample much different from the bilirubin standard solution used as a calibrator for establishment a linear regression equation.
The choice of wavelength can also affect sensing accuracy. The maximal absorption wavelength of bilirubin solution used as a calibrator lies around 450 nm. But the peak of absorption spectrum for the bile sample often shifts to 400 nm when pH is <3. So the estimation error is inevitable using the established calibration equation.
Another factor may be the precipitates resulting from bile compounds at lower pH value. The dissociation constants of conjugated bilirubin in bile are more than 3. Some components of the bile sample become insoluble when mixed with gastric juice whose pH is always 1-2. Then such a condition does not sufficiently meet with the prerequisite for the validity of Lambert-Beer law.


 (Lambert-Beer law) , L is the optic length, c is the concentration of an absorbing substance and ε is the molar absorptive coefficient. As for a certain fixed sensor, α , k and ε can be regarded as constants.
(Lambert-Beer law) , L is the optic length, c is the concentration of an absorbing substance and ε is the molar absorptive coefficient. As for a certain fixed sensor, α , k and ε can be regarded as constants.