Enhanced Hydrogen Detection in ppb-Level by Electrospun SnO2-Loaded ZnO Nanofibers
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis of SnO2-Loaded ZnO NFs
2.2. Device for Material Characterization
2.3. Gas Sensing Test
3. Results and Discussion
3.1. Morphological and Microstructural Study
3.2. Gas Sensing Study
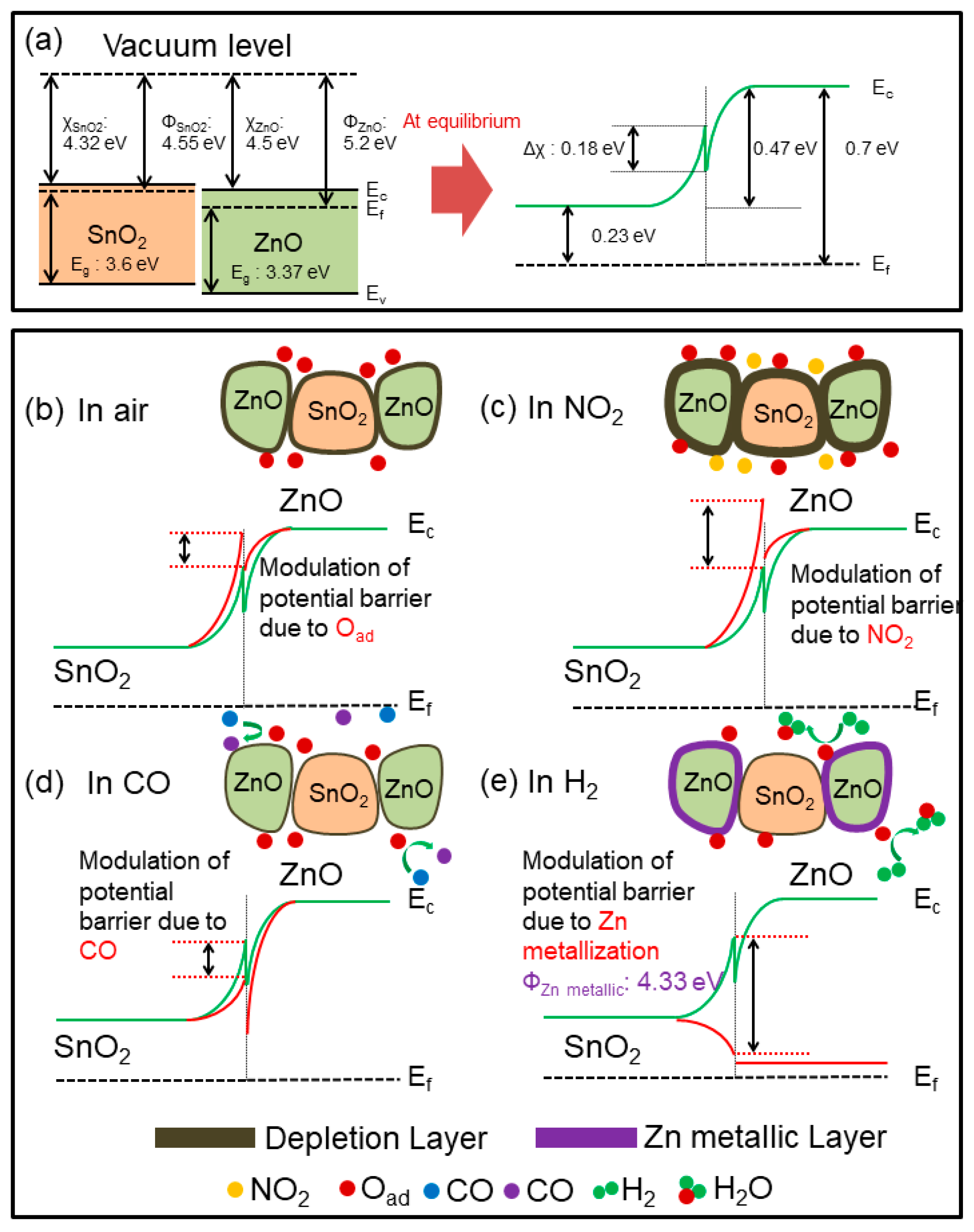
3.3. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamal, T. High performance NiO decorated graphene as a potential H2 gas sensor. J. Alloys Compd. 2017, 729, 1058–1063. [Google Scholar] [CrossRef]
- Mourya, S.; Kumar, A.; Jaiswal, J.; Malik, G.; Kumar, B.; Chandra, R. Development of Pd-Pt functionalized high performance H2 gas sensor based on silicon carbide coated porous silicon for extreme environment applications. Sens. Actuators B 2019, 283, 373–383. [Google Scholar] [CrossRef]
- Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Selectivity towards H2 gas by flame-made Pt-loaded WO3 sensing films. Sens. Actuators B 2011, 157, 290–297. [Google Scholar] [CrossRef]
- Kumar, M.K.; Ramaprabhu, S. Palladium dispersed multiwalled carbon nanotube based hydrogen sensor for fuel cell applications. Int. J. Hydrogen Energy 2007, 32, 2518–2526. [Google Scholar]
- Yang, L.; Yin, C.; Zhang, Z.; Zhou, J.; Xu, H. The investigation of hydrogen gas sensing properties of SAW gas sensor based on palladium surface modified SnO2 thin film. Mater. Sci. Semicond. Process. 2017, 60, 16–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent advancements in optical fiber hydrogen sensors. Sens. Actuators B 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Chen, M.; Zou, L.; Zhang, Z.; Shen, J.; Li, D.; Zong, Q.; Gao, G.; Wu, G.; Shen, J.; Zhang, Z. Tandem gasochromic-Pd-WO3/graphene/Si device for room-temperature high-performance optoelectronic hydrogen sensors. Carbon 2018, 130, 281–287. [Google Scholar] [CrossRef]
- Sawaguchi, N.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N. Enhanced hydrogen selectivity of thermoelectric gas sensor by modification of platinum catalyst surface. Mater. Lett. 2006, 60, 313–316. [Google Scholar] [CrossRef]
- Steinebach, H.; Kannan, S.; Rieth, L.; Solzbacher, F. H2 gas sensor performance of NiO at high temperatures in gas mixtures. Sens. Actuators B 2010, 151, 162–168. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Van Toan, N.; Chien, N.V.; Van Duy, N.; Hong, H.S.; Nguyen, H.; Hoa, N.D.; Van Hieu, N. Fabrication of highly sensitive and selective H2 gas sensor based on SnO2 thin film sensitized with microsized Pd islands. J. Hazard. Mater. 2016, 301, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, F.; Hashemi, B.; Mirzaei, A.; Fazio, E.; Neri, F.; Donato, N.; Leonardi, S.G.; Neri, G. Sm-doped cobalt ferrite nanoparticles: A novel sensing material for conductometric hydrogen leak sensor. Ceram. Int. 2017, 43, 1029–1037. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Kim, H.; Pak, Y.; Jeong, Y.; Kim, W.; Kim, J.; Jung, G.Y. Amorphous Pd-assisted H2 detection of ZnO nanorod gas sensor with enhanced sensitivity and stability. Sens. Actuators B 2018, 262, 460–468. [Google Scholar] [CrossRef]
- Tuscharoen, S.; Kulakeatmongkol, N.; Horprathum, M.; Aiampanakit, K.; Eiamchai, P.; Pattantsetakul, V.; Limwichean, S.; Chananonnawathorn, C.; Hendro; Kaewkhao, J. Low-temperature hydrothermal synthesis single-crystal ZnO nanowire for gas sensor application. Mater. Today Proc. 2018, 5, 15213–15217. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Sensing behavior to ppm-level gases and synergistic sensing mechanism in metal-functionalized rGO-loaded ZnO nanofibers. Sens. Actuators B 2018, 255, 1884–1896. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Zebarjad, S.M.; Bahrololoom, M.E.; Dabiri, E.; Arab, S.M. Synthesis of ZnO/In2O3 composite nanofibers by co-electrospinning: A comprehensive parametric investigating the process. Ceram. Int. 2019, 45, 2530–2541. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Yang, Y.; Geng, M.; Zou, Y.; Shahzad, M.B.; Dai, Y.; Qi, Y. Photocatalytic properties of Fe-doped ZnO electrospun nanofibers. Ceram. Int. 2018, 44, 19998–20005. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.-W.; Kim, S.S. Electrospun metal oxide composite nanofibers gas sensors: A review. J. Korean Ceram. Soc. 2017, 54, 366–379. [Google Scholar] [CrossRef]
- Boyadjiev, S.I.; Kéri, O.; Bárdos, P.; Firkala, T.; Gáber, F.; Nagy, Z.K.; Baji, Z.; Takács, M.; Szilágyi, I.M. TiO2/ZnO and ZnO/TiO2 core/shell nanofibers prepared by electrospinning and atomic layer deposition for photocatalysis and gas sensing. Appl. Surf. Sci. 2017, 424, 190–197. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, J.-H.; Kim, S.S. Influence of hollowness variation on the gas-sensing properties of ZnO hollow nanofibers. Sens. Actuators B 2016, 232, 698–704. [Google Scholar] [CrossRef]
- Cao, F.; Li, C.; Li, M.; Li, H.; Huang, X.; Yang, B. Direct growth of Al-doped ZnO ultrathin nanosheets on electrode for ethanol gas sensor application. Appl. Surf. Sci. 2018, 447, 173–181. [Google Scholar] [CrossRef]
- Hastir, A.; Kohli, N.; Singh, R.C. Ag doped ZnO nanowires as highly sensitive ethanol gas sensor. Mater. Today Proc. 2017, 4, 9476–9480. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Jhang, B.-Y.; Kao, C.; Hsueh, T.-J. UV-illumination and Au-nanoparticles enhanced gas sensing of p-type Na-doped ZnO nanowires operating at room temperature. Sens. Actuators B 2018, 274, 565–574. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhou, L.; Liu, D.; Liu, F.; Liu, F.; Liang, X.; Yan, X.; Gao, Y.; Lu, G. The role of Ce doping in enhancing sensing performance of ZnO-based gas sensor by adjusting the proportion of oxygen species. Sens. Actuators B 2018, 273, 991–998. [Google Scholar] [CrossRef]
- Kudo, M.; Kosaka, T.; Takahashi, Y.; Kokusen, H.; Sotani, N.; Hasegawa, S. Sensing functions to NO and O2 of Nb2O5- or Ta2O5-loaded TiO2 and ZnO. Sens. Actuators B 2000, 69, 10–15. [Google Scholar] [CrossRef]
- Diao, K.; Xiao, J.; Zheng, Z.; Cui, X. Enhanced sensing performance and mechanism of CuO nanoparticle-loaded ZnO nanowires: Comparison with ZnO-CuO core-shell nanowires. Appl. Surf. Sci. 2018, 459, 630–638. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2 (n)-NiO (p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Shao, C.; Li, X.; Ma, J.; Zhang, X.; Liu, Y. Composition-controllable p-CuO/n-ZnO hollow nanofibers for highperformance H2S detection. Sens. Actuators B 2019, 285, 495–503. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, Z.; Zeng, Z.; Zhao, H.; Wang, X.; Xu, J. Light enhanced room temperature resistive NO2 sensor based on a gold-loaded organic–inorganic hybrid perovskite incorporating tin dioxide. Microchim. Acta 2019, 186, 47. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Mola, G.T.; Oseni, S.O.; Kanimozhi, K.; Magdalane, C.M.; Kennedy, J.; Maaza, M. ZnO doped single wall carbon nanotube as an active medium for gas sensor and solar absorber. J. Mater. Sci. Mater. Electron. 2019, 30, 147–158. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Luo, W.; Cheng, X.; Zhu, Y.; El-Toni, A.H.; Khan, A.; Deng, Y.; Zhao, D. Pore engineering of mesoporous tungsten oxides for ultrasensitive gas sensing. Adv. Mater. Interfaces 2019, 6, 1801269. [Google Scholar] [CrossRef]
- Wei, D.; Jiang, W.; Gao, H.; Chuai, X.; Liu, F.; Liu, F.; Sun, P.; Liang, X.; Gao, Y.; Yan, X.; et al. Facile synthesis of La-doped In2O3 hollow microspheres and enhanced hydrogen sulfide sensing characteristics. Sens. Actuators B 2018, 276, 413–420. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.-W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Fabrication and gas sensing properties of vertically aligned Si nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Kwon, Y.J.; Kim, S.S.; Kim, T.W.; Kim, H.W. Selective NO2 sensor based on Bi2O3 branched SnO2 nanowires. Sens. Actuators B 2018, 274, 356–369. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Significant enhancement of hydrogen-sensing properties of ZnO nanofibers through NiO loading. Nanomaterials 2018, 8, 902. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Sun, J. Controlled synthesis of defect-rich ultrathin two-dimensional WO3 nanosheets for NO2 gas detection. Sens. Actuators B 2017, 245, 828–834. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Sun, G.J.; Kheel, H.; Lee, C. Fe2O3/Co3O4 composite nanoparticle ethanol sensor. J. Korean Phys. Soc. 2016, 69, 373–380. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Fan, L.; Yu, Z.; Yan, B.; Xiong, D.; Song, X.; Li, S.; Adair, K.R.; Li, D.; et al. Rational design of Sn/SnO2/porous carbon nanocomposites as anode materials for sodium-ion batteries. Appl. Surf. Sci. 2017, 412, 170–176. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Ma, Y.; Wang, Z.; Chang, Q. Surface defects control for ZnO nanorods synthesized by quenching and their anti-recombination in photocatalysis. Appl. Surf. Sci. 2015, 332, 47–54. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Kim, H.W.; Kim, S.S. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2015, 286, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Enhancement of hydrogen sensing response of ZnO nanowires for the decoration of WO3 nanoparticles. Mater. Lett. 2019, 234, 315–318. [Google Scholar] [CrossRef]
- Ab Kadir, R.; Li, Z.; Sadek, A.Z.; Abdul Rani, R.; Zoolfakar, A.S.; Field, M.R.; Ou, J.Z.; Chrimes, A.F.; Kalantar-zadeh, K. Electrospun granular hollow SnO2 nanofibers hydrogen gas sensors operating at low temperatures. J. Phys. Chem. C 2014, 118, 3129–3139. [Google Scholar] [CrossRef]
- Sun, Z.-P.; Liu, L.; Zhang, L.; Jia, D.-Z. Rapid synthesis of ZnO nano-rods by one-step, room-temperature, solid-state reaction and their gas-sensing properties. Nanotechnology 2006, 17, 2266. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Huang, H.; Lee, Y.C.; Tan, O.K.; Vayssieres, L. Hydrothermally grown oriented ZnO nanorod arrays for gas sensing applications. Nanotechnology 2006, 17, 4995. [Google Scholar] [CrossRef]
- Liu, Y.; Hang, T.; Xie, Y.; Bao, Z.; Song, J.; Zhang, H.; Xie, E. Effect of Mg doping on the hydrogen-sensing characteristics of ZnO thin films. Sens. Actuators B 2011, 160, 266–270. [Google Scholar] [CrossRef]
- Choi, K.-S.; Chang, S.-P. Effect of structure morphologies on hydrogen gas sensing by ZnO nanotubes. Mater. Lett. 2018, 230, 48–52. [Google Scholar] [CrossRef]
- Bhati, V.S.; Ranwa, S.; Fanetti, M.; Valant, M.; Kumar, M. Efficient hydrogen sensor based on Ni-doped ZnO nanostructures by RF sputtering. Sens. Actuators B 2018, 255, 588–597. [Google Scholar] [CrossRef]
- Li, Y.; Deng, D.; Chen, N.; Xing, X.; Liu, X.; Xiao, X.; Wang, Y. Pd nanoparticles composited SnO2 microspheres as sensing materials for gas sensors with enhanced hydrogen response performances. J. Alloys Compd. 2017, 710, 216–224. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Liu, L.; Xu, X.; Wang, Z.; Wang, W.; Zheng, W.; Dong, B.; Wang, C. Enhancement of hydrogen monitoring properties based on Pd–SnO2 composite nanofibers. Sens. Actuators B 2010, 147, 111–115. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Zhang, H.; Wang, Z.; Dong, B.; Jiang, T.; Wang, W.; Li, Z.; Wang, C. Effects of Al doping on SnO2 nanofibers in hydrogen sensor. Sens. Actuators B 2011, 160, 858–863. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Jiang, T.; Xu, X.; Zhang, J.; An, C.; Wang, C. N-type SnO2 nanosheets standing on p-type carbon nanofibers: A novel hierarchical nanostructures based hydrogen sensor. RSC Adv. 2015, 5, 64582–64587. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Li, T.; Li, Z.; Wu, W.; Zhou, C.; Sun, P.; Liu, F.; Yan, X.; Gao, Y.; et al. Highly efficient ethanol gas sensor based on hierarchical SnO2/Zn2SnO4 porous spheres. Sens. Actuators B 2019, 282, 339–346. [Google Scholar] [CrossRef]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO–SnO2 based composite type gas sensor for selective hydrogen sensing. Sens. Actuators B 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, H.W.; Kim, S.S. Grain size tuned highly H2-selective chemiresistive sensors based on ZnO–SnO2 composite nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 2486–2494. [Google Scholar] [CrossRef]
- Katoch, A.; Kim, J.-H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Bifunctional sensing mechanism of SnO2–ZnO composite nanofibers for drastically enhancing the sensing behavior in H2 gas. ACS Appl. Mater. Interfaces 2015, 7, 11351–11358. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B 2016, 230, 736–745. [Google Scholar] [CrossRef]






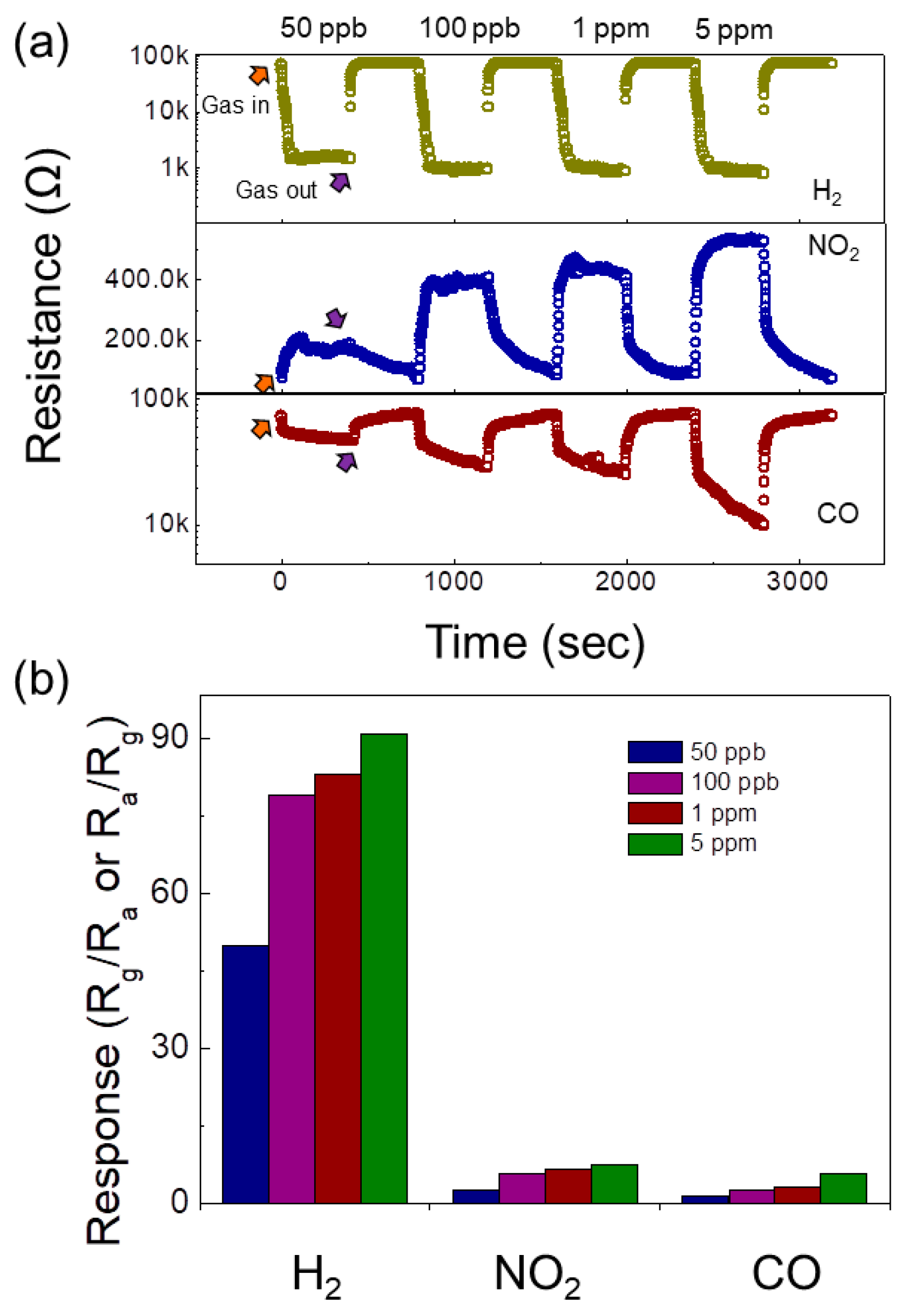
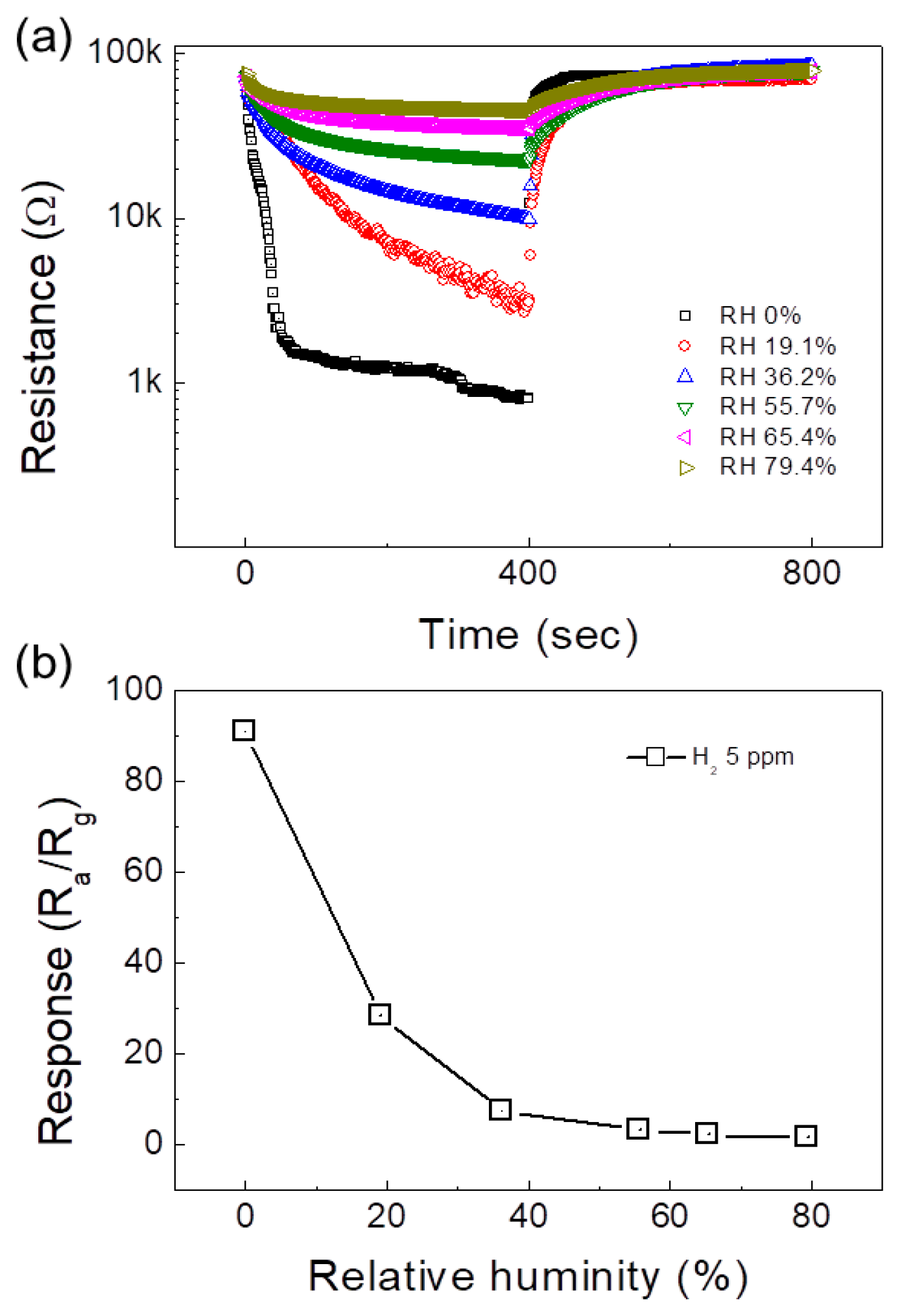
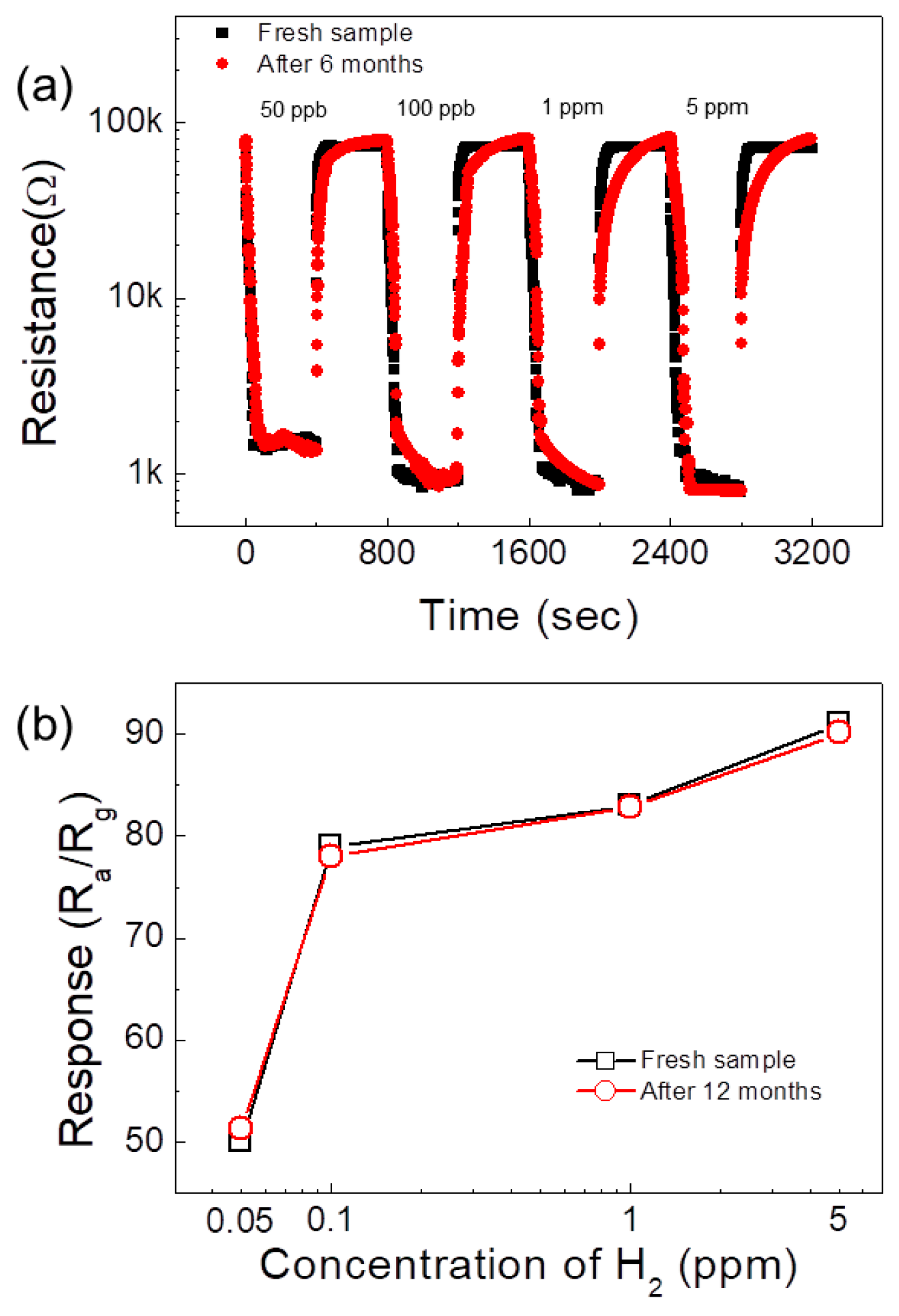
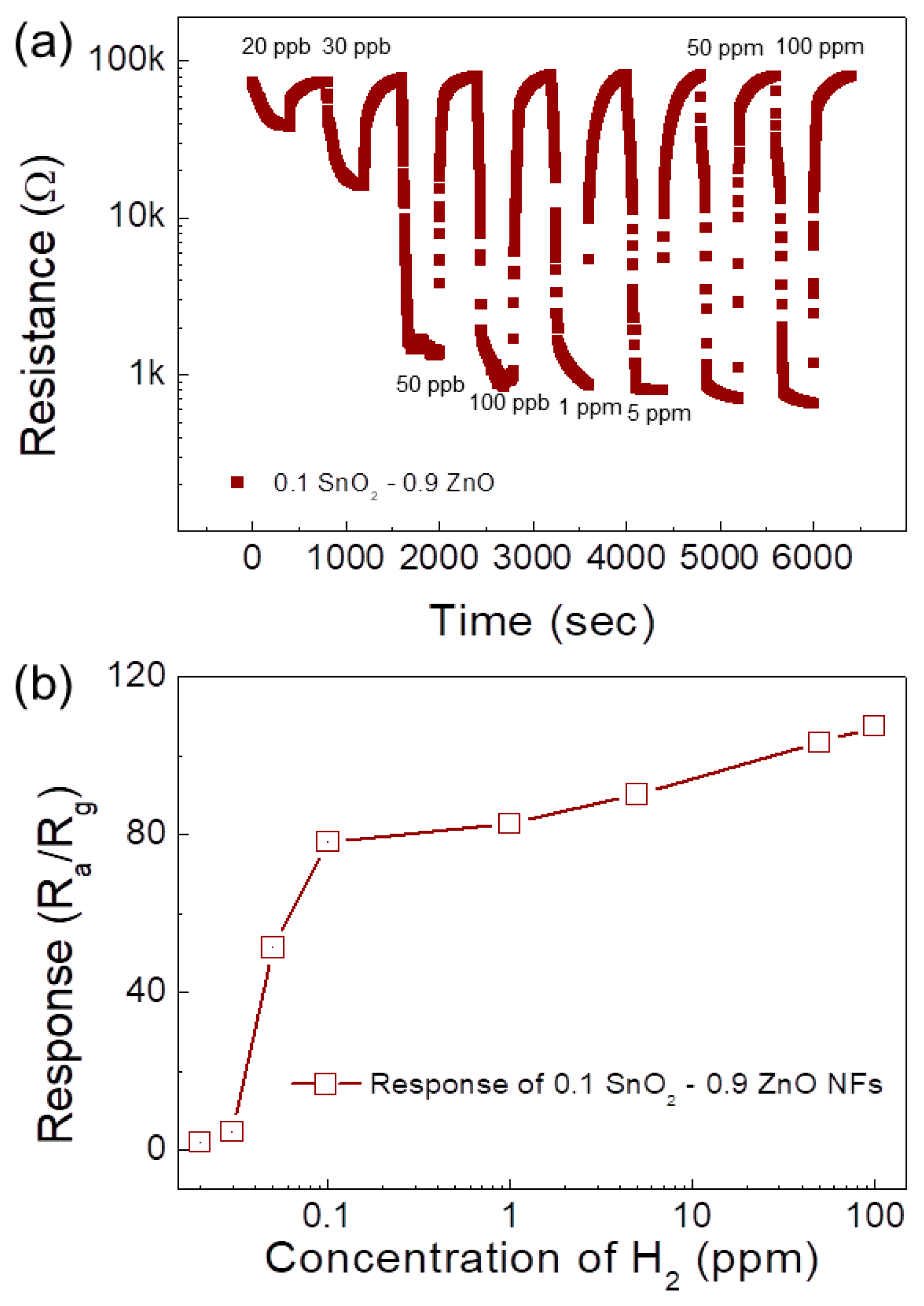
| Sensor | Conc. (ppm) | T (°C) | Response | Ref. |
|---|---|---|---|---|
| 0.1 SnO2 loaded ZnO NFs | 0.05 | 300 | 50.1 a | Present work |
| WO3-ZnO | 2000 | 200 | 13 a | [44] |
| SnO2 NFs | 1000 | 150 | 2.4 a | [45] |
| ZnO Nanorods | 100 | 340 | 5 a | [46] |
| ZnO Nanorods | 1000 | 250 | 11 a | [47] |
| Mg doped ZnO thin films | 5000 | 300 | 50 a | [48] |
| Porous ZnO nanotubes | 5000 | 200 | 8 a | [49] |
| Ni-doped ZnO | 10,000 | 150 | 43.4% b | [50] |
| Pd-SnO2 composite microspheres | 100 | 200 | 16.7 a | [51] |
| Pd-SnO2 NFs | 100 | 280 | 8.2 a | [52] |
| Al-doped SnO2 NFs | 100 | 340 | 7.7 a | [53] |
| SnO2 nanosheets/carbon NFs | 100 | 200 | 16.3 a | [54] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, J.-Y.; Kim, J.-H.; Kim, S.S. Enhanced Hydrogen Detection in ppb-Level by Electrospun SnO2-Loaded ZnO Nanofibers. Sensors 2019, 19, 726. https://doi.org/10.3390/s19030726
Lee J-H, Kim J-Y, Kim J-H, Kim SS. Enhanced Hydrogen Detection in ppb-Level by Electrospun SnO2-Loaded ZnO Nanofibers. Sensors. 2019; 19(3):726. https://doi.org/10.3390/s19030726
Chicago/Turabian StyleLee, Jae-Hyoung, Jin-Young Kim, Jae-Hun Kim, and Sang Sub Kim. 2019. "Enhanced Hydrogen Detection in ppb-Level by Electrospun SnO2-Loaded ZnO Nanofibers" Sensors 19, no. 3: 726. https://doi.org/10.3390/s19030726





